Estimation of Genetic Diversity and Number of Unique Genotypes of Cassava Germplasm from Burkina Faso Using Microsatellite Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. DNA Extraction
2.3. SSR Marker Genotyping
2.3.1. SSR Markers and Polymerase Chain Reaction (PCR)
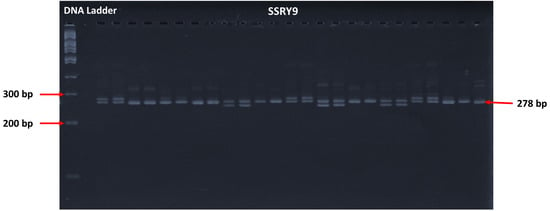
2.3.2. Gel Electrophoresis
2.3.3. Band Scoring
2.4. Analysis of Genetic Diversity
2.5. Analysis of Genetic Structure
3. Results
3.1. Genetic Diversity Parameters
3.2. Population Structure and Genetic Relationships
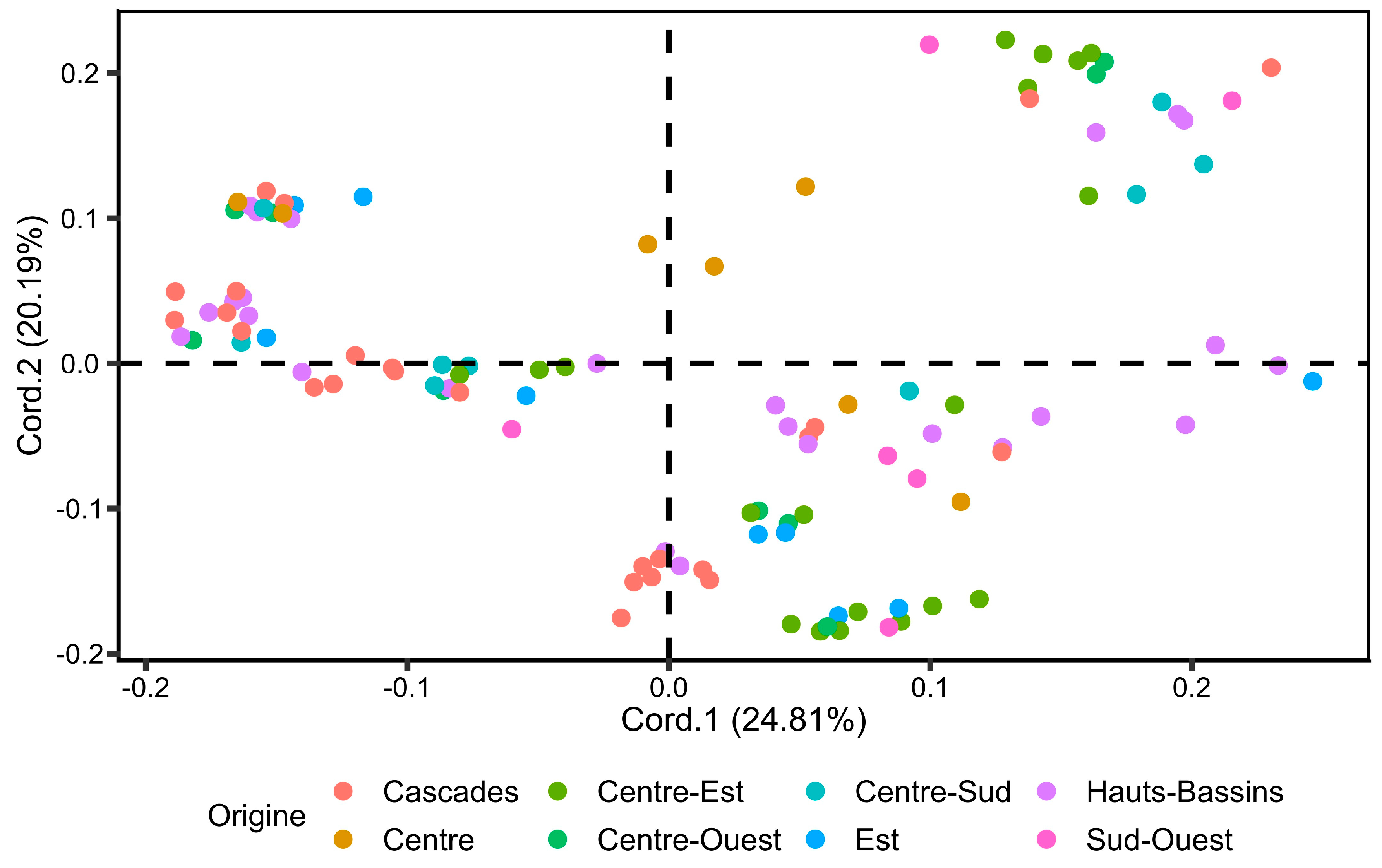
3.2.1. Principal Coordinate Analysis (PCoA)
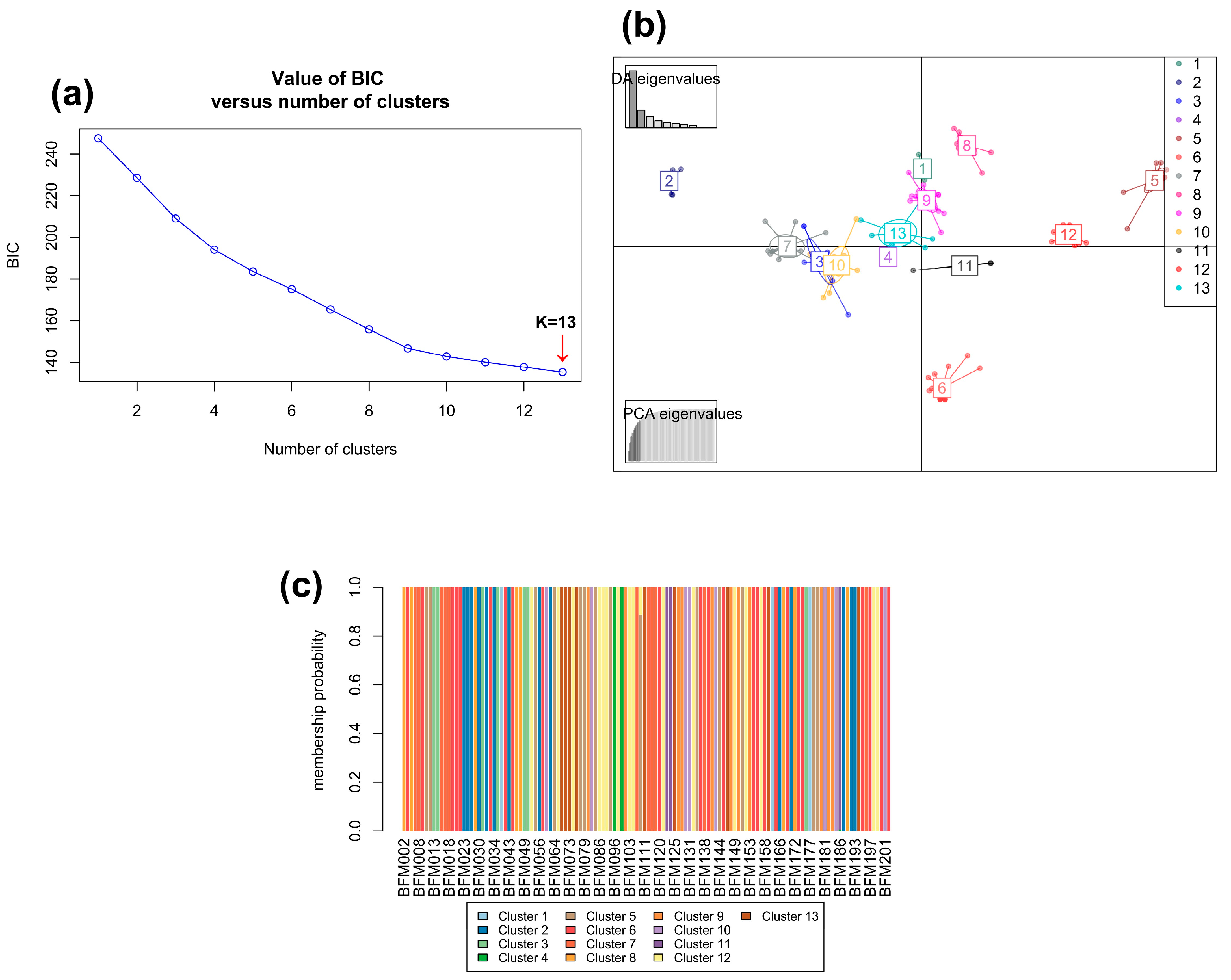
3.2.2. Hierarchical Clustering Analysis and Identification of Duplicate Accessions
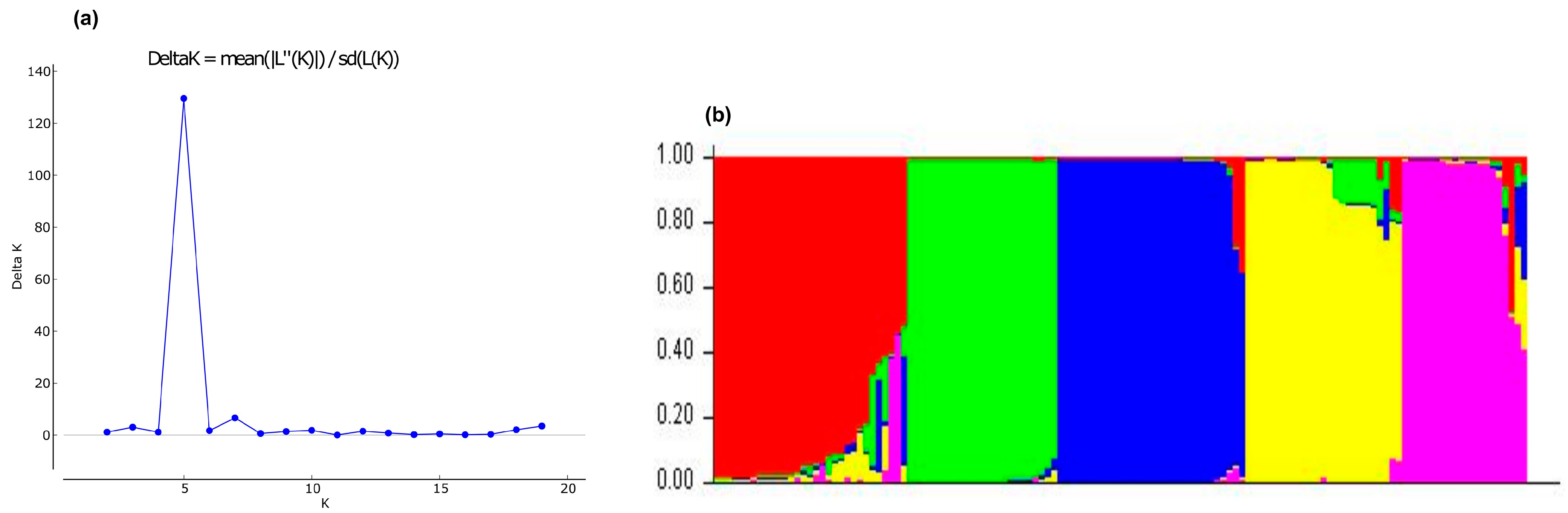
3.2.3. Bayesian Analysis
3.2.4. Discriminant Analysis of Principal Components (DAPC)
3.2.5. Analysis of Molecular Variance (AMOVA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adjebeng-Danquah, J.; Manu-Aduening, J.; Asante, I.K.; Agyare, R.Y.; Gracen, V.; Offei, S.K. Genetic diversity and population structure analysis of Ghanaian and exotic cassava accessions using simple sequence repeat (SSR) markers. Heliyon 2020, 6, e03154. [Google Scholar] [CrossRef] [PubMed]
- Montagnac, J.A.; Davis, C.R.; Tanumihardjo, S.A. Nutritional value of cassava for use as a staple food and recent advances for improvement. Compr. Rev. Food Sci. Food Saf. 2009, 8, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Salvador, E.M.; Steenkamp, V.; McCrindle, C.M.E. Production, consumption and nutritional value of cassava (Manihot esculenta, Crantz) in Mozambique: An overview. J. Agric. Biotechnol. Sustain. Dev. 2014, 6, 29–38. [Google Scholar] [CrossRef]
- Latif, S.; Müller, J. Potential of cassava leaves in human nutrition: A review. Trends Food Sci. Technol. 2015, 44, 147–158. [Google Scholar] [CrossRef]
- Ally, H.M.; Hamss, H.; Simiand, C.; Maruthi, M.N.; Colvin, J.; Omongo, C.A.; Delatte, H. What has changed in the outbreaking populations of the severe crop pest whitefly species in cassava in two decades? Sci. Rep. 2019, 9, 14796. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Organization of the United Nations Statistics Division. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 30 October 2023).
