Abstract
Fatty liver disease is one of the major causes of morbidity and mortality worldwide. Fatty liver includes non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH), now replaced by a consensus group as metabolic dysfunction-associated steatotic liver disease (MASLD). While excess nutrition and obesity are major contributors to fatty liver, the underlying mechanisms remain largely unknown and therapeutic interventions are limited. Reversible chemical modifications in RNA are newly recognized critical regulators controlling post-transcriptional gene expression. Among these modifications, N6-methyladenosine (m6A) is the most abundant and regulates transcript abundance in fatty liver disease. Modulation of m6A by readers, writers, and erasers (RWE) impacts mRNA processing, translation, nuclear export, localization, and degradation. While many studies focus on m6A RWE expression in human liver pathologies, limitations of technology and bioinformatic methods to detect m6A present challenges in understanding the epitranscriptomic mechanisms driving fatty liver disease progression. In this review, we summarize the RWE of m6A and current methods of detecting m6A in specific genes associated with fatty liver disease.
1. Introduction
Chronic nutrient overload results in impaired glucose and lipid metabolism in the liver resulting in lipid accumulation in hepatocytes (steatosis) as the starting point for the spectrum of nonalcoholic fatty liver disease (NAFLD). The prevalence of NAFLD is increasing with 36–48% of the U.S. population affected [1,2,3]. NAFLD ranges from steatosis to non-alcoholic steatohepatitis (NASH), characterized by hepatocellular cell death, inflammation, and fibrosis with accumulation of proinflammatory monocyte-derived macrophages [4]; to cirrhosis and hepatocellular cancer (HCC) [1]. A group of national liver associations recently issued a consensus statement suggested replacing NAFLD with metabolic dysfunction-associated steatotic liver disease metabolic dysfunction-associated steatotic liver disease (MASLD) [5]. The rationale, in part, for the change is that MASALD was considered less stigmatizing in nomenclature for liver disease and MASALD incorporates at least one of five cardiometabolic disease risk factors [5]. For the purposes of this review, we will use the terms NAFLD and NASH due to their prevalence in the reported literature included herein. There are currently no approved therapeutic agents approved for treating NAFLD or NASH [6]. The farnesoid X receptor (FXR) agonist obeticholic acid recently failed to gain approval by the Food and Drug Administration for the treatment of NASH, given its side effects [7,8]. NAFLD is associated with obesity-related metabolic changes, i.e., insulin-resistance and type 2 diabetes mellitus (T2DM), and its prevalence is higher in men than premenopausal women [9,10]. However, after age 70, women have a higher prevalence of NAFLD than men [11]. Women treated with tamoxifen [2,12,13,14] or aromatase inhibitors [15] as endocrine therapies to prevent recurrence of estrogen receptor α positive (ERα+) breast cancer have a higher prevalence of NAFLD, notably in the context of obesity [16]. Studies in mice have demonstrated that estrogens have preventive metabolic effects against NAFLD mediated by hepatic estrogen receptor α (ERα) [17,18]. In addition to obesity, other contributors to NAFLD include genetic variants (SNPs) [19], epigenetics, e.g., altered DNA methylation and miRNA expression [20,21]; gut microbiome dysbiosis [1,2,22], and exposure to metabolism disrupting environmental chemicals (MDCs), e.g., polychlorinated biphenyls (PCBs) [23].
Another layer of coding events in regulatory control is the “epitranscriptome”, i.e., post-transcriptional chemical RNA modifications. Over 170 chemical modifications of transcribed mammalian RNA, including mRNA, rRNA, tRNA, and non-coding (nc) RNAs that regulate transcript fate and function have been identified [24,25,26]. These chemical ‘marks’ on RNA are dynamically regulated in response to cell stress and changes in cellular homeostasis [25,27,28]. N(6)-methyladenosine (m6A) is the most common dynamic modification of the mRNA transcriptome and regulates the transcription, processing, stability and the cellular location of mRNAs, long non-coding RNAs (lncRNAs), eRNAs, rRNA, tRNA, circRNAs, and primary microRNAs (pri-miRNAs) [29,30]. m6A is the best-studied epitranscriptome mark with 45 results in PubMed in a search for “m6A and fatty liver”. m6A is regulated by proteins called “readers, writers, and erasers” (RWE). The m6A modification occurs within the conserved RRACH (DRACH) motif (R = A, G; H = A, C, U) [31] which is recognized by the METTL3 writer (methyltransferase) complex [32,33]. Protein ‘readers’ of m6A regulate transcript fate by altering splicing (AS), subcellular location, stability, degradation, and translation [29,32,34].
There is one recent review on this topic that covered the role of immune cells in the liver and how m6A modification regulates immune cells and genes in lipid homeostasis in the liver as implicated by studies with overexpression of fat mass and obesity associated (FTO), a m6A ‘eraser’, in HepG2 cells [35]. Here we will review the state of knowledge about m6A and other RNA modifications in fatty liver disease.
2. N6-Methyladenosine (m6A)
Recent research has highlighted the important role that m6A modifications play in various cellular processes, including development [36,37], differentiation [38,39], and stress responses [40,41]. In Table 1, the key functions of m6A RNA metabolism are summarized to show how m6A modifications influence the fate of RNA. Dysregulation of m6A modification has been linked to a variety of diseases including cancer [42,43,44,45] and liver disease [46,47,48]. Therefore, there is significant interest in understanding the molecular mechanisms underlying m6A regulation and the potential for targeting this pathway for therapeutic purposes.

Table 1.
Key functions of m6A in mRNA metabolism.
m6A “Writers, Readers, and Erasers”
The intricate interplay involving three groups of key m6A modifiers, referred to as “readers, writers and erasers (RWE)“, plays a crucial role in shaping the outcomes of m6A modifications on RNA metabolism and cellular processes in both normal and stress conditions [58]. These effectors are responsible for the addition, removal, and interpretation of m6A modifications, respectively. Figure 1 provides an overview of RWE involved in m6A modifications in the context of NAFLD. The m6A methyltransferase complex (the m6A “writer” complex), is responsible for catalyzing the m6A methylation on RNA [59,60,61]. The m6A writer complex includes the catalytic methyltransferase like 3 (METTL3) which forms a stable complex with methyltransferase like 14 (METTL14) forming the m6A-METTL Complex (MAC) in which METTL3 transfer the methyl group from S-adenosyl methionine (SAM) to the adenosine residue within the RRACH consensus motif in RNA [62,63]. In addition to the MAC, the m6A writer complex includes regulatory subunits, i.e., Wilms’ tumor 1 (WT1)-associating protein (WTAP) [64], Vir like m6A methyltransferase associated (VIRMA) [65,66,67], RNA binding motif protein 15 (RBM15) [68], RNA binding motif protein 15B (RBM15B) [68,69], and zinc finger CCCH-type containing 13 (ZC3H13) [70]. These proteins interact with MAC and modulate its activity and/or target specificity [71,72,73].
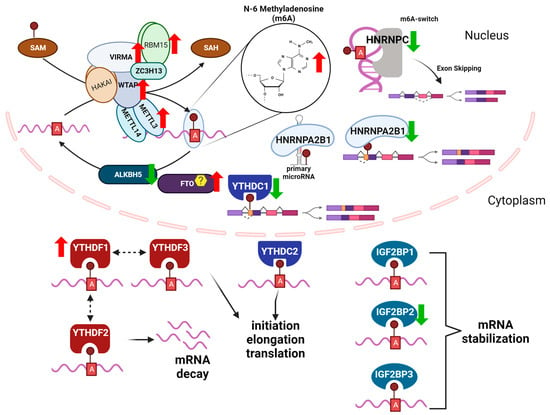
Figure 1.
m6A readers, writers, and erasers (RWE) in NAFLD, based in part on [74] RWE of m6A in mRNA regulate transcript fate. Shown is the METTL3 m6A methyltransferase (writer) complex which includes: METTL3, METT14, WTAP, VIRMA, ZC2H13,RBM15,and HAKAI and the predominant subcellular location and roles of reader proteins, including nuclear RBPS: HNRNPA2B1, HNRNPC, and YTHDC1 and cytoplasmic RBPs: YTHDF1, YTHDF2, YTHDF3, YTHDC2, IGF2BP1, IGF2BP2, and IGF2BP3. ALKBH5 is a specific m6A demethylase, whereas there is controversy as to the specificity of FTO for m6A demethylation as described in the text. The up (red) and down (green) arrows indicate the expression levels generally identified in hepatic disease conditions (see further details in Table 1). Created with BioRender.com.
m6A readers specifically bind to RNA molecules containing the m6A and mediate the downstream effects of the m6A modification on RNA metabolism, including mRNA stability [75,76], splicing [77,78], and translation [79,80]. There are several known families of m6A readers that contain specialized domains or motifs that allow them to specifically recognize and bind to m6A-modified RNA molecules [81,82]. The YTH domain family proteins (YTHDF1, YTHDF2, YTHDF3, YTHDC1, and YTHDC2) play a critical role in regulating mRNA decay and translation [83,84]. YTHDC1 and YTHDC2 are localized in the nucleus and regulate alternative splicing and mRNA export [85,86,87]. YTHDF1, YTHDF2, and YTHDF3 are localized in the cytoplasm and promote mRNA decay [75,88,89]. YTHDF2, for example, is an m6A reader that binds to m6A-modified mRNAs and recruits the CCR4-NOT deadenylase complex, leading to mRNA degradation [90]. In contrast, YTHDF1 and YTHDF3 are involved in enhancing mRNA translation by promoting the recruitment of translation initiation factors to the m6A-modified mRNA [80,91,92]. The heterogeneous nuclear ribonucleoprotein (HNRNP) family of m6A reader proteins regulate mRNA splicing and stability [93]. There are 16 HNRNP family members named alphabetically A-U, classified by their RNA binding domains and have diverse structural features and functions (reviewed in [94]). For example, HNRNPC bound to m6A-modified pre-mRNA and promoted exon skipping in activated T-cells [95]. HNRNPA2B1 (A2B1) stabilized m6A-modified AKT3 mRNA in multiple myeloma cells [96] and HNRNPA1 stabilized SIRT1 mRNA, delaying cellular senescence in HeLa cells [97]. HNRNPs have been shown to play important roles in several steps of miRNA biogenesis. A2B1 binds to pri-miRNAs and to DGCR in the DROSHA complex to promote their processing [98]. Insulin-like growth factor 2 mRNA-binding proteins (IGF2BP1, IGF2BP2, and IGF2BP3) are also m6A readers that regulate mRNA stability and translation [91]. IGF2BP2 is critical in regulating hepatic metabolism (reviewed in [99]).
m6A erasers are demethylases specifically remove the m6A modification from RNA molecules restoring the A to its unmodified RNA state [100]. There are currently two known m6A erasers for mRNA: the fat mass and obesity-associated protein (FTO) [100] and the alkB homolog 5 (ALKBH5) [101]. FTO was originally identified in genome-wide association studies (GWAS) as having a modest, but significant, contribution to childhood and adult obesity [102]. FTO was reported to share motifs with Fe(II)- and 2-oxoglutarate-dependent oxygenases and showed high mRNA expression in the hypothalamic nuclei and in the arcuate nucleus that regulates satiety and feeding behaviors [103]. FTO was later identified as an m6A demethylase in vitro and in HeLa and HEK-293T cell studies [100]. FTO regulates a variety of cellular processes, including adipogenesis [104], energy homeostasis [105], and response to stress [106]. FTO depletion in HepaRG and Huh-7 cells decreased CYP2C8 expression, demonstrating a role for post-transcriptional regulation of cytochrome P450s in drug-metabolism [107]. In a study investigating the effect of the phytoestrogen resveratrol on m6A methylation in high fat diet (HFD)-fed mice, resveratrol increased hepatic Fto transcript abundance that was downregulated with excess nutrition, thus decreasing hepatic m6A RNA methylation [108]. Fusaic acid, a mycotoxin found in contaminated foods, increased global m6A methylation in mouse livers by decreasing Fto expression [109].
There is evidence that ALKBH5 is the demethylase specificly responsible for removing the methyl group from m6A -modified mRNA, whereas FTO is the m6Am demethylase [110,111,112]. The molecular mechanisms by which m6A erasers remove the m6A modification are not yet fully understood, but it is thought that these enzymes use oxidative demethylation reactions to remove the methyl group from the N6 position of the adenosine [113,114]. Overall, the identification and characterization of m6A erasers have provided critical insights into the dynamic nature of the m6A modification and its role in regulating gene expression and cellular processes in liver.
