Development and Validation of a 54K Genome-Wide Liquid SNP Chip Panel by Target Sequencing for Dairy Goat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material, DNA Extraction, and Sequencing
2.2. Trimming, Mapping, and SNP Detection
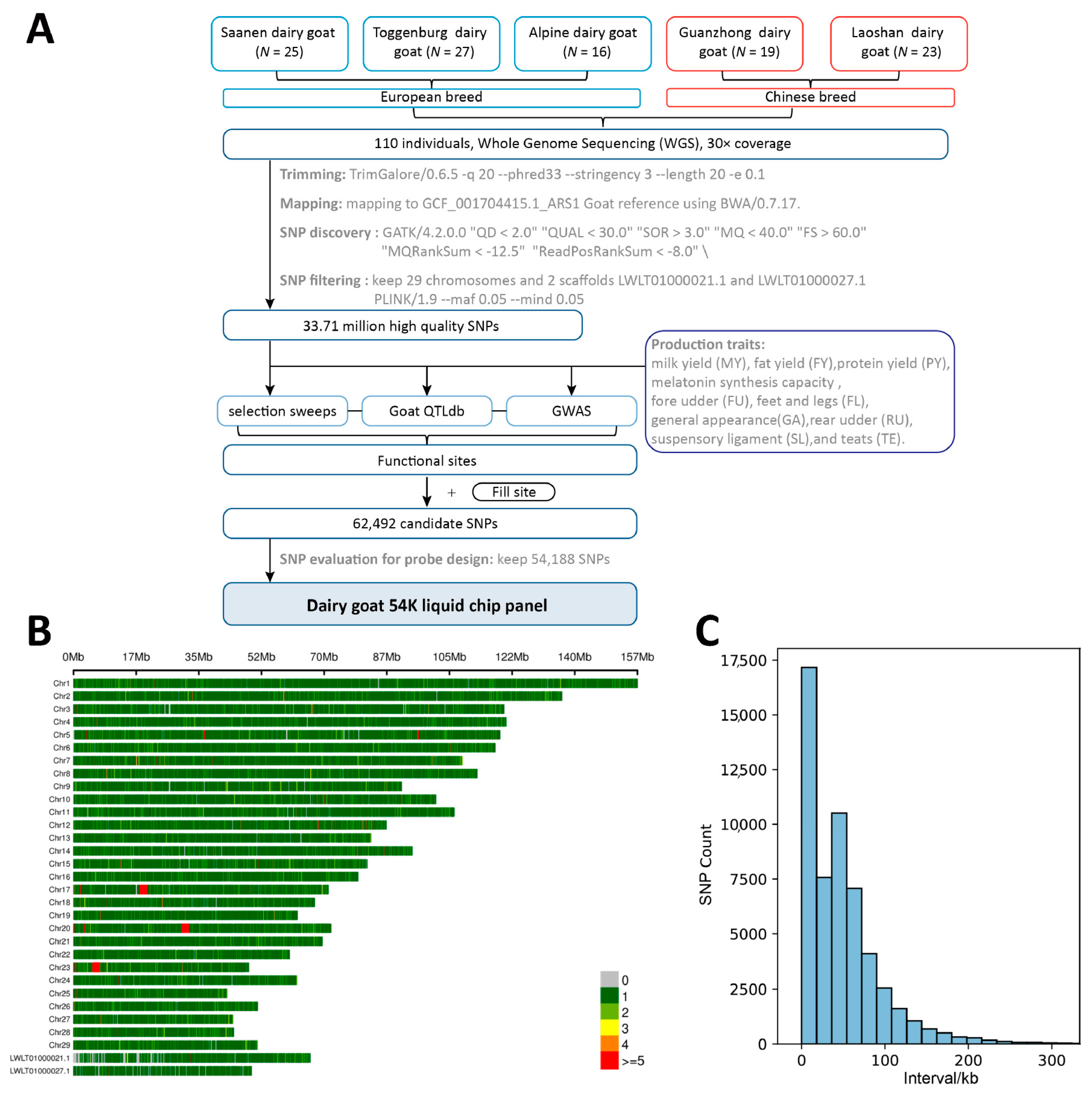
2.3. Development of the Dairy Goat SNP Panel
2.4. Validation of the 54K Liquid SNP Chip Panel
2.5. Genome-Wide Association Study
3. Results
3.1. Design and Development of the 54K Liquid SNP Chip Panel
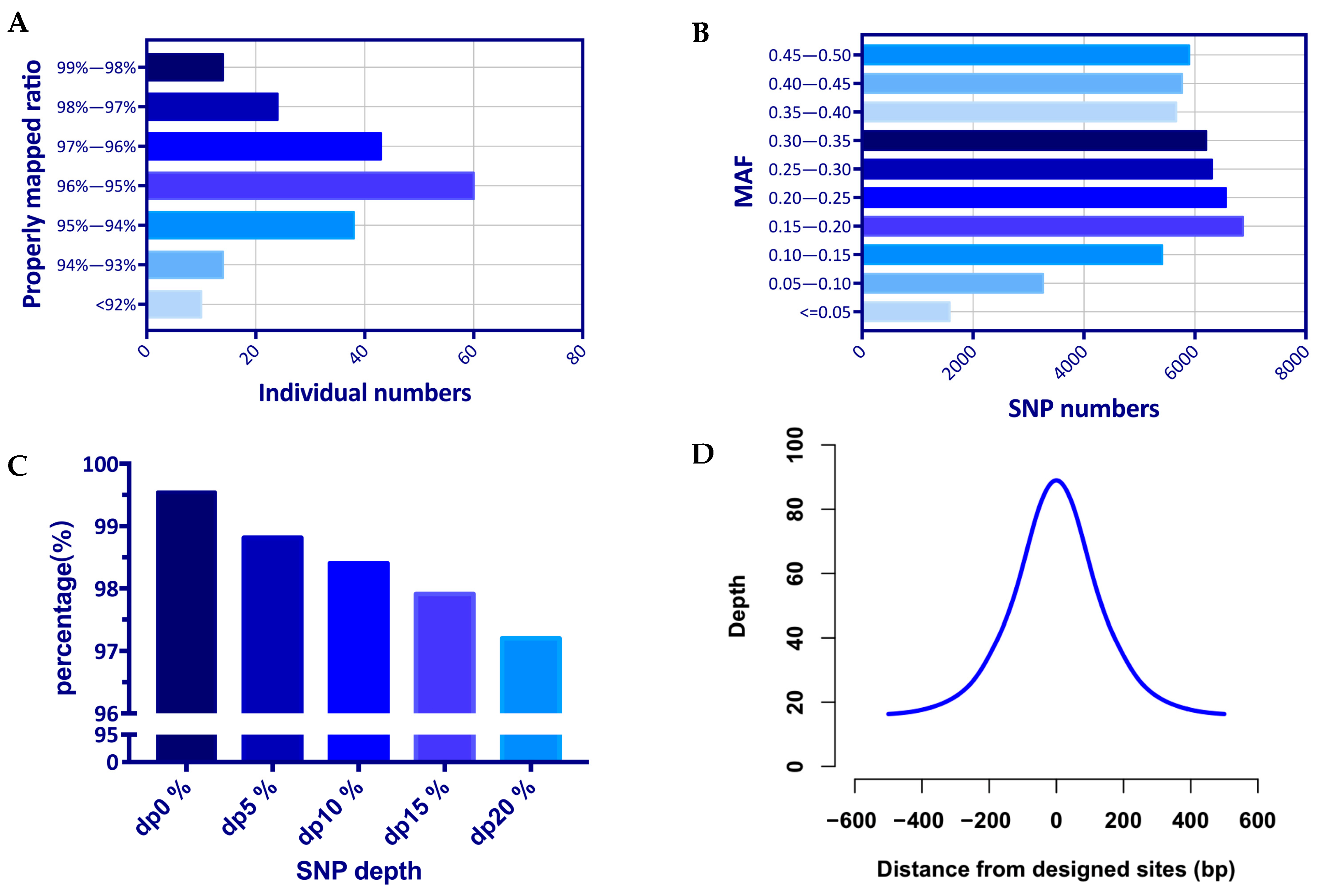
3.2. Genotyping Performance of the Liquid SNP Chip Panel
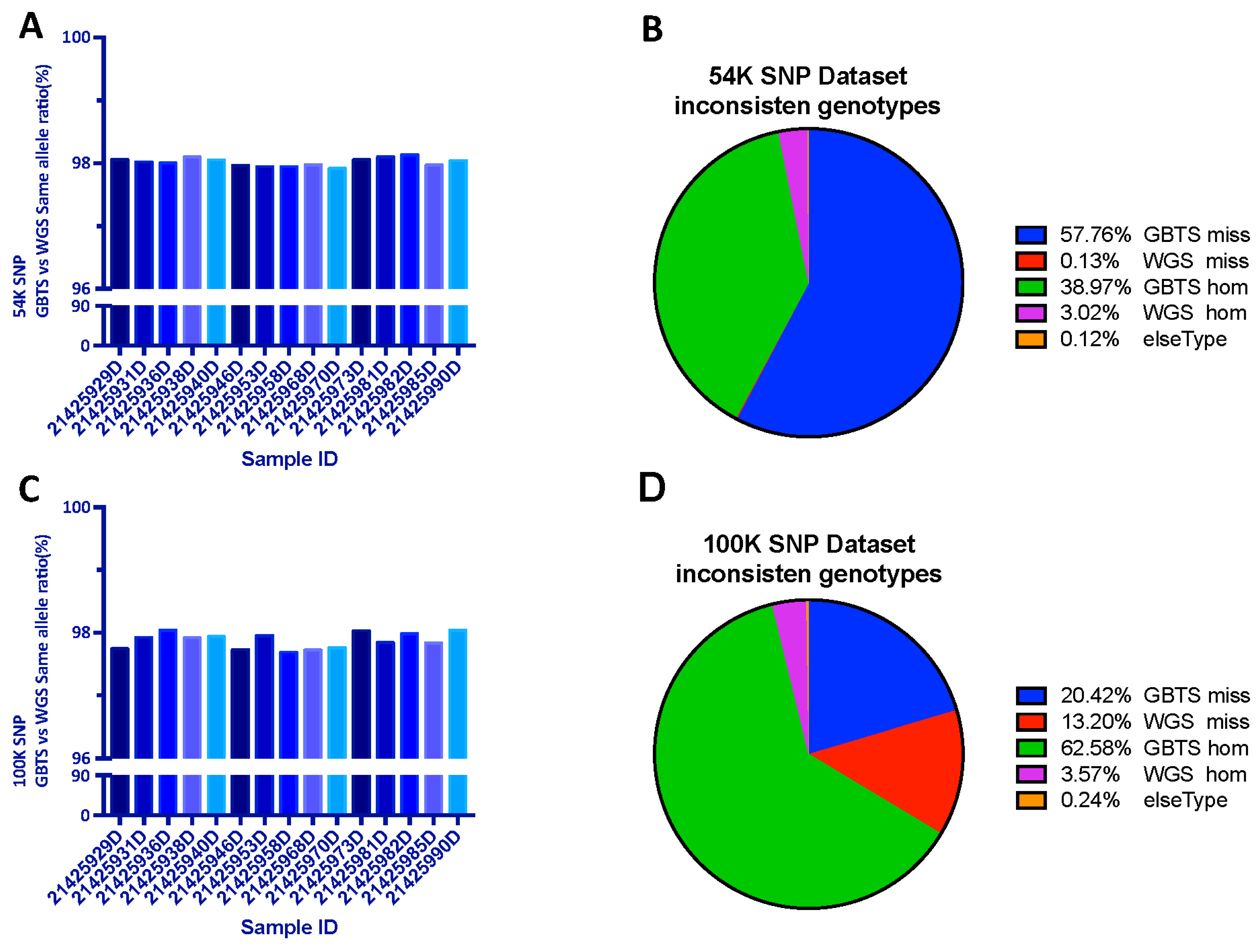
3.3. Comparison of Chip Panel and Resequencing Results
3.4. High-Resolution GWAS for Coat Color
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallace, J.G.; Rodgers-Melnick, E.; Buckler, E.S. On the Road to Breeding 4.0: Unraveling the Good, the Bad, and the Boring of Crop Quantitative Genomics. Annu. Rev. Genet. 2016, 52, 421–444. [Google Scholar] [CrossRef] [PubMed]
- Meuwissen, T.H.E.; Hayes, B.J.; Goddard, M.E. Prediction of Total Genetic Value Using Genome-Wide Dense Marker Maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Guillenea, A.; Su, G.; Lund, M.S.; Karaman, E. Genomic prediction in Nordic Red dairy cattle considering breed origin of alleles. J. Dairy Sci. 2022, 105, 2426–2438. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, R.; Calus, M.P.L.; Napel, J.T.; Veerkamp, R.F.; Michenet, A.; Savoia, S.; Cromie, A.; Vandenplas, J. International single-step SNPBLUP beef cattle evaluations for Limousin weaning weight. Genet. Sel. Evol. 2022, 54, 57. [Google Scholar] [CrossRef]
