Complete Mitochondrial Genome of Piophila casei (Diptera: Piophilidae): Genome Description and Phylogenetic Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction, Library Construction, and Sequencing
2.3. Genome Assembly and Gene Annotation
2.4. Genomic and Phylogenetic Analysis
2.5. Divergence Time Estimate
3. Results and Discussion
3.1. General Features of P. casei mt Genome
3.2. Protein-CODING Genes and Codons
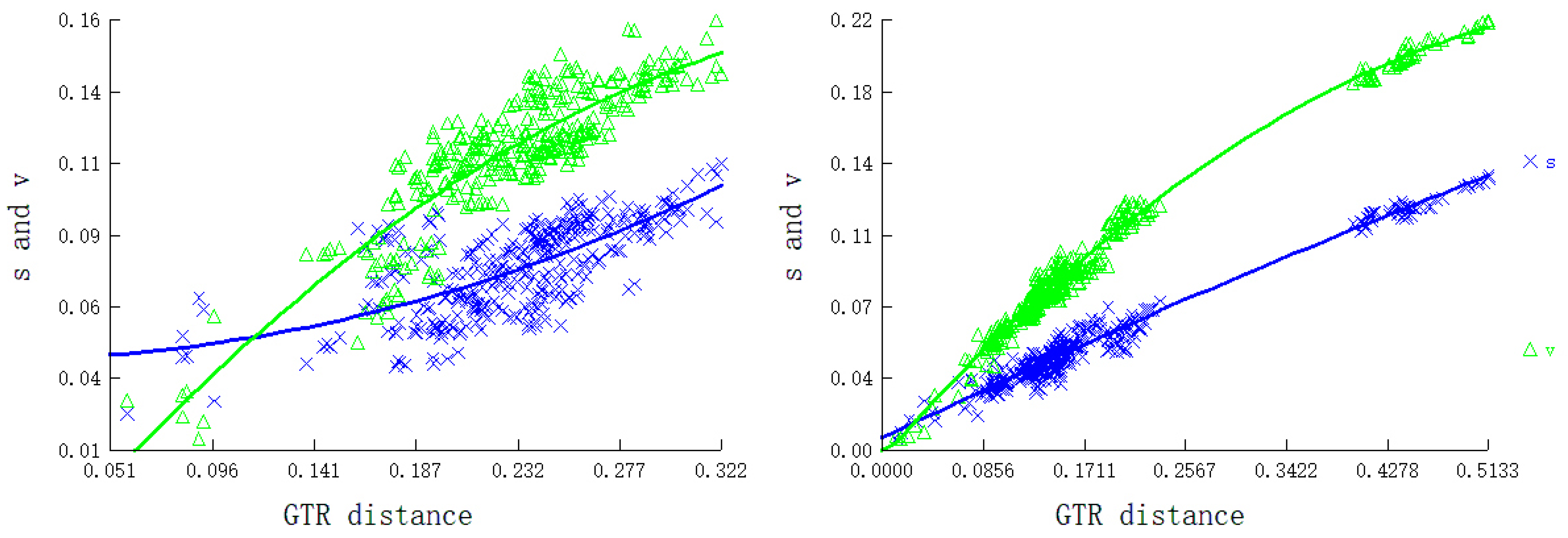
3.3. Transfer and Ribosomal RNA Genes
3.4. Phylogenetic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russo, A.; Cocuzza, G.E.; Vasta, M.C.; Simola, M.; Virone, G. Life Fertility Tables of Piophila casei L. (Diptera: Piophilidae) Reared at Five Different Temperatures. Environ. Entomol. 2006, 35, 194–200. [Google Scholar] [CrossRef]
- Simmons, P. The Cheese Skipper as a Pest in Cured Meats; US Department of Agriculture: Washington, DC, USA, 1927.