3. Methods for the Detection of m6A Modifications
Driven by a combination of technological innovations and increased understanding of the functional importance of m6A modifications in RNA, the field of m6A detection methods has recently undergone significant advancement. These developments (reviewed in [29,115]) have enabled researchers to gain deeper insights into the role of m6A in gene regulation, cellular processes, and diseases. Early methods for detecting m6A involved traditional antibody-based approaches. Immunoprecipitation (IP) techniques, such as m6A-RNA immunoprecipitation sequencing (meRIP-Seq or m6A-seq), allowed researchers to enrich m6A-modified RNA fragments using specific antibodies to selectively capture transcripts with bound m6A modifications and then sequence the enriched fragments to identify m6A sites within the transcriptome [116]. To improve the specificity and accuracy of m6A site identification, researchers introduced enhanced cross-linking techniques. m6A cross-linking and immunoprecipitation sequencing (m6A-CLIP-Seq) uses UV cross-linking to lock in the interactions between m6A-modified RNA and binding proteins, offering higher precision in identifying m6A sites [117]. Modified CLIP (miCLIP), which also employs the use of UV cross-linking, induces specific mutations (C to T conversions) during reverse transcription (RT) and enhances the precision of identifying m6A sites [117]. Researchers have developed chemical-based methods that exploit the distinct reactivity of m6A-modified adenosines. These methods involve chemical labeling or selective reactions that target m6A modifications, enabling their enrichment and subsequent sequencing. Liquid chromatography and mass spectrometry (LC-MS) can be used to identify m6A modifications [118] and deamination adjacent to RNA modification targets (DART-Seq) utilizes a fusion protein of the m6A-binding YTH domain and the cytidine deaminase APOBEC1 to guide C-to-U editing at cytidine residues adjacent to m6A sites [119,120]. The emergence of nanopore-based single-molecule sequencing technology has brought about a significant breakthrough in m6A detection. Nanopore sequencing allows for direct detection of modifications as RNA molecules pass through a nanopore. This method eliminates the need for antibodies or chemical labeling, providing a direct and unbiased approach to identifying m6A modifications [121,122,123,124].
To enhance the accuracy and comprehensive understanding of m6A modifications, researchers have begun to integrate multiple techniques. For example, combining miCLIP with RNA sequencing methods and machine learning tools provides a more holistic view of m6A dynamics within the context of RNA transcription and processing [125]. The evolution of m6A detection has led to the integration of m6A data with other omics data, such as transcriptomics, epigenomics, and proteomics. Integrating multiple layers of information offers a more complete understanding of how m6A modifications impact gene expression and cellular processes. Accurate interpretation of m6A-seq data requires sophisticated computational algorithms. Bioinformatics tools have evolved to better predict and annotate m6A sites within RNA sequences, allowing researchers to extract more meaningful insights from their experimental data [124,126,127]. Overall, the evolution of m6A detection methods in recent years has been marked by a transition from traditional antibody-based techniques to more advanced and precise approaches. These advancements have not only improved our ability to identify m6A modifications but have also deepened our understanding of their functional implications in various biological contexts.
4. m6A in NAFLD
Relatively little is known about m6A modification in NAFLD. However, emerging studies address the mechanisms of m6A regulation in hepatocellular carcinoma and other liver pathologies (Table 2). Most studies involving m6A and liver disease occurred within the last 4–5 years (Figure 2). Until recently, lack of technologies available to identify and measure transcript-specific m6A presented challenges to understanding the role of m6A modifications in human disease.

Table 2.
Evidence of m6A function in liver pathologies. The role of m6A modification and specific m6A regulators (readers, writers, and erasers) in liver disease.
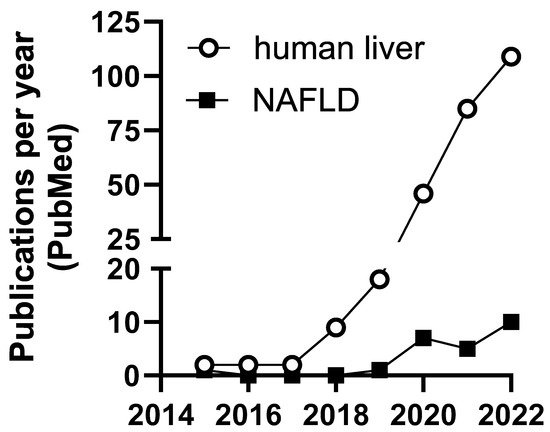
Figure 2.
Number of publications per year including m6A and liver or NAFLD in PubMed.
The first mention of m6A gene regulation in NAFLD was in 2015. In that study, hepatic SAM concentration was decreased with betaine supplementation in HFD-fed mice, resulting in decreased global m6A methylation and increased FTO expression [156]. SAM plays a role in a number of hepatic cellular pathways (reviewed in [157]). Patients with chronic liver disease and HCC have reduced SAM, due to reduce MAT1A expression in one carbon metabolism, although SAM treatment was insufficient to treat SAM deficiency since the liver upregulated compensatory mechanisms to reduce accumulation and dispose of excess SAM (reviewed in [158,159]). The potential of SAM and betaine as therapeutic interventions in MASLD remains to be fully investigated and the potenial impact of these therapies on the m6A epitranscriptome requires investigation.
The next reference to m6A in the context of NAFLD did not appear until 2020. Knockdown of FTO in a mouse hepatocyte cell line decreased Sterol Regulatory Element Binding Transcription Factor (Srebf) and Scd mRNA and protein and luciferase assays demonstrated that mutation of m6A sites in the 3′UTR of Srebf1 and Scd reduced m6A levels and increased mRNA stability in these lipogenic genes [160]. In a zebrafish model of lipid metabolism, exposure to endocrine-disrupting chemicals (EDCs) resulted in increased hepatic lipid accumulation, a decrease in global m6A, and increased FTO expression [161]. Differences in m6A modification in hepatic genes in HFD-fed mice compared to mice on a control diet were demonstrated using m6A-specific RNA immunoprecipitation sequencing (m6A-RIP-seq, also called MeRIP-seq) [162]. mRNA from the livers of mice fed a HFD had 554 differentially methylated peaks, many of those involved in biological pathways associated with lipid metabolism [162].
In a leptin-receptor deficient obese mouse model there was a global increase in m6A methylation and an increase in the expression of lipogenic genes that correlated to liver steatosis [163]. The obese mouse livers also expressed lower levels of the m6A reader Ythdc2 compared to non-obese mouse livers, suggesting a role for Ythdc2 in decreasing lipid accumulation [163]. Mettl3 transcript abundance was increased in the macrophages from obese mouse livers and myeloid-specific Mettl3-knockout resulted in inhibited HFD-induced obesity in mice with lower hepatic lipid accumulation, inflammation, and fibrosis, demonstrating m6A involvement in immune mechanisms that contribute to NAFLD and progression to NASH [164]. In a study comparing two NAFLD models (leptin-deficient db/db mice and high fructose fed (HFrD)-mice), both groups had increased lipid metabolism compared to normal diet control, however, the HFrD mice had an increased inflammatory response and increased m6A methylation of FASN mRNA [165] demonstrating diet-specific differences in m6A regulation of inflammation. In a HFD-fed mouse model, upregulation of Mettl3 and Ythdf1 and a global increase of m6A were noted in hepatocytes [166]. Knockdown of Mettl3 or Ythdf1 decreased Rubicon, an autophagosome-lysosome fusion suppressing protein, reducing clearance of autophagosomes, indicating a role for m6A and m6A-RBPs in regulating autophagy in NAFLD [166]. In a HFD-induced NAFLD mouse model, infection with an adenovirus expressing FTO (ad-FTO) increased mRNA and protein expression of SREBF1 and ChREBP [167]. SREBF1 and ChREBP stabilized these transcripts and promoted lipogenesis [167]. In a mouse model of CCl4-induced liver fibrosis, global m6A levels were increased in fibrotic livers compared to normal mouse livers and this m6A increase was inversely correlated to a significant decline in ALKBH5 expression [168]. Drp1 encodes a protein that mediates mitochondrial fission in HSC cells, promoting proliferation and migration, and YTHDF1 bound to Drp1 (gene Dnm1l, dynamin 1-lik2) mRNA at an m6A motif and promoted Drp1 translation [168]. Furthermore, forced expression of ALKBH5 in HSC-T6 immortalized rat hepatic stellate cells correlated with upregulation of Drp1 [168]. Hepatic stellate cell (HSC) activation promotes liver fibrosis through the stimulation of extracellular matrix production [169]. In a study using HSC-specific Mettl3 knockout mice, the expression of pro-fibrotic genes, including Acta2, Col1a1, and Timp1 were decreased with Mettl3 inhibition, suggesting a HSC-specific role for m6A in liver fibrosis [170].
A genome-wide expression study compared the expression of m6A writers, readers, and erasers in liver biopsies from NAFLD patients and healthy controls [171]. NAFLD liver samples had increased METTL3, METTL15, FTO, and EIF3H (an m6A writer) expression, while WTAP, RBM15, YTHDC1, YTHDC2, IGF2BP2, HNRNPC, and HNRNPA2B1 expression was decreased [171]. EIF3H was correlated to steatosis and VIRMA was correlated to degree of lobular inflammation [171]. Additionally, the authors performed co-expression analysis of the m6A reader RNA binding proteins (RBPs) and found strong interaction relationships between RBM15, HNRNPC, YTHDC2, and HNRNPA2B1 [171]. Gene set enrichment analysis (GSEA) of the co-expressed m6A regulators showed 33 KEGG pathways that were significantly enriched including, steroid hormone biosynthesis, cytochrome P450, nucleotide metabolism, and MAPK signaling [171]. The authors concluded that this analysis highlighted the effects of dysregulated m6A on steatosis and fibrosis in NAFLD.
Other exposures have been associated with altered liver m6A. For example, global hepatic m6A was significantly increased in Western diet-fed mice treated with vinyl chloride (VC) [172]. Importantly, the VC-exposed mice developed more severe steatotic liver disease and HCC at a high frequency. In addition to m6A, several other epitranscriptomic marks were significantly changed by VC exposures. We recently published on modified nucleotides and bases circulating in the blood or urine of human subjects with alcohol-associated liver disease (ALD). While numerous modified bases were significantly altered, m6A was unchanged [173]. The abundance of modified nucleobase and ribonucleoside, 7,9-dimethylguanine in urine and 2-methylthio-N6-threonylcarbamoyladenosine (ms2t6A) in serum were strongly associated with the severity of the ALD. However, m6A has been used to subtype human alcohol-related HCCs [174]. While more data are required, there appears to be an emerging relationship between environmental exposures, steatotic liver diseases, and hepatocellular carcinomas.
Taken together, these studies suggest that m6A modification affects the stability and translation of key genes involved in hepatic lipid metabolism, inflammation, and fibrosis by linking the dysregulation of m6A modification and m6A modifiers to the accumulation of fat in the liver, inflammation, and oxidative stress, all of which are key features of NAFLD. While some progress has been made in understanding the role of m6A in NAFLD, there are several areas that remain to be studied. Areas for future study include the identification of specific m6A-modified transcripts and pathways that are involved in the pathogenesis of NAFLD, validation of m6A modifications as a diagnostic or prognostic biomarkers for NAFLD, the development of therapeutic strategies targeting dysregulated m6A modifications in NAFLD, and the potential crosstalk between m6A modification and other post-transcriptional modifications, including alternative splicing and miRNA regulation.
5. m6A and Liver Physiology
5.1. m6A and Lipid Metabolism
Disruption of the balance between fatty acid uptake, de novo lipogenesis, and lipid oxidation leads to excess fat accumulation in the liver which is a hallmark of NAFLD [175,176,177]. Recent studies have recognized the role of m6A writers, erasers, and readers in the regulation of genes responsible for fatty acid oxidation [178], lipogenesis [179], and lipid transport [180]. In hepatic lipid metabolism disorders, genes with m6A hypermethylation in HFD-induced fatty livers are enriched in processes associated with lipid metabolism, whereas hypo-methylated m6A sites are linked to translation-related processes [162]. In a mouse model of HFD and toxicant exposure, genes with PCB and HFD-induced m6A changes were enriched in pathways related to lipid and lipoprotein metabolism [181]. Furthermore, m6A sequencing conducted on adult pig livers demonstrated that highly m6A-containing transcripts were enriched in pathways related to the positive regulation of metabolic processes and fatty acid transport [182]. In the livers of mice with specific knockout of the Mettl3 gene in hepatocytes, there was a significant reduction in the expression of genes which regulates fatty acid synthesis and oxidation, including Ehhadh, Fasn, Foxo1, Ppargc1a, and Sirt1, and also decreased circadian clock Bmal1 and Clock transcript and protein expression [183]. The hepatocyte-specific deletion of Mettl3 resulted in improvements in insulin sensitivity and glucose homeostasis, indicating that these effects were dependent on m6A modification [183].