- Bolormaa, S.; MacLeod, I.M.; Khansefid, M.; Marett, L.C.; Wales, W.J.; Miglior, F.; Baes, C.F.; Schenkel, F.S.; Connor, E.E.; Manzanilla-Pech, C.I.V.; et al. Sharing of either phenotypes or genetic variants can increase the accuracy of genomic prediction of feed efficiency. Genet. Sel. Evol. 2022, 54, 60. [Google Scholar] [CrossRef]
- Ros-Freixedes, R.; Johnsson, M.; Whalen, A.; Chen, C.-Y.; Valente, B.D.; Herring, W.O.; Gorjanc, G.; Hickey, J.M. Genomic prediction with whole-genome sequence data in intensely selected pig lines. Genet. Sel. Evol. 2022, 54, 65. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, Z.; Ren, D.; Cai, X.; Zhu, Q.; Ding, X.; Zhang, H.; Zhang, Z.; Li, J. Genomic Prediction Using LD-Based Haplotypes in Combined Pig Populations. Front. Genet. 2022, 13, 843300. [Google Scholar] [CrossRef]
- Jibrila, I.; Napel, J.; Vandenplas, J.; Bergsma, R.; Veerkamp, R.F.; Calus, M.P.L. Impact of genomic preselection on subsequent ssGBLUP evaluation of preselected animals for scarcely recorded feed intake in pigs. J. Anim. Breed. Genet. 2023, 140, 253–263. [Google Scholar] [CrossRef]
- Li, X.; Nie, C.; Liu, Y.; Chen, Y.; Lv, X.; Wang, L.; Zhang, J.; Yang, W.; Li, K.; Zheng, C.; et al. The Genetic Architecture of Early Body Temperature and Its Correlation With Salmonella Pullorum Resistance in Three Chicken Breeds. Front. Genet. 2020, 10, 1287. [Google Scholar] [CrossRef]
- Mastrangelo, S.; Ben-Jemaa, S.; Perini, F.; Cendron, F.; Biscarini, F.; Lasagna, E.; Penasa, M.; Cassandro, M. Genome-wide mapping of signatures of selection using a high-density array identified candidate genes for growth traits and local adaptation in chickens. Genet. Sel. Evol. 2023, 55, 20. [Google Scholar] [CrossRef]