- El-Sharkawy, M.A. Physiological characteristics of cassava tolerance to prolonged drought in the tropics: Implications for breeding cultivars adapted to seasonally dry and semiarid environments. Brazilian J. Plant Physiol. 2007, 19, 257–286. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A. Cassava biology and physiology. Plant Mol. Biol. 2004, 56, 481–501. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.J.; Ferreira, C.F.; Silva, S.D.V.; Jesus, O.D.N.; Oliveira, G.A.F.; Silva, M.D.S. Potential of SNP markers for the characterization of Brazilian cassava germplasm. Theor. Appl. Genet 2014, 127, 1423–1440. [Google Scholar] [CrossRef]
- Elias, M.; Penet, L.; Vindry, P.; McKey, D.; Panaud, O.; Robert, T. Unmanaged sexual reproduction and the dynamics of genetic diversity of a vegetatively propagated crop plant, cassava (Manihot esculenta Crantz), in a traditional farming system. Mol. Ecol. 2001, 10, 1895–1907. [Google Scholar] [CrossRef]
- Park, Y.; Dixit, A.; Ma, K.; Kang, J.; Rao, V.R.; Cho, E. On-farm Conservation Strategy to Ensure Crop Genetic Diversity in Changing Agro-ecosystems in the Republic of Korea. J. Agron. Crop Sci. 2005, 191, 401–410. [Google Scholar] [CrossRef]
- Rao, S.A.; Bounphanousay, C.; Schiller, J.M.; Alcantara, A.P.; Jackson, M.T. Naming of traditional rice varieties by farmers in the Lao PDR. Genet. Resour. Crop Evol. 2002, 49, 83–88. [Google Scholar]
- Ferguson, M.E.; Shah, T.; Kulakow, P.; Ceballos, H. A global overview of cassava genetic diversity. PLoS ONE 2019, 14, e0224763. [Google Scholar] [CrossRef] [PubMed]
- Kawuki, R.S.; Ferguson, M.; Labuschagne, M.T.; Herselman, L.; Orone, J.; Ralimanana, I.; Bidiaka, M.; Lukombo, S.; Kanyange, M.C.; Gashaka, G.; et al. Variation in qualitative and quantitative traits of cassava germplasm from selected national breeding programmes in sub-Saharan Africa. Field Crops Res. 2011, 122, 151–156. [Google Scholar] [CrossRef]
- Kamanda, I.; Blay, E.T.; Asante, I.K.; Danquah, A.; Ifie, B.E.; Parkes, E.; Kulakow, P.; Rabbi, I.; Conteh, A.; Kamara, J.S.; et al. Genetic diversity of provitamin-A cassava (Manihot esculenta Crantz) in Sierra Leone. Genet. Resour. Crop Evol. 2020, 67, 1193–1208. [Google Scholar] [CrossRef]
- Gonçalves, T.M.; Filho, P.S.V.; Vidigal, M.C.G.; Ferreira, R.C.U.; Rocha, V.P.C.; Ortiz, A.H.T.; Moiana, L.D.; Kvitschal, M.V. Genetic diversity and population structure of traditional sweet cassava accessions from Southern of Minas Gerais State, Brazil, using microsatellite markers. Afr. J. Biotechnol. 2017, 16, 346–358. [Google Scholar] [CrossRef]