Additionally, the circadian clock influences hepatic lipid metabolism by affecting the decay of peroxisome proliferator-activated receptor-α (PPARα) mRNA through YTHDF2 mediation [184], establishing a connection between the circadian clock and metabolic diseases. Another study showed that carbon tetrachloride (CCl4)-induced liver fibrosis in mice resulted in increased total liver m6A with specific increased m6A in NR1D1 [49]. Three changes in RWE were detected at the mRNA transcript level in CCl4-exposed livers: increased ZC3H13 and YTHDC1 and decreased FTO. NR1D1 encodes the ‘orphan nuclear receptor’ REV-ERBα, a transcriptional repressor that regulates diurnal de novo lipogenesis in the liver [185]. RNA immunoprecipitation showed that the NR1D1 transcript associated with YTHDC1 in the CCl4-induced fibrotic liver and in hepatic stellate cells (HSC) [49]. The investigators identified one m6A position in the coding sequence of the NR1D1 transcript. They cloned a 22 nt fragment with the A or a G in the DRACH motif into a luciferase reporter and showed that siYTHDC1 co-transfection of HSC-LX2 cells resulted in increased luciferase activity only when A was present. These data were suggested to indicate that YTHDC1 binding m6A in the NR1D1 transcript resulted in transcript degradation and reduced NR1D1 protein in the CCL4-exposed livers. The decrease in NR1D1 was associated with de-phosphorylation of the mitochondrial fission protein DRP1, increased reactive oxygen species (ROS), reduced mitochondrial fission, mitochondrial DNA release, and activation of cyclic GMP-AMP synthesis (cGAS) inflammatory signaling, HSC activation, and hepatic fibrosis [49]. However, how NR1D1 regulated DRP1 phosphorylation was not elucidated.
FTO is recognized for its impact on adipogenesis and metabolism. FTO expression is significantly higher in the livers of rats with NAFLD and patients with NASH [186,187]. In T2DM patients, glucose increases FTO expression, and decreases global m6A content [188]. In mouse models, FTO depletion correlates to a significant decrease in body weight and adipose tissue, while mice with increased levels of FTO exhibit heightened food intake [189]. When FTO is overexpressed in HepG2 cells, the expression of genes involved in lipid metabolism such as FASN, SCD1, and MGAT1 are increased [190]. Conversely, FTO reduces the expression of genes associated with lipid transport such as MTTP, APOB, and LIPC, which results in lipid accumulation [190]. However, these effects are not observed when the FTO R316A mutant, lacking demethylase activity, is present [190]. Additionally, the overexpression of FTO can elevate the levels key lipogenic regulators, SREBP1c and CIDEC in hepatocytes [191]. Importantly, using short hairpin RNA (shRNA) to knock down FTO diminished dexamethasone-induced fatty liver in mice [160]. In summary, these findings indicate that under lipotoxic conditions, FTO may have a detrimental role in hepatocytes by modifying m6A patterns. These studies suggest that the dynamic process of m6A methylation may play a crucial role in liver metabolism (Figure 3).
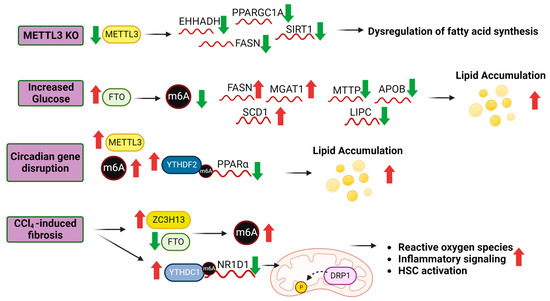
Figure 3.
Role of m6A modifications in lipid metabolism. The liver expression of m6A RWE is perturbed by physiological and disease processes, resulting in changes in m6A levels and subsequently, transcript processing and protein translation regulated in part by m6A readers. Experimental hepatocyte-specific knockout of Mettl3 in mice increased fibrosis and steatosis and downregulated the indicated transcripts [192]. In T2DM patients, glucose increases FTO expression [188]. Overexpression of FTO in HepG2 cells altered the expression of the indicated transcripts which resulted in increased lipid accumulation [190]. Circadian gene disruption in mice increased Mettl3 and Ythdf2 expression which mediated Ppara mRNA decay [184]. CCl4-induced liver fibrosis in HSCs increased ZC2H13 and decreased FTO increasing m6A levels. YTHDC1 bound to m6A on the NR1D1 transcript, decreasing NR1D1 expression which resulted in disrupted fatty acid regulation, lipid accumulation and liver inflammation [49]. Abbreviations: Enoyl-CoA Hydratase And 3-Hydroxyacyl CoA Dehydrogenase (EHHADH), PPARG Coactivator 1 α (PPARGC1A), Sirtuin 1 (SIRT1), Fatty Acid Synthase (FASN), α-1,3-Mannosyl-Glycoprotein 2-β-N-Acetylglucosaminyltransferase (MGAT1), Stearoyl-CoA Desaturase (SCD1), Microsomal Triglyceride Transfer Protein (MTTP), Apolipoprotein B (APOB), Lipase C, Hepatic Type (LIPC), Peroxisome Proliferator Activated Receptor α (PPARα), Nuclear Receptor Subfamily 1 Group D Member 1 (NR1D1). Further details from these studies are provided in the text.
5.2. m6A and Glucose Metabolism
Aberrant glucose metabolism has emerged as a key player in the development and progression of NAFLD. Glucose metabolism is a tightly regulated and is disrupted by insulin resistance, a hallmark feature of NAFLD [193,194]. Insulin resistance increases production of glucose in the liver (gluconeogenesis), further exacerbating hyperglycemia [195]. Further, dysregulated glucose metabolism in NAFLD is closely associated with lipogenesis [193]. Elevated levels of glucose promote the hepatic synthesis of fatty acids through de novo lipogenesis, leading to the accumulation of triglycerides within hepatocytes [193]. The excess triglycerides further contribute to hepatocellular lipid accumulation, leading to the characteristic hepatic steatosis observed in NAFLD [196]. A study using mouse hepatocytes demonstrated that depletion of Mettl3 reduced glycogen storage by decreasing m6A levels on the glycogen synthase 2 (Gys2) transcript which is stabilized by IGF2BP2 [153]. In another study, mice fed a HFD had increased Mettl3 transcript expression and higher m6A levels which correlated to altered expression of glucose metabolism genes including Lpin1, a liver metabolism regulator [197]. Conversely, the expression of m6A erasers, FTO and ALKBH5, was decreased in obese and T2DM patients compared to healthy controls [198]. Additional m6A readers have been implicated in the regulation of glucose metabolism. For example, YTHDF3 was reported to promote glycolysis by increasing phosphofructokinase (PFKL) expression in HCC cells [150]. Additionally, YTHDC1 has been implicated in the regulation of glucose metabolism and insulin resistance by interacting with serine/arginine splicing factor (Srsf3) and cleavage and polyadenylation specific factor 6 (Cpsf6) to regulate mRNA splicing of in mouse β-cells [199]. Prior to the discovery of their role as m6A readers, IGF2BPs were identified by genome-wide association studies as T2DM associated genes [200,201,202]. As a result of conserved IGF2BP binding sites enriched in “GGAC”, there is considerable overlap with the m6A motif, strengthening preferential recognition of m6A-modified mRNAs [79,203,204]. These findings suggest that m6A RWE modulate glucose utilization by affecting key metabolic genes or signaling pathways involved in glucose metabolism.
5.3. m6A and Hepatic Stellate Cell Activation
Hepatic stellate cells (HSC) are undifferentiated cells that play a role in liver regeneration by differentiating into endothelial cells in response to liver damage [205]. HSC activation is a driver of liver fibrosis [206], a critical step in developing cirrhosis [207,208]. As activated HSCs develop into myofibroblasts, they deposit excess extracellular matrix which results in the production of scar tissue contributing to liver fibrosis [206]. Recent studies have identified potential mRNA targets of m6A and related pathways using in vivo and in vitro models of HSC activation [209,210,211]. In a CCl4-induced mouse model of liver fibrosis, 3315 genes had altered m6A levels and genes with differential m6A were enriched in pathways associated with liver fibrosis and HSC activation, including; endoplasmic reticulum (ER) stress, PPAR signaling, and TGF-β signaling [47]. In another study, ALKBH5 was found to be decreased in fibrotic mouse livers which resulted in increased HSC activation. Conversely, overexpression of ALKBH5 inhibited HSC activation and suppressed liver fibrosis [209]. Transforming growth factor-β1 (TGF-β1) is a fibrogenic cytokine commonly used to activate HSC in vitro [212]. Liver fibrosis is increased in Prdx3 (peroxiredoxin 3) knockdown mice; however, mutation of three m6A sites in the Prdx3 transcript resulted in a loss of YTHDF3-mediated down-regulation of PRDX3 protein, thus increasing PRDX3 [91]. Although no studies have shown a direct interaction between m6A and TGF-β1 expression, loss of m6A methylation mediated by FTO and RALY RNA binding protein-like (RALYL), increased the stability of TGF-β2 in HCCs promoting cancer stemness and cell proliferation [213].
5.4. m6A and Hepatic Immune Response Signaling
The accumulation of lipids in NAFLD promotes lipotoxicity in the liver, resulting in upregulated immune response signaling and the recruitment of immune cells, inflammasomes, and macrophages [214]. Kupffer cells (KCs) are hepatic macrophages, located in liver sinusoids, that contribute to activation of HSCs and the progression from NAFL to NASH by secreting TGF-β1 [215]. KCs are activated in response to hepatic injury, inflammation, and damage [216] and produce tumor necrosis factor-α (TNF-α), interlukin-1β (IL-1β), and reactive oxygen species (ROS) [217,218,219]. In lipopolysaccharide (LPS)-induced activation of KCs, overexpression of METTL3 and global m6A hypermethylation increased TGF-β1 secretion [220]. In response to radiation, hTERT (telomerase reverse transcriptase)-HSC cells had increased expression of HMGB1, protein that induces interferon expression. In irradiated hTERT-HSC cells, ALKBH5 demethylated HMGB1 mRNA, and inhibited YTHDF2-mediated transcript degradation, demonstrating that HMGB1 transcript stability was regulated by m6A methylation [221]. A study observing m6A modification on inflammatory signaling pathways in piglets, found that LPS initiated an inflammatory response through the NOD1/NFкB signaling pathway and caused dramatic changes in m6A modifications of transcripts in NFкB, Toll-like receptor, and HIF-1 signaling, suggesting that m6A regulates hepatic immune response signaling [222].
6. Other mRNA Modifications in Liver
Three common mRNA transcript modifications [36] are less well-characterized than m6A and are included here to provide the reader references for further reading.
6.1. Pseudouridine (Ψ)
Pseudouridine is the most abundant modified nucleotide in RNA and is enriched in ncRNAs, tRNAs, and has also been identified in mRNA, with 74% in introns, in human HepG2 hepatocellular carcinoma cells [223]. Ψ was round enriched near alternative splice regions and splice sites where RBPs, i.e., U2AF2, U2AF1, SF3A3, PRPF8, HNRNPC, PTBP1, and were shown to bind [223]. Knockout of the pseudouridine synthases PUS1 and PUS2 altered splicing of distinct pre-mRNA targets in HepG2 cells [223]. The role for Ψ modification of mRNA transcripts in NAFLD is unexplored.
6.2. 1-Methyladenosine (m1A)
The m1A modification is found in rRNA, tRNA, and mRNA is catalyzed by the writers TRMT61A and TRMT6, read by HRSP12, and erased by FTO, ALKBH5, and ALKBH3. m1A methylation is increased in human HCC tumors and in liver cancer stem cells (CSC) [224]. Increased m1A in tRNAs was reported to increase PPARγ translation in CSCs to increase cholesterol synthesis, activate Hedgehog (Hh) signaling, and drive self-renewal of liver cancer stem cells (CSC) in HCC [224]. No studies on m1A in NAFLD were found.
6.3. 5-Methylcytidine (m5C)
m5C is found in mRNA, tRNA, and rRNA. Cholangiocarcinoma (CCA) is the second most common primary liver cancer and has poor prognosis [225]. In cholangiocarcinoma (CCA) cell lines, the lncRNA NF-kappa B interacting lncRNA (NKILA), which is overexpressed and associated with reduced overall survival of CCA patients, was modified by m5C which stabilized NKILA [226]. Mechanistically, NKILA was identified as a competing endogenous RNA (ceRNA) for miR-582-3p; thus ‘sponging miR-582-3p, and relieving its repression of YAP1 protein expression, a driver of CCA [227].
7. Conclusions
Data reported in the literature support the conclusion that changes in m6A in mRNA alter transcript stability of genes involved in liver pathologies and relate to genes and pathways in hepatic regulation of glucose and fat metabolism. Further, expression of the m6A METTL3 writing complex, erasers (demethylases: FTO and ALKBH5), and m6A readers (RBPs) are altered in human NAFLD and in experimental models of liver disease and contribute to NAFLD, HCC, and CCA. Further studies are needed to investigate the specific mechanisms by which mRNA transcript-specific m6A interact with RBPs and alter translation and RNA degradation in the liver and the mechanisms regulating the levels of m6A RWE in response to diet and environmental-induced liver disease.