- Mamanova, L.; Coffey, A.J.; Scott, C.E.; Kozarewa, I.; Turner, E.H.; Kumar, A.; Howard, E.; Shendure, J.; Turner, D.J. Target-enrichment strategies for next-generation sequencing. Nat. Methods 2010, 7, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Sekine, D.; Oku, S.; Nunome, T.; Hirakawa, H.; Tsujimura, M.; Terachi, T.; Toyoda, A.; Shigyo, M.; Sato, S.; Tsukazaki, H. Development of a genome-wide marker design workflow for onions and its application in target amplicon sequencing-based genotyping. Dna. Res. Int. J. Rapid Publ. Rep. Genes Genomes 2022, 29, dsac020. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yang, Q.; Huang, F.; Zheng, H.; Sang, Z.; Xu, Y.; Zhang, C.; Wu, K.; Tao, J.; Prasanna, B.M.; et al. Development of high-resolution multiple-SNP arrays for genetic analyses and molecular breeding through genotyping by target sequencing and liquid chip. Plant Commun. 2021, 2, 100230. [Google Scholar] [CrossRef] [PubMed]
- Tosser-Klopp, G.; Bardou, P.; Bouchez, O.; Cabau, C.; Crooijmans, R.; Dong, Y.; Donnadieu-Tonon, C.; Eggen, A.; Heuven, H.C.M.; Jamli, S.; et al. Design and Characterization of a 52K SNP Chip for Goats. PLoS ONE 2014, 9, e86227. [Google Scholar] [CrossRef]
- Massender, E.; Oliveira, H.R.; Brito, L.F.; Maignel, L.; Jafarikia, M.; Baes, C.F.; Sullivan, B.; Schenkel, F.S. Genome-wide association study for milk production and conformation traits in Canadian Alpine and Saanen dairy goats. J. Dairy Sci. 2023, 106, 1168–1189. [Google Scholar] [CrossRef]
- Andrews, S.; Krueger, F.; Segonds-Pichon, A.; Biggins, L.; Krueger, C.; Wingett, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: https://qubeshub.org/resources/fastqc (accessed on 10 January 2022.).