- Albuquerque, H.D.Y.G.; Oliveira, E.D.J.; Brito, A.C.; Andrade, L.D.R.B.; Carmo, C.D.D.; Morgante, C.V.; Vieira, E.A.; Moura, E.F.; Faleiro, F.G. Identification of duplicates in cassava germplasm banks based on single-nucleotide polymorphisms (SNPs). Sci. Agric. 2019, 76, 328–336. [Google Scholar] [CrossRef]
- Prempeh, R.N.A.; Manu-Aduening, J.A.; Quain, M.D.; Asante, I.K.; Offei, S.K.; Danquah, E.Y. Assessment of genetic diversity among cassava landraces using single nucleotide polymorphic markers. Afr. J. Biotechnol. 2020, 19, 383–391. [Google Scholar]
- Asare, P.A.; Galyuon, I.K.A.; Sarfo, J.K.; Tetteh, J.P. Morphological and molecular based diversity studies of some cassava (Manihot esculenta crantz) germplasm in Ghana. Afr. J. Biotechnol. 2011, 10, 13900–13908. [Google Scholar] [CrossRef]
- Mezette, T.F.; Blumer, C.G.; Veasey, E.A. Morphological and molecular diversity among cassava genotypes. Pesqui. Agropecuária Bras. 2013, 48, 510–518. [Google Scholar] [CrossRef]
- Fregene, M.; Angel, F.; Gómez, R.; Rodriguez, F.; Chavarriaga, P.; Roca, W.; Tohme, J.; Bonierbale, M. A molecular genetic map of cassava (Manihot esculenta Crantz). Theor. Appl. Genet. 1997, 95, 431–441. [Google Scholar] [CrossRef]
- Elias, M.; Panaud, O.; Robert, T. Assessment of genetic variability in a traditional cassava (Manihot esculenta Crantz) farming system, using AFLP markers. Heredity 2000, 85, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Kawuki, R.S.; Herselman, L.; Labuschagne, M.T.; Nzuki, I.; Ralimanana, I.; Bidiaka, M.; Kanyange, M.C.; Gashaka, G.; Masumba, E.; Mkamilo, G.; et al. Genetic diversity of cassava (Manihot esculenta Crantz) landraces and cultivars from southern, eastern and central Africa. Plant Genet. Resour. 2013, 11, 170–181. [Google Scholar] [CrossRef]
- Albuquerque, H.D.Y.G.; Carmo, C.D.D.; Brito, A.C.; Oliveira, E.D.J. Genetic diversity of Manihot esculenta Crantz germplasm based on single-nucleotide polymorphism markers. Ann. Appl. Biol. 2018, 173, 271–284. [Google Scholar] [CrossRef]
- Adu, B.G.; Yeboah, A.; Akromah, R.; Bobobee, E.; Amoah, S.; Kena, A.W.; Amoah, R.A. Whole genome SNPs and phenotypic characterization of cassava (Manihot esculenta Crantz) germplasm in the semi-deciduous forest ecology of Ghana. Ecol. Genet. Genom. 2020, 17, 100068. [Google Scholar] [CrossRef]