Author Contributions
B.J.P. writing, M.C.C. writing and editing, C.M.K. writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported, in part by NIH R21ES031510, ES031510-01S1, P30ES030283, R35ES028373, R01ES032189, T32ES011564, P42ES023716, P20GM113226; and P20GM103436.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ALD | alcohol-associated liver disease |
| ALKBH5 | alkB homolog 5 |
| AS | alternative splicing |
| ERα | estrogen receptor α |
| FTO | fat mass obesity-associated protein |
| FXR | farnesoid X receptor |
| HCC | hepatocellular carcinoma |
| HFD | high fat diet |
| HNRNP | heteronuclear family RNA binding proteins |
| HNRNPA1, HNRNPA2B1, HNRNPC, HNRNPG | |
| HSC | Hepatic stellate cells |
| IG2BP | Insulin-like growth factor 2 mRNA binding proteins |
| IGF2BP1, IGF2BP2, IGF2BP3 | |
| KC | Kupffer cells |
| lncRNAs | long non-coding RNAs |
| m6A | N-6-methyladenosine |
| MAC | m6A-METTL complex |
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| MDCs | metabolism disrupting chemicals |
| METTL3 | methyltransferase like 3 |
| METTL14 | methyltransferase like 14 |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| PBXs | polychlorinated biphenyls |
| RBPs | RNA binding proteins |
| RWE | readers, writers, and erasers |
| SAM | S-adenosyl methionine |
| T2DM | type 2 diabetes mellitus |
| VIRMA | Vir like m6A methyltransferase associated |
| WTAP | Wilm’s tumor 1 (WT1)-associating protein |
| YTH | domain family proteins |
| YTHDF1, YTHDF2, YTHDF3, YTHDC1, and YTHDC2 | |
| ZC3H13 | zinc finger CCCH-type containing 13 |
References
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.G.; Rinella, M. NAFLD 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.S.; Alvarez, C.S.; Graubard, B.I.; McGlynn, K.A. Agreement between the Prevalence of Nonalcoholic Fatty Liver Disease Determined by Transient Elastography and Fatty Liver Indices. Clin. Gastroenterol. Hepatol. 2022, 20, 227–229.e2. [Google Scholar] [CrossRef] [PubMed]
- Hirsova, P.; Bamidele, A.O.; Wang, H.; Povero, D.; Revelo, X.S. Emerging Roles of T Cells in the Pathogenesis of Nonalcoholic Steatohepatitis and Hepatocellular Carcinoma. Front. Endocrinol. 2021, 12, 760860. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023. online ahead of print. [Google Scholar]
- Basu, R.; Noureddin, M.; Clark, J.M. Nonalcoholic Fatty Liver Disease: Review of Management for Primary Care Providers. Mayo Clin. Proc. 2022, 97, 1700–1716. [Google Scholar] [CrossRef]
- Dolgin, E. NASH therapies head toward landmark approval. Nat. Biotechnol. 2023, 41, 587–590. [Google Scholar] [CrossRef]
- Ng, C.H.; Tang, A.S.P.; Xiao, J.; Wong, Z.Y.; Yong, J.N.; Fu, C.E.; Zeng, R.W.; Tan, C.; Wong, G.H.Z.; Teng, M.; et al. Safety and tolerability of obeticholic acid in chronic liver disease: A pooled analysis of 1878 individuals. Hepatol. Commun. 2023, 7, e0005. [Google Scholar] [CrossRef]
- Cvitanović Tomaš, T.; Urlep, Ž.; Moškon, M.; Mraz, M.; Rozman, D. LiverSex Computational Model: Sexual Aspects in Hepatic Metabolism and Abnormalities. Front. Physiol. 2018, 9, 360. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in NAFLD: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef]
- DiStefano, J.K. NAFLD and NASH in Postmenopausal Women: Implications for Diagnosis and Treatment. Endocrinology 2020, 161, bqaa134. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Kim, K.M.; An, J.H.; Lee, D.B.; Shim, J.H.; Lim, Y.-S.; Lee, H.C.; Lee, Y.S.; Ahn, J.-H.; Jung, K.H.; et al. Clinical significance of fatty liver disease induced by tamoxifen and toremifene in breast cancer patients. Breast 2016, 28, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Jung, E.-A.; Yoo, J.-J.; Kim, S.G.; Lee, C.-B.; Kim, Y.S.; Jeong, S.W.; Jang, J.Y.; Lee, S.H.; Kim, H.S.; et al. Prevalence, incidence and risk factors of tamoxifen-related non-alcoholic fatty liver disease: A systematic review and meta-analysis. Liver Int. 2020, 40, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.; Yoon, H.G.; Seo, D.H.; Park, S.; Kim, S.I.; Sohn, J.H.; Rhee, Y. Different patterns in the risk of newly developed fatty liver and lipid changes with tamoxifen versus aromatase inhibitors in postmenopausal women with early breast cancer: A propensity score–matched cohort study. Eur. J. Cancer 2017, 82, 103–114. [Google Scholar] [CrossRef]
- Lee, J.I.; Yu, J.-H.; Anh, S.G.; Lee, H.W.; Jeong, J.; Lee, K.S. Aromatase Inhibitors and Newly Developed Nonalcoholic Fatty Liver Disease in Postmenopausal Patients with Early Breast Cancer: A Propensity Score-Matched Cohort Study. Oncologist 2019, 24, e653–e661. [Google Scholar] [CrossRef]
- Scalzo, R.L.; Foright, R.M.; Hull, S.E.; Knaub, L.A.; Johnson-Murguia, S.; Kinanee, F.; Kaplan, J.; Houck, J.A.; Johnson, G.; Sharp, R.R.; et al. Breast Cancer Endocrine Therapy Promotes Weight Gain with Distinct Adipose Tissue Effects in Lean and Obese Female Mice. Endocrinology 2021, 162, bqab174. [Google Scholar] [CrossRef] [PubMed]
- Meda, C.; Barone, M.; Mitro, N.; Lolli, F.; Pedretti, S.; Caruso, D.; Maggi, A.; Della Torre, S. Hepatic ERα accounts for sex differences in the ability to cope with an excess of dietary lipids. Mol. Metab. 2020, 32, 97–108. [Google Scholar] [CrossRef]
- Hart-Unger, S.; Arao, Y.; Hamilton, K.J.; Lierz, S.L.; Malarkey, D.E.; Hewitt, S.C.; Freemark, M.; Korach, K.S. Hormone signaling and fatty liver in females: Analysis of estrogen receptor α mutant mice. Int. J. Obes. 2017, 41, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, B.; Lindén, D.; Brolén, G.; Liljeblad, M.; Bjursell, M.; Romeo, S.; Loomba, R. Review article: The emerging role of genetics in precision medicine for patients with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2020, 51, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
- Jonas, W.; Schurmann, A. Genetic and epigenetic factors determining NAFLD risk. Mol. Metab. 2021, 50, 101111. [Google Scholar] [CrossRef]
- Maude, H.; Sanchez-Cabanillas, C.; Cebola, I. Epigenetics of Hepatic Insulin Resistance. Front. Endocrinol. 2021, 12, 681356. [Google Scholar] [CrossRef]
- Wahlang, B.; Alexander, N.C.; Li, X.; Rouchka, E.C.; Kirpich, I.A.; Cave, M.C. Polychlorinated biphenyls altered gut microbiome in CAR and PXR knockout mice exhibiting toxicant-associated steatohepatitis. Toxicol. Rep. 2021, 8, 536–547. [Google Scholar] [CrossRef]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef]
- Sanoudou, D.; Gkouskou, K.K.; Eliopoulos, A.G.; Mantzoros, C.S. Epitranscriptomic challenges and promises in metabolic diseases. Metabolism 2022, 132, 155219. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.E.; Pazos, M.A., 2nd; Curcio, M.J.; Fabris, D. Global Epitranscriptomics Profiling of RNA Post-Transcriptional Modifications as an Effective Tool for Investigating the Epitranscriptomics of Stress Response. Mol. Cell. Proteom. 2016, 15, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Destefanis, E.; Avşar, G.; Groza, P.; Romitelli, A.; Torrini, S.; Pir, P.; Conticello, S.G.; Aguilo, F.; Dassi, E. A mark of disease: How mRNA modifications shape genetic and acquired pathologies. RNA 2021, 27, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Pham, P.; Dedon, P.C.; Begley, T.J. Lifestyle modifications: Coordinating the tRNA epitranscriptome with codon bias to adapt translation during stress responses. Genome Biol. 2018, 19, 228. [Google Scholar] [CrossRef]
- Sarkar, A.; Gasperi, W.; Begley, U.; Nevins, S.; Huber, S.M.; Dedon, P.C.; Begley, T.J. Detecting the epitranscriptome. WIREs RNA 2021, 12, e1663. [Google Scholar] [CrossRef]
- Zaccara, S.; Ries, R.J.; Jaffrey, S.R. Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Wang, R.; Xiong, F.; Krakowiak, J.; Liao, Z.; Nguyen, P.T.; Moroz-Omori, E.V.; Shao, J.; Zhu, X.; Bolt, M.J.; et al. Enhancer RNA m6A methylation facilitates transcriptional condensate formation and gene activation. Mol. Cell 2021, 81, 3368–3385.e3369. [Google Scholar] [CrossRef]
- Jenjaroenpun, P.; Wongsurawat, T.; Wadley, T.D.; Wassenaar, T.M.; Liu, J.; Dai, Q.; Wanchai, V.; Akel, N.S.; Jamshidi-Parsian, A.; Franco, A.T.; et al. Decoding the epitranscriptional landscape from native RNA sequences. Nucleic Acids Res. 2020, 49, e7. [Google Scholar] [CrossRef]
- Licht, K.; Jantsch, M.F. Rapid and dynamic transcriptome regulation by RNA editing and RNA modifications. J. Cell Biol. 2016, 213, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Knuckles, P.; Bühler, M. Adenosine methylation as a molecular imprint defining the fate of RNA. FEBS Lett. 2018, 592, 2845–2859. [Google Scholar] [CrossRef] [PubMed]
- Cayir, A.; Byun, H.-M.; Barrow, T.M. Environmental epitranscriptomics. Environ. Res. 2020, 189, 109885. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Meng, J.; Su, R.; Zhang, J.; Chen, J.; Ma, X.; Xia, Q. Epitranscriptomics in liver disease: Basic concepts and therapeutic potential. J. Hepatol. 2020, 73, 664–679. [Google Scholar] [CrossRef]
- Roundtree, I.A.; Evans, M.E.; Pan, T.; He, C. Dynamic RNA Modifications in Gene Expression Regulation. Cell 2017, 169, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Toth, J.I.; Petroski, M.D.; Zhang, Z.; Zhao, J.C. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol. 2014, 16, 191–198. [Google Scholar] [CrossRef]
- Huang, M.; Xu, S.; Liu, L.; Zhang, M.; Guo, J.; Yuan, Y.; Xu, J.; Chen, X.; Zou, J. m6A Methylation Regulates Osteoblastic Differentiation and Bone Remodeling. Front. Cell Dev. Biol. 2021, 9, 783322. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Wang, X.; Sun, C.; Zheng, J.; Li, J.; Yi, G.; Yang, N. The m6A methylation regulates gonadal sex differentiation in chicken embryo. J. Anim. Sci. Biotechnol. 2022, 13, 52. [Google Scholar] [CrossRef]
- Engel, M.; Eggert, C.; Kaplick, P.M.; Eder, M.; Roh, S.; Tietze, L.; Namendorf, C.; Arloth, J.; Weber, P.; Rex-Haffner, M.; et al. The Role of m(6)A/m-RNA Methylation in Stress Response Regulation. Neuron 2018, 99, 389–403.e9. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhang, Y.; Zhang, J.; Wang, J.; Li, Q. The alteration of N6-methyladenosine (m6A) modification at the transcriptome-wide level in response of heat stress in bovine mammary epithelial cells. BMC Genom. 2022, 23, 829. [Google Scholar] [CrossRef]
- Ling, Z.; Chen, L.; Zhao, J. m6A-dependent up-regulation of DRG1 by METTL3 and ELAVL1 promotes growth, migration, and colony formation in osteosarcoma. Biosci. Rep. 2020, 40, BSR20200282. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Ji, J. N6-methyladenosine (m6A) RNA modification in cancer stem cells. Stem Cells 2020, 38, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.J.; Zhao, X.; Li, H.; Sun, L.P.; Yuan, Y. Expression profiles and prognostic roles of m6A writers, erasers and readers in gastric cancer. Future Oncol. 2021, 17, 2605–2620. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, X.; Yang, P.; Zhang, X.; Peng, Y.; Li, D.; Yu, Y.; Wu, Y.; Wang, Y.; Zhang, J.; et al. RNA m6A methylation orchestrates cancer growth and metastasis via macrophage reprogramming. Nat. Commun. 2021, 12, 1394. [Google Scholar] [CrossRef]