- Krueger, F.; James, F.; Ewels, P.; Afyounian, E.; Schuster-Boeckler, B. FelixKrueger/TrimGalore: V0.6.7-DOI via Zenodo (0.6.7). Zenodo 2021. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows—Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- DePristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; Del Angel, G.; Rivas, M.A.; Hanna, M.; et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef]
- Van der Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; del Angel, G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From FastQ Data to High-Confidence Variant Calls: The Genome Analysis Toolkit Best Practices Pipeline. Curr. Protoc. Bioinform. 2013, 43, 11.10.1–11.10.33. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Patterson, N.; Reich, D. Population differentiation as a test for selective sweeps. Genome Res. 2010, 20, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-L.; Park, C.A.; Reecy, J.M. Bringing the Animal QTLdb and CorrDB into the future: Meeting new challenges and providing updated services. Nucleic Acids Res. 2021, 50, D956–D961. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 2012, 44, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Garrick, D.J.; Taylor, J.F.; Fernando, R.L. Deregressing estimated breeding values and weighting information for genomic regression analyses. Genet. Sel. Evol. 2009, 41, 55. [Google Scholar] [CrossRef]
- Yang, M.; Shi, J.; Tian, J.; Tao, J.; Chai, M.; Wang, J.; Xu, Z.; Song, Y.; Zhu, K.; Ji, P.; et al. Exogenous melatonin reduces somatic cell count of milk in Holstein cows. Sci. Rep. 2017, 7, 43280. [Google Scholar] [CrossRef]
- Tao, J.; Yang, M.; Wu, H.; Ma, T.; He, C.; Chai, M.; Zhang, X.; Zhang, J.; Ding, F.; Wang, S.; et al. Effects of AANAT overexpression on the inflammatory responses and autophagy activity in the cellular and transgenic animal levels. Autophagy 2018, 14, 1850–1869. [Google Scholar] [CrossRef]