- Karim, K.Y.; Ifie, B.; Dzidzienyo, D.; Danquah, E.Y.; Blay, E.T.; Whyte, J.B.A.; Kulakow, P.; Rabbi, I.; Parkes, E.; Omoigui, L.; et al. Genetic characterization of cassava (Manihot esculenta Crantz) genotypes using agro-morphological and single nucleotide polymorphism markers. Physiol. Mol. Biol. Plants 2020, 26, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Nelimor, C.; Badu-Apraku, B.; Garcia-Oliveira, A.L.; Tetteh, A.; Paterne, A.; N’guetta, A.S.-P.; Gedil, M. Genomic Analysis of Selected Maize Landraces from Sahel and Coastal West Africa Reveals Their. Genes 2020, 11, 1054. [Google Scholar] [CrossRef] [PubMed]
- Pierre, N.; Wamalwa, L.N.; Muiru, W.M.; Simon, B.; Kanju, E.; Ferguson, M.E.; Ndavi, M.M.; Tumwegamire, S. Genetic diversity of local and introduced cassava germplasm in Burundi using DArTseq molecular analyses. PLoS ONE 2022, 17, e0256002. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qiao, L.; Chen, B.; Zheng, Y.; Zhi, C.; Zhang, S.; Pan, Y. Plant Diversity SSR markers development and their application in genetic diversity evaluation of garlic (Allium sativum) germplasm. Plant Divers. 2022, 44, 481–491. [Google Scholar] [CrossRef]
- Luo, Z.; Yao, Z.; Yang, Y.; Wang, Z.; Zou, H.; Zhang, X.; Chen, J. Genetic fingerprint construction and genetic diversity analysis of sweet potato (Ipomoea batatas) germplasm resources. BMC Plant Biol. 2023, 23, 355. [Google Scholar] [CrossRef]
- Suvi, W.T.; Shimelis, H.; Laing, M.; Mathew, I.; Titus, W.; Shimelis, H.; Laing, M.; Mathew, I. Assessment of the genetic diversity and population structure of rice genotypes using SSR markers. Acta Agric. Scand. Sect. B—Soil. Plant Sci. 2020, 70, 76–86. [Google Scholar] [CrossRef]
- Soro, M.; Pita, J.S.; Somé, K.; Otron, D.H.; Yéo, E.; Mutuku, J.M.; Néya, J.B.; Tiendrébéogo, F.; Koné, D. Genomic analysis and identification of potential duplicate accessions in Burkina Faso cassava germplasm based on single nucleotide polymorphism. Front. Sustain. Food Syst. 2023, 7, 1202015. [Google Scholar] [CrossRef]
- Singh, N.; Choudhury, D.R.; Singh, A.K.; Kumar, S.; Srinivasan, K.; Tyagi, R.K.; Singh, N.K.; Singh, R. Comparison of SSR and SNP Markers in Estimation of Genetic Diversity and Population Structure of Indian Rice Varieties. PLoS ONE 2013, 8, e84136. [Google Scholar] [CrossRef] [PubMed]
- Permingeat, H.R.; Romagnoli, M.V.; Juliana, I.; Vallejos, R.H. A Simple Method for Isolating DNA of High Yield and Quality from Cotton (Gossypium hirsutum L.) Leaves. Plant Mol. Biol. Report 1998, 16, 89. [Google Scholar] [CrossRef]