- Chen, M.; Wong, C.M. The emerging roles of N6-methyladenosine (m6A) deregulation in liver carcinogenesis. Mol. Cancer 2020, 19, 44. [Google Scholar] [CrossRef]
- Fan, C.; Ma, Y.; Chen, S.; Zhou, Q.; Jiang, H.; Zhang, J.; Wu, F. Comprehensive Analysis of the Transcriptome-Wide m6A Methylation Modification Difference in Liver Fibrosis Mice by High-Throughput m6A Sequencing. Front. Cell Dev. Biol. 2021, 9, 767051. [Google Scholar] [CrossRef]
- Malovic, E.; Ealy, A.; Kanthasamy, A.; Kanthasamy, A.G. Emerging Roles of N6-Methyladenosine (m6A) Epitranscriptomics in Toxicology. Toxicol. Sci. 2021, 181, 13–22. [Google Scholar] [CrossRef]
- Chen, L.; Xia, S.; Wang, F.; Zhou, Y.; Wang, S.; Yang, T.; Li, Y.; Xu, M.; Zhou, Y.; Kong, D.; et al. m(6)A methylation-induced NR1D1 ablation disrupts the HSC circadian clock and promotes hepatic fibrosis. Pharmacol. Res. 2023, 189, 106704. [Google Scholar] [CrossRef]
- Winkler, R.; Gillis, E.; Lasman, L.; Safra, M.; Geula, S.; Soyris, C.; Nachshon, A.; Tai-Schmiedel, J.; Friedman, N.; Le-Trilling, V.T.K.; et al. m(6)A modification controls the innate immune response to infection by targeting type I interferons. Nat. Immunol. 2019, 20, 173–182. [Google Scholar] [CrossRef]
- Huang, X.T.; Li, J.H.; Zhu, X.X.; Huang, C.S.; Gao, Z.X.; Xu, Q.C.; Zhao, W.; Yin, X.Y. HNRNPC impedes m(6)A-dependent anti-metastatic alternative splicing events in pancreatic ductal adenocarcinoma. Cancer Lett. 2021, 518, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, N.S.; McIntyre, A.B.R.; Mattocks, M.D.; Holley, C.L.; Lazear, H.M.; Mason, C.E.; Horner, S.M. Altered m(6)A Modification of Specific Cellular Transcripts Affects Flaviviridae Infection. Mol. Cell 2020, 77, 542–555.e8. [Google Scholar] [CrossRef] [PubMed]
- Flamand, M.N.; Meyer, K.D. m6A and YTHDF proteins contribute to the localization of select neuronal mRNAs. Nucleic Acids Res. 2022, 50, 4464–4483. [Google Scholar] [CrossRef] [PubMed]
- Roundtree, I.A.; Luo, G.Z.; Zhang, Z.; Wang, X.; Zhou, T.; Cui, Y.; Sha, J.; Huang, X.; Guerrero, L.; Xie, P.; et al. YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. eLife 2017, 6, e31311. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, M.; Huang, H.; Zhu, J.; Song, H.; Zhu, J.; Park, J.; Ji, S.J. Dynamic m6A modification regulates local translation of mRNA in axons. Nucleic Acids Res. 2018, 46, 1412–1423. [Google Scholar] [CrossRef]
- Mao, Y.; Dong, L.; Liu, X.M.; Guo, J.; Ma, H.; Shen, B.; Qian, S.B. m(6)A in mRNA coding regions promotes translation via the RNA helicase-containing YTHDC2. Nat. Commun. 2019, 10, 5332. [Google Scholar] [CrossRef]
- Chen, X.Y.; Liang, R.; Yi, Y.C.; Fan, H.N.; Chen, M.; Zhang, J.; Zhu, J.S. The m(6)A Reader YTHDF1 Facilitates the Tumorigenesis and Metastasis of Gastric Cancer via USP14 Translation in an m(6)A-Dependent Manner. Front. Cell Dev. Biol. 2021, 9, 647702. [Google Scholar] [CrossRef]
- Yang, Y.; Hsu, P.J.; Chen, Y.S.; Yang, Y.G. Dynamic transcriptomic m(6)A decoration: Writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018, 28, 616–624. [Google Scholar] [CrossRef]
- Liu, J.; Yue, Y.; Han, D.; Wang, X.; Fu, Y.; Zhang, L.; Jia, G.; Yu, M.; Lu, Z.; Deng, X.; et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014, 10, 93–95. [Google Scholar] [CrossRef]
- Narayan, P.; Rottman, F.M. An in vitro system for accurate methylation of internal adenosine residues in messenger RNA. Science 1988, 242, 1159–1162. [Google Scholar] [CrossRef]
- Bokar, J.A.; Shambaugh, M.E.; Polayes, D.; Matera, A.G.; Rottman, F.M. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA 1997, 3, 1233–1247. [Google Scholar]
- Wei, C.M.; Moss, B. Nucleotide sequences at the N6-methyladenosine sites of HeLa cell messenger ribonucleic acid. Biochemistry 1977, 16, 1672–1676. [Google Scholar] [CrossRef]
- Harper, J.E.; Miceli, S.M.; Roberts, R.J.; Manley, J.L. Sequence specificity of the human mRNA N6-adenosine methylase in vitro. Nucleic Acids Res. 1990, 18, 5735–5741. [Google Scholar] [CrossRef] [PubMed]
- Ping, X.L.; Sun, B.F.; Wang, L.; Xiao, W.; Yang, X.; Wang, W.J.; Adhikari, S.; Shi, Y.; Lv, Y.; Chen, Y.S.; et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014, 24, 177–189. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.J.; Xia, L.; Zhang, H. The biological function of m6A methyltransferase KIAA1429 and its role in human disease. PeerJ 2022, 10, e14334. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Jie, Y.; Zhao, H. m6A ‘writer’ KIAA1429 regulates the proliferation and migration of endothelial cells in atherosclerosis. Mol. Biotechnol. 2022, 65, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.; Mumbach, M.R.; Jovanovic, M.; Wang, T.; Maciag, K.; Bushkin, G.G.; Mertins, P.; Ter-Ovanesyan, D.; Habib, N.; Cacchiarelli, D.; et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5’ sites. Cell Rep. 2014, 8, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.P.; Chen, C.K.; Pickering, B.F.; Chow, A.; Jackson, C.; Guttman, M.; Jaffrey, S.R. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature 2016, 537, 369–373. [Google Scholar] [CrossRef]
- Li, T.; Wang, T.; Jing, J.; Sun, L. Expression Pattern and Clinical Value of Key m6A RNA Modification Regulators in Abdominal Aortic Aneurysm. J. Inflamm. Res. 2021, 14, 4245–4258. [Google Scholar] [CrossRef]
- Wen, J.; Lv, R.; Ma, H.; Shen, H.; He, C.; Wang, J.; Jiao, F.; Liu, H.; Yang, P.; Tan, L.; et al. Zc3h13 Regulates Nuclear RNA m(6)A Methylation and Mouse Embryonic Stem Cell Self-Renewal. Mol. Cell 2018, 69, 1028–1038.e6. [Google Scholar] [CrossRef]
- Knuckles, P.; Lence, T.; Haussmann, I.U.; Jacob, D.; Kreim, N.; Carl, S.H.; Masiello, I.; Hares, T.; Villasenor, R.; Hess, D.; et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m(6)A machinery component Wtap/Fl(2)d. Genes Dev. 2018, 32, 415–429. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Q.; Wang, K.; Zhang, X.; Yang, R.; Bi, K.; Chen, W.; Diao, H. RBM15-mediated N6-methyladenosine modification affects COVID-19 severity by regulating the expression of multitarget genes. Cell Death Dis. 2021, 12, 732. [Google Scholar] [CrossRef]
- Cheng, X.; Li, M.; Rao, X.; Zhang, W.; Li, X.; Wang, L.; Huang, G. KIAA1429 regulates the migration and invasion of hepatocellular carcinoma by altering m6A modification of ID2 mRNA. OncoTargets Ther. 2019, 12, 3421–3428. [Google Scholar] [CrossRef] [PubMed]
- Petri, B.J.; Klinge, C.M. m6A readers, writers, erasers, and the m6A epitranscriptome in breast cancer. J. Mol. Endocrinol. 2023, 70, e220110. [Google Scholar] [CrossRef]
- Lee, Y.; Choe, J.; Park, O.H.; Kim, Y.K. Molecular Mechanisms Driving mRNA Degradation by m(6)A Modification. Trends Genet. 2020, 36, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; He, C.; Dickinson, B.C. Targeted m(6)A Reader Proteins To Study Epitranscriptomic Regulation of Single RNAs. J. Am. Chem. Soc. 2018, 140, 11974–11981. [Google Scholar] [CrossRef]
- Alarcon, C.R.; Goodarzi, H.; Lee, H.; Liu, X.; Tavazoie, S.; Tavazoie, S.F. HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell 2015, 162, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Xiao, W.; Zhao, Y.L.; Yang, Y.G. m(6)A: Signaling for mRNA splicing. RNA Biol. 2016, 13, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Weng, H.; Sun, W.; Qin, X.; Shi, H.; Wu, H.; Zhao, B.S.; Mesquita, A.; Liu, C.; Yuan, C.L. Recognition of RNA N 6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2018, 20, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Chen, Y.S.; Ping, X.L.; Yang, X.; Xiao, W.; Yang, Y.; Sun, H.Y.; Zhu, Q.; Baidya, P.; Wang, X.; et al. Cytoplasmic m(6)A reader YTHDF3 promotes mRNA translation. Cell Res. 2017, 27, 444–447. [Google Scholar] [CrossRef]
- Patil, D.P.; Pickering, B.F.; Jaffrey, S.R. Reading m(6)A in the Transcriptome: m(6)A-Binding Proteins. Trends Cell Biol. 2018, 28, 113–127. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, Y.; Shen, H.; Xie, W. m(6)A-binding proteins: The emerging crucial performers in epigenetics. J. Hematol. Oncol. 2020, 13, 35. [Google Scholar] [CrossRef]
- Liao, J.; Wei, Y.; Liang, J.; Wen, J.; Chen, X.; Zhang, B.; Chu, L. Insight into the structure, physiological function, and role in cancer of m6A readers-YTH domain-containing proteins. Cell Death Discov. 2022, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Sun, H.; Xu, C. YTH Domain: A Family of N(6)-methyladenosine (m(6)A) Readers. Genom. Proteom. Bioinform. 2018, 16, 99–107. [Google Scholar] [CrossRef]
- Kasowitz, S.D.; Ma, J.; Anderson, S.J.; Leu, N.A.; Xu, Y.; Gregory, B.D.; Schultz, R.M.; Wang, P.J. Nuclear m6A reader YTHDC1 regulates alternative polyadenylation and splicing during mouse oocyte development. PLoS Genet. 2018, 14, e1007412. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Sun, H.; Chen, K.; Gu, X.; Chen, H.; Jiang, L.; Chen, L.; Zhang, S.; Liu, Y.; Shi, D.; et al. Depletion of m(6) A reader protein YTHDC1 induces dilated cardiomyopathy by abnormal splicing of Titin. J. Cell. Mol. Med. 2021, 25, 10879–10891. [Google Scholar] [CrossRef] [PubMed]
- Widagdo, J.; Anggono, V.; Wong, J.J. The multifaceted effects of YTHDC1-mediated nuclear m(6)A recognition. Trends Genet. 2022, 38, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.S.; Zhu, Y.Q.; Xu, Q.C.; Chen, S.; Huang, Y.; Zhao, G.; Ni, X.; Liu, B.; Zhao, W.; Yin, X.Y. YTHDF2 promotes intrahepatic cholangiocarcinoma progression and desensitises cisplatin treatment by increasing CDKN1B mRNA degradation. Clin. Transl. Med. 2022, 12, e848. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.; Lu, Z.; Zhao, B.S.; Ma, H.; Hsu, P.J.; Liu, C.; He, C. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 2017, 27, 315–328. [Google Scholar] [CrossRef]
- Du, H.; Zhao, Y.; He, J.; Zhang, Y.; Xi, H.; Liu, M.; Ma, J.; Wu, L. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun. 2016, 7, 12626. [Google Scholar] [CrossRef]
- Sun, R.; Tian, X.; Li, Y.; Zhao, Y.; Wang, Z.; Hu, Y.; Zhang, L.; Wang, Y.; Gao, D.; Zheng, S.; et al. The m6A reader YTHDF3-mediated PRDX3 translation alleviates liver fibrosis. Redox Biol. 2022, 54, 102378. [Google Scholar] [CrossRef]
- Chang, G.; Shi, L.; Ye, Y.; Shi, H.; Zeng, L.; Tiwary, S.; Huse, J.T.; Huo, L.; Ma, L.; Ma, Y.; et al. YTHDF3 Induces the Translation of m(6)A-Enriched Gene Transcripts to Promote Breast Cancer Brain Metastasis. Cancer Cell 2020, 38, 857–871.e857. [Google Scholar] [CrossRef]
- Wang, J.; Sun, D.; Wang, M.; Cheng, A.; Zhu, Y.; Mao, S.; Ou, X.; Zhao, X.; Huang, J.; Gao, Q.; et al. Multiple functions of heterogeneous nuclear ribonucleoproteins in the positive single-stranded RNA virus life cycle. Front. Immunol. 2022, 13, 989298. [Google Scholar] [CrossRef] [PubMed]