- Wu, H.; Yao, S.; Wang, T.; Wang, J.; Ren, K.; Yang, H.; Ma, W.; Ji, P.; Lu, Y.; Ma, H.; et al. Effects of Melatonin on Dairy Herd Improvement (DHI) of Holstein Cow with High SCS. Molecules 2021, 26, 834. [Google Scholar] [CrossRef]
- Si, Z.; Jin, S.; Li, J.; Han, Z.; Li, Y.; Wu, X.; Ge, Y.; Fang, L.; Zhang, T.; Hu, Y. The design, validation, and utility of the “ZJU CottonSNP40K” liquid chip through genotyping by target sequencing. Ind. Crop. Prod. 2022, 188, 115629. [Google Scholar] [CrossRef]
- Qiao, X.; Su, R.; Wang, Y.; Wang, R.; Yang, T.; Li, X.; Chen, W.; He, S.; Jiang, Y.; Xu, Q.; et al. Genome-wide Target Enrichment-aided Chip Design: A 66 K SNP Chip for Cashmere Goat. Sci. Rep. 2017, 7, 8621. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, H.; Tao, J.; Ren, Y.; Xu, C.; Wu, K.; Zou, C.; Zhang, J.; Xu, Y. Development of multiple SNP marker panels affordable to breeders through genotyping by target sequencing (GBTS) in maize. Mol. Breed. 2019, 39, 37. [Google Scholar] [CrossRef]
- Zhang, C.; Li, M.; Liang, L.; Xiang, J.; Zhang, F.; Zhang, C.; Li, Y.; Liang, J.; Zheng, T.; Zhang, F.; et al. Rice3K56 is a high-quality SNP array for genome-based genetic studies and breeding in rice (Oryza sativa L.). Crop. J. 2023. [Google Scholar] [CrossRef]
- Liu, J.; Peng, W.; Yu, F.; Lin, W.; Shen, Y.; Yu, W.; Gong, S.; Huang, H.; You, W.; Luo, X.; et al. Development and validation of a 40-K multiple-SNP array for Pacific abalone (Haliotis discus hannai). Aquaculture 2022, 558, 738393. [Google Scholar] [CrossRef]