- Mba, R.E.C.; Stephenson, P.; Edwards, K.; Melzer, S.; Nkumbira, J.; Gullberg, U.; Apel, K.; Gale, M.; Tohme, J.; Fregene, M. Simple sequence repeat (SSR) markers survey of the cassava (Manihot esculenta Crantz) genome: Towards an SSR-based molecular genetic map of cassava. Theor. Appl. Genet. 2001, 102, 21–31. [Google Scholar] [CrossRef]
- Twumasi, P.; Acquah, E.W.; Quain, M.D.; Parkes, E.Y. Use of simple sequence repeat (SSR) markers to establish genetic relationships among cassava cultivars released by different research groups in Ghanaian. Int. J. Genet. Mol. Biol. 2014, 6, 29–36. [Google Scholar]
- Beovides, Y.; Fregene, M.; Gutiérrez, J.P.; Milián, M.D.; Coto, O.; Buitrago, C.; Cruz, J.A.; Ruiz, E.; Basail, M.; Rayas, A.; et al. Molecular diversity of Cuban cassava (Manihot esculenta Crantz) cultivars assessed by simple sequences repeats (SSR). Biotechnol. Agron. Soc. Environ. 2015, 19, 364–377. [Google Scholar]
- Acquah, W.E.; Quain, D.M.; Twumasi, P. Genetic relationships between some released and elite Ghanaian cassava cultivars based on distance matrices. Afr. J. Biotechnol. 2011, 10, 913–921. [Google Scholar]
- Grünwald, N.J.; Everhart, S.E.; Kamvar, Z.N. Best Practices for Population Genetic Analyses. Phytopathology 2017, 107, 1000–1010. [Google Scholar] [CrossRef]
- Arnaud-Haond, S.; Duarte, C.M.; Alberto, F.; Serrao, E.A. Standardizing methods to address clonality in population studies. Mol. Ecol. 2007, 16, 5115–5139. [Google Scholar] [CrossRef]
- Liu, K.; Muse, S.V. PowerMaker: An integrated analysis environment for genetic maker analysis. Bioinforma Appl. Note 2005, 21, 2128–2129. [Google Scholar] [CrossRef]
- Goudet, J.; Jombart, T.; Kamvar, Z.N.; Archer, E.; Hardy, O. Estimation and Tests of Hierarchical F-Statistics. 2020, pp. 1–73. Available online: https://cran.r-project.org/web/packages/hierfstat/hierfstat.pdf (accessed on 30 October 2023).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R Package Version 2.5-7; 2020. Available online: https://www.researchgate.net/publication/346579465_vegan_community_ecology_package_version_25-7_November_2020 (accessed on 30 October 2023).
- Villanueva, R.A.M.; Chen, Z.J. ggplot2: Elegant Graphics for Data Analysis (2nd ed.). Meas. Interdiscip. Res. Perspect. 2019, 17, 160–167. [Google Scholar] [CrossRef]
- Larmarange, J. JLutils: Collection of R Functions. R Package Version 1.22.0. 2021. Available online: https://github.com/larmarange/JLutils(accessed on 30 October 2023).