- Geuens, T.; Bouhy, D.; Timmerman, V. The hnRNP family: Insights into their role in health and disease. Hum. Genet. 2016, 135, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.S.; Preussner, M.; Bunse, M.; Karni, R.; Heyd, F. Activation-Dependent TRAF3 Exon 8 Alternative Splicing Is Controlled by CELF2 and hnRNP C Binding to an Upstream Intronic Element. Mol. Cell. Biol. 2017, 37, e00488-16. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Tang, X.; Tang, C.; Hua, Z.; Ke, M.; Wang, C.; Zhao, J.; Gao, S.; Jurczyszyn, A.; Janz, S.; et al. HNRNPA2B1 promotes multiple myeloma progression by increasing AKT3 expression via m6A-dependent stabilization of ILF3 mRNA. J. Hematol. Oncol. 2021, 14, 54. [Google Scholar] [CrossRef]
- Wang, H.; Han, L.; Zhao, G.; Shen, H.; Wang, P.; Sun, Z.; Xu, C.; Su, Y.; Li, G.; Tong, T.; et al. hnRNP A1 antagonizes cellular senescence and senescence-associated secretory phenotype via regulation of SIRT1 mRNA stability. Aging Cell 2016, 15, 1063–1073. [Google Scholar] [CrossRef]
- Alarcon, C.R.; Lee, H.; Goodarzi, H.; Halberg, N.; Tavazoie, S.F. N6-methyladenosine marks primary microRNAs for processing. Nature 2015, 519, 482–485. [Google Scholar] [CrossRef]
- Dai, W.; Sun, Y.; Jiang, Z.; Du, K.; Xia, N.; Zhong, G. Key genes associated with non-alcoholic fatty liver disease and acute myocardial infarction. Med. Sci. Monit. 2020, 26, e922492. [Google Scholar] [CrossRef]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.G.; et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Dahl, J.A.; Niu, Y.; Fedorcsak, P.; Huang, C.M.; Li, C.J.; Vagbo, C.B.; Shi, Y.; Wang, W.L.; Song, S.H.; et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 2013, 49, 18–29. [Google Scholar] [CrossRef]
- Blakemore, A.I.F.; Froguel, P. Is Obesity Our Genetic Legacy? J. Clin. Endocrinol. Metab. 2008, 93, s51–s56. [Google Scholar] [CrossRef] [PubMed]
- Church, C.; Lee, S.; Bagg, E.A.L.; McTaggart, J.S.; Deacon, R.; Gerken, T.; Lee, A.; Moir, L.; Mecinović, J.; Quwailid, M.M.; et al. A Mouse Model for the Metabolic Effects of the Human Fat Mass and Obesity Associated FTO Gene. PLoS Genet. 2009, 5, e1000599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, Y.; Sun, B.F.; Shi, Y.; Yang, X.; Xiao, W.; Hao, Y.J.; Ping, X.L.; Chen, Y.S.; Wang, W.J.; et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014, 24, 1403–1419. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Koch, L.; Emmerling, C.; Vierkotten, J.; Peters, T.; Bruning, J.C.; Ruther, U. Inactivation of the Fto gene protects from obesity. Nature 2009, 458, 894–898. [Google Scholar] [CrossRef]
- Yang, S.; Wei, J.; Cui, Y.H.; Park, G.; Shah, P.; Deng, Y.; Aplin, A.E.; Lu, Z.; Hwang, S.; He, C.; et al. m(6)A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nat. Commun. 2019, 10, 2782. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Ondo, K.; Takemoto, S.; Fukami, T.; Nakajima, M. Methylation of adenosine at the N6 position post-transcriptionally regulates hepatic P450s expression. Biochem. Pharmacol. 2020, 171, 113697. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Y.; Yu, J.; Gan, Z.; Wei, W.; Wang, C.; Zhang, L.; Wang, T.; Zhong, X. Resveratrol Attenuates High-Fat Diet Induced Hepatic Lipid Homeostasis Disorder and Decreases m(6)A RNA Methylation. Front. Pharmacol. 2020, 11, 568006. [Google Scholar] [CrossRef]
- Ghazi, T.; Nagiah, S.; Chuturgoon, A.A. Fusaric acid induces hepatic global m6A RNA methylation and differential expression of m6A regulatory genes in vivo—A pilot study. Epigenetics 2022, 17, 695–703. [Google Scholar] [CrossRef]
- Mauer, J.; Luo, X.; Blanjoie, A.; Jiao, X.; Grozhik, A.V.; Patil, D.P.; Linder, B.; Pickering, B.F.; Vasseur, J.-J.; Chen, Q.; et al. Reversible methylation of m6Am in the 5′ cap controls mRNA stability. Nature 2017, 541, 371–375. [Google Scholar] [CrossRef]
- Mauer, J.; Sindelar, M.; Despic, V.; Guez, T.; Hawley, B.R.; Vasseur, J.-J.; Rentmeister, A.; Gross, S.S.; Pellizzoni, L.; Debart, F.; et al. FTO controls reversible m6Am RNA methylation during snRNA biogenesis. Nat. Chem. Biol. 2019, 15, 340–347. [Google Scholar] [CrossRef]
- Mauer, J.; Jaffrey, S.R. FTO, m(6) A(m), and the hypothesis of reversible epitranscriptomic mRNA modifications. FEBS Lett. 2018, 592, 2012–2022. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yan, J.; Li, Q.; Li, J.; Gong, S.; Zhou, H.; Gan, J.; Jiang, H.; Jia, G.F.; Luo, C.; et al. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res. 2015, 43, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.J.; Wang, R.L.; Wang, D.; Liu, L.; Cheng, L. Visible-light-mediated oxidative demethylation of N(6)-methyl adenines. Chem. Commun. 2017, 53, 10734–10737. [Google Scholar] [CrossRef]
- Meng, Q.; Schatten, H.; Zhou, Q.; Chen, J. Crosstalk between m6A and coding/non-coding RNA in cancer and detection methods of m6A modification residues. Aging 2023, 15, 6577–6619. [Google Scholar] [CrossRef]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Salmon-Divon, M.; Amariglio, N.; Rechavi, G. Transcriptome-wide mapping of N(6)-methyladenosine by m(6)A-seq based on immunocapturing and massively parallel sequencing. Nat. Protoc. 2013, 8, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.T.; Porman, A.M.; Johnson, A.M. Identification of m6A residues at single-nucleotide resolution using eCLIP and an accessible custom analysis pipeline. RNA 2020, 27, 527–541. [Google Scholar] [CrossRef]
- Thuring, K.; Schmid, K.; Keller, P.; Helm, M. Analysis of RNA modifications by liquid chromatography-tandem mass spectrometry. Methods 2016, 107, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.D. DART-seq: An antibody-free method for global m(6)A detection. Nat. Methods 2019, 16, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Tegowski, M.; Zhu, H.; Meyer, K.D. Detecting m(6)A with In Vitro DART-Seq. Methods Mol. Biol. 2022, 2404, 363–374. [Google Scholar] [PubMed]
- Kono, N.; Arakawa, K. Nanopore sequencing: Review of potential applications in functional genomics. Dev. Growth Differ. 2019, 61, 316–326. [Google Scholar] [CrossRef]
- Parker, M.T.; Knop, K.; Sherwood, A.V.; Schurch, N.J.; Mackinnon, K.; Gould, P.D.; Hall, A.J.; Barton, G.J.; Simpson, G.G. Nanopore direct RNA sequencing maps the complexity of Arabidopsis mRNA processing and m(6)A modification. eLife 2020, 9, e49658. [Google Scholar] [CrossRef] [PubMed]
- Pratanwanich, P.N.; Yao, F.; Chen, Y.; Koh, C.W.Q.; Wan, Y.K.; Hendra, C.; Poon, P.; Goh, Y.T.; Yap, P.M.L.; Chooi, J.Y.; et al. Identification of differential RNA modifications from nanopore direct RNA sequencing with xPore. Nat. Biotechnol. 2021, 39, 1394–1402. [Google Scholar] [CrossRef]
- Hendra, C.; Pratanwanich, P.N.; Wan, Y.K.; Goh, W.S.S.; Thiery, A.; Goke, J. Detection of m6A from direct RNA sequencing using a multiple instance learning framework. Nat. Methods 2022, 19, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Körtel, N.; Rücklé, C.; Zhou, Y.; Busch, A.; Hoch-Kraft, P.; Sutandy, F.X.R.; Haase, J.; Pradhan, M.; Musheev, M.; Ostareck, D.; et al. Deep and accurate detection of m6A RNA modifications using miCLIP2 and m6Aboost machine learning. Nucleic Acids Res. 2021, 49, e92. [Google Scholar] [CrossRef]
- Li, C.; Liu, W.; Liu, C.; Luo, Q.; Luo, K.; Wei, C.; Li, X.; Qin, J.; Zheng, C.; Lan, C.; et al. Integrating machine learning and bioinformatics analysis to m6A regulator-mediated methylation modification models for predicting glioblastoma patients’ prognosis and immunotherapy response. Aging 2023, 15, 4051–4070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hamada, M. DeepM6ASeq: Prediction and characterization of m6A-containing sequences using deep learning. BMC Bioinform. 2018, 19, 524. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, Y.; Lu, J.; Peng, C.; Ling, Z.; Chen, Y.; Chen, D.; Tong, R.; Zheng, S.; Wu, J. m(6)A-modification regulated circ-CCT3 acts as the sponge of miR-378a-3p to promote hepatocellular carcinoma progression. Epigenetics 2023, 18, 2204772. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Li, X.; Yin, B.; Huang, J.; Lu, S.; Wang, C.; Ke, S.; Xu, Y.; Qian, B.; Feng, Z.; et al. Comprehensive analysis of transcriptome-wide M(6)A methylation for hepatic ischaemia reperfusion injury in mice. Epigenetics 2023, 18, 2201716. [Google Scholar] [CrossRef] [PubMed]
- Deblois, G.; St-Pierre, J.; Giguere, V. The PGC-1/ERR signaling axis in cancer. Oncogene 2013, 32, 3483–3490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiong, L.; Wei, T.; Liu, Q.; Yan, L.; Chen, J.; Dai, L.; Shi, L.; Zhang, W.; Yang, J.; et al. Hypoxia-responsive PPARGC1A/BAMBI/ACSL5 axis promotes progression and resistance to lenvatinib in hepatocellular carcinoma. Oncogene 2023, 42, 1509–1523. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, H.; Dong, W.; Zhang, C.; Hu, M.; Ma, W.; Jiang, X.; Li, H.; Yang, P.; Xiang, D. N6-Methyladenosine–Mediated Up-Regulation of FZD10 Regulates Liver Cancer Stem Cells’ Properties and Lenvatinib Resistance Through WNT/β-Catenin and Hippo Signaling Pathways. Gastroenterology 2023, 164, 990–1005. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, Q.; Zhou, Q.; Fang, F.; Lei, K.; Liu, Z.; Zheng, G.; Zhu, L.; Huo, J.; Li, X.; et al. METTL3-m6A-EGFR-axis drives lenvatinib resistance in hepatocellular carcinoma. Cancer Lett. 2023, 559, 216122. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, A.; Cui, Y.; Gong, H.; Li, H. LRPPRC facilitates tumor progression and immune evasion through upregulation of m(6)A modification of PD-L1 mRNA in hepatocellular carcinoma. Front. Immunol. 2023, 14, 1144774. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, M.; Wang, G.; Mai, Z.; Zhou, B.; Han, Y.; Zhuang, J.; Xia, W. lncRNA miR4458HG modulates hepatocellular carcinoma progression by activating m6A-dependent glycolysis and promoting the polarization of tumor-associated macrophages. Cell. Mol. Life Sci. 2023, 80, 99. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, G.; Zhao, Y.; Gao, H.; Li, L.; Yin, Y.; Jiang, J.; Wang, L.; Mang, Y.; Gao, Y.; et al. Exosome-derived circCCAR1 promotes CD8 + T-cell dysfunction and anti-PD1 resistance in hepatocellular carcinoma. Mol. Cancer 2023, 22, 55. [Google Scholar] [CrossRef]
- Kuang, Y.; Cheng, Y.; Wang, J.; Li, H.; Cao, X.; Wang, Y. KIAA1429 mediates epithelial mesenchymal transition in sorafenib-resistant hepatocellular carcinoma through m6A methylation modification. Cancer Med. 2023, 12, 7222–7233. [Google Scholar] [CrossRef]
- Lan, T.; Li, H.; Zhang, D.; Xu, L.; Liu, H.; Hao, X.; Yan, X.; Liao, H.; Chen, X.; Xie, K.; et al. KIAA1429 contributes to liver cancer progression through N6-methyladenosine-dependent post-transcriptional modification of GATA3. Mol. Cancer 2019, 18, 186. [Google Scholar] [CrossRef]