- Alshanbari, F.; Castaneda, C.; Juras, R.; Hillhouse, A.; Mendoza, M.N.; Gutiérrez, G.A.; De León, F.A.P.; Raudsepp, T. Comparative FISH-Mapping of MC1R, ASIP, and TYRP1 in New and Old World Camelids and Association Analysis With Coat Color Phenotypes in the Dromedary (Camelus dromedarius). Front. Genet. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Ding, P.; Chen, S.; Zhao, S.; Wang, J.; Lai, S. Analysis of MC1R, MITF, TYR, TYRP1, and MLPH Genes Polymorphism in Four Rabbit Breeds with Different Coat Colors. Animals 2021, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Otto, M.; Ammann, P.; Keller, I.; Drögemüller, C.; Leeb, T. The brown coat colour of Coppernecked goats is associated with a non-synonymous variant at the TYRP1 locus on chromosome. Anim. Genet. 2014, 46, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liu, X.; Geng, L.; Ma, R.; Li, L.; Li, J.; Zhang, C.; Liu, Z.; Gong, Y.; Li, X. Illumina-sequencing based transcriptome study of coat color phenotypes in domestic goats. Genes Genom. 2017, 39, 817–830. [Google Scholar] [CrossRef]
- Shestak, A.G.; Bukaeva, A.A.; Saber, S.; Zaklyazminskaya, E.V. Allelic Dropout Is a Common Phenomenon That Reduces the Diagnostic Yield of PCR-Based Sequencing of Targeted Gene Panels. Front. Genet. 2021, 12, 620337. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, S.; Li, W.; Jin, H.; Zhang, L.; Liu, G. Development and Validation of a 54K Genome-Wide Liquid SNP Chip Panel by Target Sequencing for Dairy Goat. Genes 2023, 14, 1122. https://doi.org/10.3390/genes14051122
Guan S, Li W, Jin H, Zhang L, Liu G. Development and Validation of a 54K Genome-Wide Liquid SNP Chip Panel by Target Sequencing for Dairy Goat. Genes. 2023; 14(5):1122. https://doi.org/10.3390/genes14051122
Chicago/Turabian StyleGuan, Shengyu, Weining Li, Hai Jin, Lu Zhang, and Guoshi Liu. 2023. "Development and Validation of a 54K Genome-Wide Liquid SNP Chip Panel by Target Sequencing for Dairy Goat" Genes 14, no. 5: 1122. https://doi.org/10.3390/genes14051122