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Earl, D.A.; vonHoldt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Jombart, T.; Devillard, S.; Balloux, F. Discriminant analysis of principal components: A new method for the analysis of genetically structured populations. BMC Genet. 2010, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Collard, B.C.Y.; Jahufer, M.Z.Z.; Brouwer, J.B.; Pang, E.C.K. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica 2005, 142, 169–196. [Google Scholar] [CrossRef]
- Anne, C. Choosing the right molecular genetic markers for studying biodiversity: From molecular evolution to practical aspects. Genetica 2006, 127, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Amiteye, S. Basic Concepts And Methodologies Of DAN Marker Systems in Plant Molecular Breeding. Heliyon 2021, 7, e08093. [Google Scholar] [CrossRef]
- Moyib, O.K.; Odunola, O.A.; Dixon, A.G.O. SSR markers reveal genetic variation between improved cassava cultivars and landraces within a collection of Nigerian cassava germplasm. Afr. J. Biotechnol. 2007, 6, 2666–2674. [Google Scholar]
- Turyagyenda, L.F.; Kizito, E.B.; Ferguson, M.E.; Baguma, Y.; Harvey, J.W.; Gibson, P.; Wanjala, B.W.; Osiru, D.S.O. Genetic diversity among farmer-preferred cassava landraces in uganda. Afr. Crop Sci. J. 2012, 20, 15–30. [Google Scholar]
- Pedri, E.C.M.; Hoogerheide, E.S.S.; Tiago, A.V.; Cardoso, E.S.; Pinto, J.M.A.; Santos, L.L.; Yamashita, O.M.; Rossi, A.A.B. Genetic diversity of cassava landraces cultivated in northern Mato Grosso State, Brazil, using microsatellite markers. Genet. Mol. Res. 2019, 18, gmr18315. [Google Scholar] [CrossRef]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef]
- Rabbi, I.Y.; Kulakow, P.A.; Manu-aduening, J.A.; Dankyi, A.A.; Asibuo, J.Y.; Parkes, E.Y.; Abdoulaye, T.; Girma, G.; Gedil, M.A.; Ramu, P.; et al. Tracking crop varieties using genotyping-by-sequencing markers: A case study using cassava (Manihot esculenta Crantz). BMC Genet. 2015, 16, 115. [Google Scholar] [CrossRef]
- Yang, X.; Xu, Y.; Shah, T. Comparison of SSRs and SNPs in assessment of genetic relatedness in maize. Genetica 2011, 139, 1045–1054. [Google Scholar] [CrossRef]
- Emanuelli, F.; Lorenzi, S.; Grzeskowiak, L.; Catalano, V.; Stefanini, M.; Troggio, M.; Myles, S.; Martinez-zapater, J.M.; Zyprian, E.; Moreira, F.M.; et al. Genetic diversity and population structure assessed by SSR and SNP markers in a large germplasm collection of grape. BMC Plant Biol. 2013, 13, 39. [Google Scholar] [CrossRef]



| Markers | Forward Primer | Reverse Primer | PS (pb) | AT (°C) |
|---|---|---|---|---|
| SSRY4 | ATAGAGCAGAAGTGCAGGCG | CTAACGCACACGACTACGGA | 287 | 55 |
| SSRY9 | ACAATTCATCATGAGTCATCAACT | CCGTTATTGTTCCTGGTCCT | 278 | 55 |
| SSRY12 | AACTGTCAAACCATTCTACTTGC | GCCAGCAAGGTTTGCTACAT | 266 | 55 |
| SSRY19 | TGTAAGGCATTCCAAGAATTATCA | TCTCCTGTGAAAAGTGCATGA | 214 | 55 |
| SSRY20 | CATTGGACTTCCTACAAATATGAAT | TGATGGAAAGTGGTTATGTCCTT | 143 | 55 |
| SSRY21 | CCTGCCACAATATTGAAATGG | CAACAATTGGACTAAGCAGCA | 192 | 55 |
| SSRY34 | TTCCAGACCTGTTCCACCAT | ATTGCAGGGATTATTGCTCG | 279 | 55 |