- Fang, J.; Wu, X.; He, J.; Zhang, H.; Chen, X.; Zhang, H.; Novakovic, B.; Qi, H.; Yu, X. RBM15 suppresses hepatic insulin sensitivity of offspring of gestational diabetes mellitus mice via m6A-mediated regulation of CLDN4. Mol. Med. 2023, 29, 23. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, Y.; Yin, J.; Tang, N.; Wang, K.; Huang, L.; Hu, J.; Feng, Z.; Gao, Q.; Huang, A. O-GlcNAcylation of YTHDF2 promotes HBV-related hepatocellular carcinoma progression in an N(6)-methyladenosine-dependent manner. Signal Transduct. Target. Ther. 2023, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, P.; Deng, Y.; Cai, X.; Sun, M.; Sun, Y.; Wu, D. ALKBH5-mediated m(6) A demethylation of TIRAP mRNA promotes radiation-induced liver fibrosis and decreases radiosensitivity of hepatocellular carcinoma. Clin. Transl. Med. 2023, 13, e1198. [Google Scholar] [CrossRef]
- Lyu, Z.; Huang, B.; Zhang, J.; Qian, Q.; Pu, X.; Cui, N.; Ou, Y.; Li, B.; You, Z.; Lian, M.; et al. Suppression of YTHDF2 attenuates autoimmune hepatitis by expansion of myeloid-derived suppressor cells. J. Autoimmun. 2023, 135, 102993. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, K.; Du, Y.; Liu, Z.; Gong, Y. HDAC1 overexpression promoted by METTL3-IGF2BP2 inhibits FGF21 expression in metabolic syndrome-related liver injury. Biochem. Cell Biol. 2023, 101, 52–63. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, K.; Chen, S.; Lian, G.; Huang, Y.; Yao, H.; Zhao, Y.; Huang, K.; Yin, D.; Lin, H.; et al. CCL3 secreted by hepatocytes promotes the metastasis of intrahepatic cholangiocarcinoma by VIRMA-mediated N6-methyladenosine (m(6)A) modification. J. Transl. Med. 2023, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Hou, J.; Xu, H.; Lei, Z.; Li, Z.; Zhu, H.; Yu, X.; Yang, Z.; Jin, X.; Sun, J. The Prognostic Value of a lncRNA Risk Model Consists of 9 m6A Regulator-Related lncRNAs in Hepatocellular Carcinoma (HCC). Evol. Bioinform. 2023, 19, 11769343221142013. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Du, M.; Meng, L.; Yang, Y.; He, S.; Zhu, Y.; Ren, X.; Wei, M.; Dong, R.; Zheng, S.; et al. Integrative analysis implicates the significance of m6A in the liver fibrosis of biliary atresia by regulating THY1. Hepatol. Commun. 2023, 7, e0004. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, L.; Guo, H.; Zhou, S.; Wang, C.; Ji, C.; Meng, F.; Liang, S.; Zhang, B.; Yuan, Y.; et al. Targeting SLP2-mediated lipid metabolism reprograming restricts proliferation and metastasis of hepatocellular carcinoma and promotes sensitivity to Lenvatinib. Oncogene 2023, 42, 374–388. [Google Scholar] [CrossRef]
- Chen, S.; Xia, H.; Sheng, L. WTAP-mediated m6A modification on circCMTM3 inhibits hepatocellular carcinoma ferroptosis by recruiting IGF2BP1 to increase PARK7 stability. Dig. Liver Dis. 2022, 55, 967–981. [Google Scholar] [CrossRef]
- Wang, L.; Yi, X.; Xiao, X.; Zheng, Q.; Ma, L.; Li, B. Exosomal miR-628-5p from M1 polarized macrophages hinders m6A modification of circFUT8 to suppress hepatocellular carcinoma progression. Cell. Mol. Biol. Lett. 2022, 27, 106. [Google Scholar] [CrossRef]
- Zhou, R.; Ni, W.; Qin, C.; Zhou, Y.; Li, Y.; Huo, J.; Bian, L.; Zhou, A.; Li, J. A functional loop between YTH domain family protein YTHDF3 mediated m(6)A modification and phosphofructokinase PFKL in glycolysis of hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 334. [Google Scholar] [CrossRef]
- Yang, I.; Oh, S.Y.; Jang, S.; Kim, I.Y.; Sung, Y.M.; Seong, J.K. Mettl14 mutation restrains liver regeneration by attenuating mitogens derived from non-parenchymal liver cells. BMB Rep. 2022, 55, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Wang, K.; Wang, L. Systematic analysis of the expression profile and prognostic significance of m6A regulators and PD-L1 in hepatocellular carcinoma. Discov. Oncol. 2022, 13, 131. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, H.; Zhang, X.; Jiang, X.; Liu, Y.; Zhang, H.; Peng, Y.; Li, D.; Yu, Y.; Zhang, J.; et al. N6-methyladenosine modification governs liver glycogenesis by stabilizing the glycogen synthase 2 mRNA. Nat. Commun. 2022, 13, 7038. [Google Scholar] [CrossRef]
- Gao, H.; Wang, X.; Ma, H.; Lin, S.; Zhang, D.; Wu, W.; Liao, Z.; Chen, M.; Li, Q.; Lin, M.; et al. METTL16 regulates m(6)A methylation on chronic hepatitis B associated gene HLA-DPB1 involved in liver fibrosis. Front. Genet. 2022, 13, 996245. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, N.; Zeng, J.; Wang, T.; Shen, Y.; Ma, C.; Yang, M. N(6)-methyladenosine-modified lncRNA ARHGAP5-AS1 stabilises CSDE1 and coordinates oncogenic RNA regulons in hepatocellular carcinoma. Clin. Transl. Med. 2022, 12, e1107. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, X.; Wu, W.; Wang, X.; Wang, Y. FTO-dependent function of N6-methyladenosine is involved in the hepatoprotective effects of betaine on adolescent mice. J. Physiol. Biochem. 2015, 71, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.M.; Simile, M.M.; Calvisi, D.F.; Feo, C.F.; Feo, F. S-Adenosylmethionine: From the Discovery of Its Inhibition of Tumorigenesis to Its Use as a Therapeutic Agent. Cells 2022, 11, 409. [Google Scholar] [CrossRef]
- Mato, J.M.; Martínez-Chantar, M.L.; Lu, S.C. S-adenosylmethionine metabolism and liver disease. Ann. Hepatol. 2013, 12, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ramos, D.; Lopitz-Otsoa, F.; Millet, O.; Alonso, C.; Lu, S.C.; Mato, J.M. One Carbon Metabolism and S-Adenosylmethionine in Non-Alcoholic Fatty Liver Disease Pathogenesis and Subtypes. Livers 2022, 2, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, Y.; Zhang, L.; Jia, Y.; Cai, D.; Qian, S.B.; Du, M.; Zhao, R. GR-mediated FTO transactivation induces lipid accumulation in hepatocytes via demethylation of m(6)A on lipogenic mRNAs. RNA Biol. 2020, 17, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ling, Y.; Jiang, J.; Wang, D.; Wang, J.; Li, J.; Wang, X.; Wang, H. Differential mechanisms regarding triclosan vs. bisphenol A and fluorene-9-bisphenol induced zebrafish lipid-metabolism disorders by RNA-Seq. Chemosphere 2020, 251, 126318. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhang, Z.; Tai, L.; Zhang, L.; Sun, Z.; Zhou, L. Comprehensive analysis of differences of N(6)-methyladenosine RNA methylomes between high-fat-fed and normal mouse livers. Epigenomics 2019, 11, 1267–1282. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Liu, C.; Xu, L.; Yuan, Y.; Zhao, J.; Zhao, W.; Chen, Y.; Qiu, J.; Meng, M.; Zheng, Y.; et al. N6-Methyladenosine Reader Protein YT521-B Homology Domain-Containing 2 Suppresses Liver Steatosis by Regulation of mRNA Stability of Lipogenic Genes. Hepatology 2021, 73, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, B.; Arumugam, S.; Lu, Q.; Mankash, S.M.; Li, J.; Sun, B.; Li, J.; Flavell, R.A.; Li, H.B.; et al. m(6)A mRNA methylation-directed myeloid cell activation controls progression of NAFLD and obesity. Cell Rep. 2021, 37, 109968. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Z.; Xiang, L.; Zhou, B.; Wang, X.; Lin, Y.; Ding, X.; Liu, F.; Lu, Y.; Peng, Y. Analysis of N6-Methyladenosine Methylation Modification in Fructose-Induced Non-Alcoholic Fatty Liver Disease. Front. Endocrinol. 2021, 12, 780617. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Gong, Y.; Wang, X.; He, W.; Wu, L.; Zhang, L.; Xiong, L.; Huang, Y.; Su, L.; Shi, P.; et al. METTL3-m(6)A-Rubicon axis inhibits autophagy in nonalcoholic fatty liver disease. Mol. Ther. 2022, 30, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Sun, C.; Yan, Y.; Niu, Z.; Li, Y.; Xu, X.; Zhang, J.; Wu, Y.; Li, Y.; Wang, L.; et al. Aberrant elevation of FTO levels promotes liver steatosis by decreasing the m6A methylation and increasing the stability of SREBF1 and ChREBP mRNAs. J. Mol. Cell Biol. 2023, 14, mjac061. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Y.; Sun, F.; Luo, Y.; Yang, Y.; Li, J.; Hu, W.; Tao, H.; Lu, C.; Yang, J.-J. ALKBH5 attenuates mitochondrial fission and ameliorates liver fibrosis by reducing Drp1 methylation. Pharmacol. Res. 2023, 187, 106608. [Google Scholar] [CrossRef]
- Wang, S.; Friedman, S.L. Hepatic fibrosis: A convergent response to liver injury that is reversible. J. Hepatol. 2020, 73, 210–211. [Google Scholar] [CrossRef]
- Li, Y.; Kang, X.; Zhou, Z.; Pan, L.; Chen, H.; Liang, X.; Chu, J.; Dong, S.; Liu, C.; Yu, S.; et al. The m6A methyltransferase Mettl3 deficiency attenuates hepatic stellate cell activation and liver fibrosis. Mol. Ther. 2022, 30, 3714–3728. [Google Scholar] [CrossRef]
- Cheng, W.; Li, M.; Zhang, L.; Zhou, C.; Yu, S.; Peng, X.; Zhang, W.; Zhang, W. New roles of N6-methyladenosine methylation system regulating the occurrence of non-alcoholic fatty liver disease with N6-methyladenosine-modified MYC. Front. Pharmacol. 2022, 13, 973116. [Google Scholar] [CrossRef]
- Liu, S.; He, L.; Bannister, O.B.; Li, J.; Schnegelberger, R.D.; Vanderpuye, C.-M.; Althouse, A.D.; Schopfer, F.J.; Wahlang, B.; Cave, M.C.; et al. Western diet unmasks transient low-level vinyl chloride-induced tumorigenesis; potential role of the (epi-)transcriptome. Toxicol. Appl. Pharmacol. 2023, 468, 116514. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Vatsalya, V.; Ma, X.; Klinge, C.M.; Cave, M.C.; Feng, W.; McClain, C.J.; Zhang, X. Metabolic Analysis of Nucleosides/Bases in the Urine and Serum of Patients with Alcohol-Associated Liver Disease. Metabolites 2022, 12, 1187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, F.; Zeng, M.; Han, X.; Cai, L.; Zhang, J.; Weng, J.; Gao, Y. Identification and Characterization of Alcohol-related Hepatocellular Carcinoma Prognostic Subtypes based on an Integrative N6-methyladenosine methylation Model. Int. J. Biol. Sci. 2021, 17, 3554–3572. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Kim, D.-Y.; Lee, Y.-G.; Lee, Y.-S.; Truong, X.T.; Lee, J.-H.; Song, D.-K.; Kwon, T.K.; Park, S.-H.; Jung, C.H. SREBP-1c impairs ULK1 sulfhydration-mediated autophagic flux to promote hepatic steatosis in high-fat-diet-fed mice. Mol. Cell 2021, 81, 3820–3832.e27. [Google Scholar] [CrossRef]
- Jeon, T.-I.; Osborne, T.F. SREBPs: Metabolic integrators in physiology and metabolism. Trends Endocrinol. Metab. 2012, 23, 65–72. [Google Scholar] [CrossRef]
- Yadav, P.K.; Rajvanshi, P.K.; Rajasekharan, R. The role of yeast m6A methyltransferase in peroxisomal fatty acid oxidation. Curr. Genet. 2018, 64, 417–422. [Google Scholar] [CrossRef]
- Yang, Y.; Cai, J.; Yang, X.; Wang, K.; Sun, K.; Yang, Z.; Zhang, L.; Yang, L.; Gu, C.; Huang, X.; et al. Dysregulated m6A modification promotes lipogenesis and development of non-alcoholic fatty liver disease and hepatocellular carcinoma. Mol. Ther. 2022, 30, 2342–2353. [Google Scholar] [CrossRef]
- Xu, Z.; Qin, Y.; Lv, B.; Tian, Z.; Zhang, B. Intermittent Fasting Improves High-Fat Diet-Induced Obesity Cardiomyopathy via Alleviating Lipid Deposition and Apoptosis and Decreasing m6A Methylation in the Heart. Nutrients 2022, 14, 251. [Google Scholar] [CrossRef]