| SSRY38 | GGCTGTTCGTGATCCTTATTAAC | GTAGTTGAGAAAACTTTGCATGAG | 122 | 55 |
| SSRY51 | AGGTTGGATGCTTGAAGGAA | GGATGCAGGAGTGCTCAACT | 298 | 55 |
| SSRY59 | GCAATGCAGTGAACCATCTTT | CGTTTGTCCTTTCTGATGTTC | 158 | 55 |
| SSRY63 | TCAGAATCATCTACCTTGGCA | AAGACAATCATTTTGTGCTCCA | 290 | 55 |
| SSRY64 | CGACAAGTCGTATATGTAGTATTC | GCAGAGGTGGCTAACGAGAC | 194 | 55 |
| SSRY69 | CGATCTCAGTCGATACCCAAG | CACTCCGTTGCAGGCATTA | 239 | 55 |
| SSRY82 | TGTGACAATTTTCAGATAGCTTCA | CACCATCGGCATTAAACTTTG | 211 | 55 |
| SSRY100 | ATCCTTGCCTGACATTTTGC | TTCGCAGAGTCCAATTGTTG | 210 | 55 |
| SSRY102 | TTGGCTGCTTTCACTAATGC | TTGAACACGTTGAACAACCA | 179 | 55 |
| SSRY103 | TGAGAAGGAAACTGCTTGCAC | CAGCAAGACCATCACCAGTTT | 272 | 55 |
| SSRY105 | CAAACATCTGCACTTTTGGC | TCGAGTGGCTTCTGGTCTTC | 225 | 55 |
| SSRY106 | GGAAACTGCTTGCACAAAGA | CAGCAAGACCATCACCAGTTT | 270 | 55 |
| SSRY108 | ACGCTATGATGTCCAAAGGC | CATGCCACATAGTTCGTGCT | 203 | 55 |
| SSRY110 | TTGAGTGGTGAATGCGAAAG | AGTGCCACCTTGAAAGAGCA | 247 | 55 |
| SSRY127 | GCTGAACTGCTTTGCCAACT | CTTCGGCCTCTACAAAAGGA | 130 | 45 |
| SSRY132 | CTTTTTGCCAGTCTTCCTGC | TGTCCAATGTCTTCCTTTCCTT | 196 | 55 |
| SSRY135 | CCAGAAACTGAAATGCATCG | AACATGTGCGACAGTGATTG | 253 | 45 |
| SSRY147 | GTACATCACCACCAACGGGC | AGAGCGGTGGGGCGAAGAGC | 113 | 55 |
| SSRY148 | GGCTTCATCATGGAAAAACC | CAATGCTTTACGGAAGAGCC | 114 | 55 |
| SSRY151 | AGTGGAAATAAGCCATGTGATG | CCCATAATTGATGCCAGGTT | 182 | 55 |
| SSRY155 | CGTTGATAAAGTGGAAAGAGCA | ACTCCACTCCCGATGCTCGC | 158 | 55 |
| SSRY161 | AAGGAACACCTCTCCTAGAATCA | CCAGCTGTATGTTGAGTGAGC | 220 | 55 |
| SSRY169 | TCAAACAAGAATTAGCAGAACTGG | TGAGATTTCGTAATATTCATTTCACTT | 187 | 45 |
| SSRY171 | ACAGCTCTAAAAACTGCAGCC | AACGTAGGCCCTAACTAACCC | 100 | 55 |
| SSRY177 | ACTGTGCCAAAATAGCCAAATAGT | TCATGAGTGTGGGATGTTTTTATG | 291 | 55 |
| SSRY179 | ACCACAAACATAGGCACGAG | CACCCAATTCACCAATTACCA | 268 | 45 |
| SSRY180 | CAGGCTCAGGTGAAGTAAAGG | GCGAAAGTAAGTCTACAACTTTTCTAA | 226 | 55 |
| SSRY181 | CCTTGGCAGAGATGAATTAGAG | GGGGCATTCTACATGATCAATAA | 163 | 55 |
| SSRY182 | GGTAGATCTGGATCGAGGAGG | CAATCGAAACCGACGATACA | 199 | 55 |
| Markers | MaF | AnL | He | Ho | FIT | FIS | FST | PIC |
|---|---|---|---|---|---|---|---|---|
| SSRY4 | 0.35 | 5 | 0.74 | 0.65 | 0.05 | 0.03 | 0.02 | 0.69 |
| SSRY9 | 0.67 | 4 | 0.51 | 0.53 | −0.01 | −0.05 | 0.04 | 0.48 |
| SSRY12 | 0.51 | 3 | 0.58 | 0.98 | −0.70 | −0.74 | 0.02 | 0.49 |
| SSRY19 | 0.58 | 4 | 0.58 | 0.75 | −0.24 | −0.31 | 0.05 | 0.51 |
| SSRY20 | 0.50 | 6 | 0.68 | 0.77 | −0.16 | −0.20 | 0.04 | 0.65 |
| SSRY21 | 0.72 | 4 | 0.43 | 0.54 | −0.25 | −0.28 | 0.02 | 0.39 |
| SSRY34 | 0.94 | 2 | 0.12 | 0.11 | 0.20 | 0.19 | 0.01 | 0.11 |
| SSRY38 | 0.98 | 3 | 0.03 | 0.03 | −0.02 | −0.08 | 0.06 | 0.03 |
| SSRY51 | 0.38 | 3 | 0.66 | 0.90 | −0.35 | −0.35 | 0.00 | 0.59 |
| SSRY59 | 0.46 | 4 | 0.62 | 0.98 | −0.57 | −0.58 | 0.01 | 0.54 |
| SSRY63 | 0.60 | 3 | 0.55 | 0.81 | −0.46 | −0.50 | 0.02 | 0.48 |
| SSRY64 | 0.70 | 2 | 0.42 | 0.25 | 0.39 | 0.33 | 0.09 | 0.33 |
| SSRY69 | 0.50 | 5 | 0.68 | 0.62 | 0.13 | 0.12 | 0.01 | 0.64 |
| SSRY82 | 0.40 | 4 | 0.68 | 0.78 | −0.12 | −0.15 | 0.03 | 0.62 |
| SSRY100 | 0.36 | 5 | 0.69 | 0.63 | 0.02 | 0.02 | 0.00 | 0.63 |