- Petri, B.J.; Piell, K.M.; Wahlang, B.; Head, K.Z.; Andreeva, K.; Rouchka, E.C.; Cave, M.C.; Klinge, C.M. Polychlorinated biphenyls alter hepatic m6A mRNA methylation in a mouse model of environmental liver disease. Environ. Res. 2022, 216, 114686. [Google Scholar] [CrossRef]
- He, S.; Wang, H.; Liu, R.; He, M.; Che, T.; Jin, L.; Deng, L.; Tian, S.; Li, Y.; Lu, H. mRNA N6-methyladenosine methylation of postnatal liver development in pig. PLoS ONE 2017, 12, e0173421. [Google Scholar] [CrossRef]
- Xie, W.; Ma, L.L.; Xu, Y.Q.; Wang, B.H.; Li, S.M. METTL3 inhibits hepatic insulin sensitivity via N6-methyladenosine modification of Fasn mRNA and promoting fatty acid metabolism. Biochem. Biophys. Res. Commun. 2019, 518, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Yu, J.; Frazier, K.; Weng, X.; Li, Y.; Cham, C.M.; Dolan, K.; Zhu, X.; Hubert, N.; Tao, Y. Circadian clock regulation of hepatic lipid metabolism by modulation of m6A mRNA methylation. Cell Rep. 2018, 25, 1816–1828.e1814. [Google Scholar] [CrossRef] [PubMed]
- Adlanmerini, M.; Lazar, M.A. The REV-ERB Nuclear Receptors: Timekeepers for the Core Clock Period and Metabolism. Endocrinology 2023, 164, bqad069. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ren, W.; Li, A.; Ding, Y.; Guo, W.; Su, D.; Hu, C.; Xu, K.; Chen, H.; Xu, X. Fat mass and obesity-associated gene enhances oxidative stress and lipogenesis in nonalcoholic fatty liver disease. Dig. Dis. Sci. 2013, 58, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Zhou, J.; Sinha, R.A.; Singh, B.K.; Ghosh, S.; Lim, K.-H.; Chow, P.K.-H.; Woon, E.C.; Yen, P.M. Hepatic FTO expression is increased in NASH and its silencing attenuates palmitic acid-induced lipotoxicity. Biochem. Biophys. Res. Commun. 2016, 479, 476–481. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, F.; Huang, W.; Qin, S.; Huang, J.-T.; Sergi, C.; Yuan, B.-F.; Liu, S.-M. Glucose Is Involved in the Dynamic Regulation of m6A in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 104, 665–673. [Google Scholar] [CrossRef]
- Church, C.; Moir, L.; McMurray, F.; Girard, C.; Banks, G.T.; Teboul, L.; Wells, S.; Brüning, J.C.; Nolan, P.M.; Ashcroft, F.M. Overexpression of Fto leads to increased food intake and results in obesity. Nat. Genet. 2010, 42, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Zhang, Z.; Yu, L.; Li, Y.; Liang, M.; Zhou, L. FTO reduces mitochondria and promotes hepatic fat accumulation through RNA demethylation. J. Cell. Biochem. 2018, 119, 5676–5685. [Google Scholar] [CrossRef]
- Chen, A.; Chen, X.; Cheng, S.; Shu, L.; Yan, M.; Yao, L.; Wang, B.; Huang, S.; Zhou, L.; Yang, Z. FTO promotes SREBP1c maturation and enhances CIDEC transcription during lipid accumulation in HepG2 cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 538–548. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Z.; Kang, X.; Pan, L.; Liu, C.; Liang, X.; Chu, J.; Dong, S.; Li, Y.; Liu, Q.; et al. Mettl3-mediated mRNA m(6)A modification controls postnatal liver development by modulating the transcription factor Hnf4a. Nat. Commun. 2022, 13, 4555. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, L.P.; Hannivoort, R.A.; Gerken, G.; Hotamisligil, G.S.; Trauner, M.; Canbay, A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 2012, 56, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, P.; Fu, S.; Calay, E.S.; Hotamisligil, G.S. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010, 11, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Ceriello, A.; Esposito, K. Glucose metabolism and hyperglycemia. Am. J. Clin. Nutr. 2008, 87, 217S–222S. [Google Scholar] [CrossRef] [PubMed]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar]
- Li, Y.; Zhang, Q.; Cui, G.; Zhao, F.; Tian, X.; Sun, B.F.; Yang, Y.; Li, W. m(6)A Regulates Liver Metabolic Disorders and Hepatogenous Diabetes. Genom. Proteom. Bioinform. 2020, 18, 371–383. [Google Scholar] [CrossRef]
- Onalan, E.; Yakar, B.; Onalan, E.E.; Karakulak, K.; Kaymaz, T.; Donder, E. m(6)A RNA, FTO, ALKBH5 Expression in Type 2 Diabetic and Obesity Patients. J. Coll. Physicians Surg. Pak. 2022, 32, 1143–1148. [Google Scholar]
- Yang, K.; Sun, J.; Zhang, Z.; Xiao, M.; Ren, D.; Liu, S.-M. Reduction of mRNA m6A associates with glucose metabolism via YTHDC1 in human and mice. Diabetes Res. Clin. Pract. 2023, 198, 110607. [Google Scholar] [CrossRef]
- Ng, M.C.; Shriner, D.; Chen, B.H.; Li, J.; Chen, W.M.; Guo, X.; Liu, J.; Bielinski, S.J.; Yanek, L.R.; Nalls, M.A.; et al. Meta-analysis of genome-wide association studies in African Americans provides insights into the genetic architecture of type 2 diabetes. PLoS Genet. 2014, 10, e1004517. [Google Scholar] [CrossRef]
- Fuchsberger, C.; Flannick, J.; Teslovich, T.M.; Mahajan, A.; Agarwala, V.; Gaulton, K.J.; Ma, C.; Fontanillas, P.; Moutsianas, L.; McCarthy, D.J.; et al. The genetic architecture of type 2 diabetes. Nature 2016, 536, 41–47. [Google Scholar] [CrossRef]
- García-Chapa, E.G.; Leal-Ugarte, E.; Peralta-Leal, V.; Durán-González, J.; Meza-Espinoza, J.P. Genetic Epidemiology of Type 2 Diabetes in Mexican Mestizos. Biomed. Res. Int. 2017, 2017, 3937893. [Google Scholar] [CrossRef] [PubMed]
- Hafner, M.; Landthaler, M.; Burger, L.; Khorshid, M.; Hausser, J.; Berninger, P.; Rothballer, A.; Ascano, M.; Jungkamp, A.-C.; Munschauer, M. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 2010, 141, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Conway, A.E.; Van Nostrand, E.L.; Pratt, G.A.; Aigner, S.; Wilbert, M.L.; Sundararaman, B.; Freese, P.; Lambert, N.J.; Sathe, S.; Liang, T.Y. Enhanced CLIP uncovers IMP protein-RNA targets in human pluripotent stem cells important for cell adhesion and survival. Cell Rep. 2016, 15, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Senoo, H. Structure and function of hepatic stellate cells. Med. Electron Microsc. 2004, 37, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xie, L.; Yang, D.; Luo, K.; Li, X. Small-molecule natural plants for reversing liver fibrosis based on modulation of hepatic stellate cells activation: An update. Phytomedicine 2023, 113, 154721. [Google Scholar] [CrossRef]
- Puche, J.E.; Saiman, Y.; Friedman, S.L. Hepatic stellate cells and liver fibrosis. Compr. Physiol. 2013, 3, 1473–1492. [Google Scholar]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Wang, J.; Yang, Y.; Yang, Y.; Li, J.; Lu, D.; Lu, C. ALKBH5 ameliorated liver fibrosis and suppressed HSCs activation via triggering PTCH1 activation in an m(6)A dependent manner. Eur. J. Pharmacol. 2022, 922, 174900. [Google Scholar] [CrossRef]
- Shen, M.; Li, Y.; Wang, Y.; Shao, J.; Zhang, F.; Yin, G.; Chen, A.; Zhang, Z.; Zheng, S. N(6)-methyladenosine modification regulates ferroptosis through autophagy signaling pathway in hepatic stellate cells. Redox Biol. 2021, 47, 102151. [Google Scholar] [CrossRef]
- Shen, M.; Guo, M.; Li, Y.; Wang, Y.; Qiu, Y.; Shao, J.; Zhang, F.; Xu, X.; Yin, G.; Wang, S.; et al. m(6)A methylation is required for dihydroartemisinin to alleviate liver fibrosis by inducing ferroptosis in hepatic stellate cells. Free. Radic. Biol. Med. 2022, 182, 246–259. [Google Scholar] [CrossRef]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, N. TGF-β in hepatic stellate cell activation and liver fibrogenesis—Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Tsui, Y.M.; Shi, C.; Wang, Y.; Zhang, X.; Yan, Q.; Chen, M.; Jiang, C.; Yuan, Y.F.; et al. RALYL increases hepatocellular carcinoma stemness by sustaining the mRNA stability of TGF-β2. Nat. Commun. 2021, 12, 1518. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: The central role of nontriglyceride fatty acid metabolites. Hepatology 2010, 52, 774–788. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Scott, C.L. Liver macrophages in health and disease. Immunity 2022, 55, 1515–1529. [Google Scholar] [CrossRef]
- Zwicker, C.; Bujko, A.; Scott, C.L. Hepatic macrophage responses in inflammation, a function of plasticity, heterogeneity or both? Front. Immunol. 2021, 12, 690813. [Google Scholar] [CrossRef]
- Ramadori, G.; Armbrust, T. Cytokines in the liver. Eur. J. Gastroenterol. Hepatol. 2001, 13, 777–784. [Google Scholar] [CrossRef]
- Rivera, C.A.; Adegboyega, P.; van Rooijen, N.; Tagalicud, A.; Allman, M.; Wallace, M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J. Hepatol. 2007, 47, 571–579. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Seki, E.; Brenner, D.A. Toll-like receptor signaling in the liver. Gastroenterology 2006, 130, 1886–1900. [Google Scholar] [CrossRef]
- Feng, Y.; Dong, H.; Sun, B.; Hu, Y.; Yang, Y.; Jia, Y.; Jia, L.; Zhong, X.; Zhao, R. METTL3/METTL14 Transactivation and m6A-Dependent TGF-β1 Translation in Activated Kupffer Cells. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 839–856. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, Q.; Yuan, B.; Wang, B.; Zhang, Y.; Li, Z.; Du, S.; Zeng, Z. ALKBH5-Modified HMGB1-STING Activation Contributes to Radiation Induced Liver Disease via Innate Immune Response. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 491–501. [Google Scholar] [CrossRef]
- Xu, M.; Zhuo, R.; Tao, S.; Liang, Y.; Liu, C.; Liu, Q.; Wang, T.; Zhong, X. M(6)A RNA Methylation Mediates NOD1/NF-kB Signaling Activation in the Liver of Piglets Challenged with Lipopolysaccharide. Antioxidants 2022, 11, 1954. [Google Scholar] [CrossRef] [PubMed]
- Martinez, N.M.; Su, A.; Burns, M.C.; Nussbacher, J.K.; Schaening, C.; Sathe, S.; Yeo, G.W.; Gilbert, W.V. Pseudouridine synthases modify human pre-mRNA co-transcriptionally and affect pre-mRNA processing. Mol. Cell 2022, 82, 645–659.e9. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Li, X.; Xiong, X.; Wang, J.; Zhou, Z.; Zhu, X.; Gu, Y.; Dominissini, D.; He, L.; et al. N(1)-methyladenosine methylation in tRNA drives liver tumourigenesis by regulating cholesterol metabolism. Nat. Commun. 2021, 12, 6314. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Y.; Jiang, X.-W.; Li, W.-F.; Guo, G.; Gong, J.-P.; Ding, X. Clinicopathological and prognostic significance of Yes-associated protein expression in hepatocellular carcinoma and hepatic cholangiocarcinoma. Tumor Biol. 2016, 37, 13499–13508. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhu, M.; Li, W.; Zhou, Z.; Wan, X. m5C and m6A modification of long noncoding NKILA accelerates cholangiocarcinoma progression via the miR-582-3p-YAP1 axis. Liver Int. 2022, 42, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- Tóth, M.; Wehling, L.; Thiess, L.; Rose, F.; Schmitt, J.; Weiler, S.M.E.; Sticht, C.; De La Torre, C.; Rausch, M.; Albrecht, T.; et al. Co-expression of YAP and TAZ associates with chromosomal instability in human cholangiocarcinoma. BMC Cancer 2021, 21, 1079. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).