| SSRY102 | 0.78 | 2 | 0.35 | 0.45 | −0.26 | −0.26 | 0.00 | 0.29 |
| SSRY103 | 0.58 | 3 | 0.57 | 0.60 | −0.03 | −0.05 | 0.01 | 0.51 |
| SSRY105 | 0.93 | 3 | 0.14 | 0.13 | 0.18 | 0.16 | 0.03 | 0.13 |
| SSRY106 | 0.72 | 3 | 0.43 | 0.47 | −0.11 | −0.15 | 0.04 | 0.37 |
| SSRY108 | 0.76 | 4 | 0.39 | 0.48 | −0.23 | −0.22 | −0.01 | 0.36 |
| SSRY110 | 0.97 | 2 | 0.06 | 0.06 | −0.02 | −0.02 | 0.00 | 0.06 |
| SSRY135 | 0.69 | 3 | 0.47 | 0.40 | 0.09 | 0.04 | 0.05 | 0.43 |
| SSRY147 | 0.61 | 2 | 0.48 | 0.66 | −0.36 | −0.37 | 0.01 | 0.36 |
| SSRY148 | 0.95 | 2 | 0.09 | 0.09 | −0.05 | −0.09 | 0.03 | 0.08 |
| SSRY151 | 0.32 | 5 | 0.73 | 0.87 | −0.17 | −0.16 | −0.01 | 0.68 |
| SSRY155 | 0.58 | 2 | 0.49 | 0.26 | 0.47 | 0.45 | 0.02 | 0.37 |
| SSRY161 | 0.97 | 2 | 0.06 | 0.06 | −0.03 | −0.04 | 0.01 | 0.06 |
| SSRY169 | 0.96 | 2 | 0.08 | 0.08 | −0.04 | −0.06 | 0.02 | 0.08 |
| SSRY177 | 0.47 | 4 | 0.67 | 0.46 | 0.28 | 0.27 | 0.00 | 0.61 |
| SSRY179 | 0.63 | 3 | 0.52 | 0.21 | 0.47 | 0.43 | 0.06 | 0.44 |
| SSRY180 | 0.84 | 2 | 0.27 | 0.28 | −0.13 | −0.17 | 0.03 | 0.23 |
| SSRY182 | 0.45 | 4 | 0.65 | 0.53 | 0.17 | 0.12 | 0.06 | 0.58 |
| Mean | 0.65 | 3.3 | 0.45 | 0.48 | −0.07 | −0.10 | 0.03 | 0.40 |
| Source of Variation | Geographical Origin | Source of Variation | Breeding Patterns | ||||
| df | Mean Sq | % of Variation | df | Mean Sq | % of Variation | ||
| Between clusters | 7 | 13.04 | 6.31 | Between groups | 1 | 14.83 | 3.33 |
| Within individuals | 122 | 6.40 | 93.69 | Within individuals | 128 | 6.70 | 96.67 |
| Total | 129 | 6.76 | 100.00 | Total | 129 | 6.78 | 100.00 |
| Source of Variation | DAPC | Source of Variation | Bayesian Approach | ||||
| Df | Mean Sq | % of Variation | df | Mean Sq | % of Variation | ||
| Between groups | 12 | 51.55 | 70.09 | Between groups | 4 | 94.06 | 46.73 |
| Within individuals | 117 | 2.17 | 29.91 | Within individuals | 125 | 3.97 | 53.27 |
| Total | 129 | 6.76 | 100.00 | Total | 129 | 6.76 | 100.00 |
| SNP Markers | |
|---|---|
| Type of Clustering | FST |
| Geographical origin | 0.025 |
| Breeding patterns | 0.008 |
| DAPC clusters | 0.307 |
| Bayesian clusters | 0.192 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soro, M.; Zida, S.M.F.W.-P.; Somé, K.; Tiendrébéogo, F.; Otron, D.H.; Pita, J.S.; Néya, J.B.; Koné, D. Estimation of Genetic Diversity and Number of Unique Genotypes of Cassava Germplasm from Burkina Faso Using Microsatellite Markers. Genes 2024, 15, 73. https://doi.org/10.3390/genes15010073
Soro M, Zida SMFW-P, Somé K, Tiendrébéogo F, Otron DH, Pita JS, Néya JB, Koné D. Estimation of Genetic Diversity and Number of Unique Genotypes of Cassava Germplasm from Burkina Faso Using Microsatellite Markers. Genes. 2024; 15(1):73. https://doi.org/10.3390/genes15010073
Chicago/Turabian StyleSoro, Monique, Serge Marie Felicien Wend-Pagnagdé Zida, Koussao Somé, Fidèle Tiendrébéogo, Daniel H. Otron, Justin S. Pita, James B. Néya, and Daouda Koné. 2024. "Estimation of Genetic Diversity and Number of Unique Genotypes of Cassava Germplasm from Burkina Faso Using Microsatellite Markers" Genes 15, no. 1: 73. https://doi.org/10.3390/genes15010073