- Arnaldos, M.; Sánchez, F.; Álvarez, P.; García, M. A forensic entomology case from the Southeastern Iberian Peninsula. Anil Aggrawal’s Internet J. Forensic Med. Toxicol. 2004, 5, 22–25. [Google Scholar]
- Zhao, Y.; Abbar, S.; Amoah, B.; Phillips, T.; Schilling, M. Controlling pests in dry-cured ham: A review. Meat Sci. 2016, 111, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vega, D. Skipping clues: Forensic importance of the family Piophilidae (Diptera). Forensic Sci. Int. 2011, 212, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vega, D.; Gómez-Gómez, A.; Baz, A.; Díaz-Aranda, L. New piophilid in town: The first Palaearctic record of Piophila megastigmata and its coexistence with Piophila casei in central Spain. Med. Vet. Entomol. 2011, 25, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Garrood, J.R. Note on a case of intestinal myiasis. Parasitology 1910, 3, 315–318. [Google Scholar] [CrossRef]
- Peckenschneider, L.E.; Pokorny, C.; Hellwig, C.A. Intestinal infestation with maggots of the "cheese fly" (Piophila casei). J. Am. Med. Assoc. 1952, 149, 262–263. [Google Scholar] [CrossRef]
- Kirinoki, M.; Hitosugi, M.; Kato-Hayashi, N.; Iwasa, M.; Chigusa, Y. Discovery of Liopiophila varipes and Protopiophila contecta (Diptera: Piophilidae) from human cadavers. Forensic Sci. Int. 2015, 248, e8–e12. [Google Scholar] [CrossRef]
- Ozerov, A. On the classification of the Family Piophilidae (Diptera). Zool. Zhurnal 2004, 83, 1353–1360. [Google Scholar]
- Zajac, B.K.; Martin-Vega, D.; Feddern, N.; Fremdt, H.; e Castro, C.P.; Szpila, K.; Reckel, F.; Schuett, S.; Verhoff, M.A.; Amendt, J. Molecular identification and phylogenetic analysis of the forensically important family Piophilidae (Diptera) from different European locations. Forensic Sci. Int. 2016, 259, 77–84. [Google Scholar] [CrossRef]
- e Castro, C.P.; Cunha, E.; Serrano, A.; García, M.D. Piophila megastigmata (Diptera: Piophilidae): First records on human corpses. Forensic Sci. Int. 2012, 214, 23–26. [Google Scholar] [CrossRef]
- Paños, A.; Arnaldos, M.; García, M.; Ubero-Pascal, N. Ultrastructure of preimaginal stages of Piophila megastigmata McAlpine, 1978 (Diptera, Piophilidae): A fly of forensic importance. Parasitol. Res. 2013, 112, 3771–3788. [Google Scholar] [CrossRef]
- Barros-Cordeiro, K.; Pujol-Luz, J.; Báo, S. A study of the pupal development of five forensically important flies (Diptera: Brachycera). J. Med. Entomol. 2021, 58, 1643–1653. [Google Scholar] [CrossRef]
- Du, Z.; Hasegawa, H.; Cooley, J.R.; Simon, C.; Yoshimura, J.; Cai, W.; Sota, T.; Li, H. Mitochondrial genomics reveals shared phylogeographic patterns and demographic history among three periodical cicada species groups. Mol. Biol. Evol. 2019, 36, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Li, W.N.; Shao, R.; Zhang, Q.; Deng, W.; Xue, X.F. Mitochondrial genome reorganization characterizes various lineages of mesostigmatid mites (Acari: Parasitiformes). Zool. Scr. 2019, 48, 679–689. [Google Scholar] [CrossRef]
- Xue, X.-F.; Dong, Y.; Deng, W.; Hong, X.-Y.; Shao, R. The phylogenetic position of eriophyoid mites (superfamily Eriophyoidea) in Acariformes inferred from the sequences of mitochondrial genomes and nuclear small subunit (18S) rRNA gene. Mol. Phylogenetics Evol. 2017, 109, 271–282. [Google Scholar] [CrossRef]
- Su, X.; Fang, Y.; Xu, J.-Y.; Fang, W.-X.; Zhan, X.-B.; Fang, Y.; Chu, L.-M.; Feng, R.; Jin, Y.-L.; Sun, E.-T. The complete mitochondrial genome of the storage mite pest Tyrophagus fanetzhangorum (Acari: Acaridae). Syst. Appl. Acarol. 2020, 25, 1693–1701. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Z.; Bai, R.; Yu, Z.; Liu, J. The mitochondrial genome and phylogenetic analysis of the tick Haemaphysalis qinghaiensis Teng, 1980 (Acari: Ixodidae). Syst. Appl. Acarol. 2022, 27, 81–93. [Google Scholar] [CrossRef]
- Lan, Y.-M.; Feng, S.-Q.; Xia, L.-Y.; Li, Z.-H.; Cao, Y.; Stejskal, V.; Aulicky, R.; Wu, Y. The first complete mitochondrial genome of Cheyletus malaccensis (Acari: Cheyletidae): Gene rearrangement. Syst. Appl. Acarol. 2020, 25, 1433–1443. [Google Scholar] [CrossRef]
- Ye, S.; Zhang, H.-Y.; Song, Y.-F.; Yang, M.-F.; Li, L.-T.; Yu, L.-C.; Liu, J.-F. Complete mitochondrial genome of Pyemotes zhonghuajia (Acari: Pyemotidae). Syst. Appl. Acarol. 2022, 27, 1677–1686. [Google Scholar] [CrossRef]
- Meng, G.; Li, Y.; Yang, C.; Liu, S. MitoZ: A toolkit for mitochondrial genome assembly, annotation and visualization. Nucleic Acids Res. 2019, 47, e63. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wu, G.; Tang, J.; Luo, R.; Patterson, J.; Liu, S.; Huang, W.; He, G.; Gu, S.; Li, S.; et al. SOAPdenovo-Trans: De novo transcriptome assembly with short RNA-Seq reads. Bioinformatics 2014, 30, 1660–1666. [Google Scholar] [CrossRef]
- Gertz, E.M.; Yu, Y.-K.; Agarwala, R.; Schäffer, A.A.; Altschul, S.F. Composition-based statistics and translated nucleotide searches: Improving the TBLASTN module of BLAST. BMC Biol. 2006, 4, 41. [Google Scholar] [CrossRef]
- Birney, E.; Clamp, M.; Durbin, R. GeneWise and Genomewise. Genome Res. 2004, 14, 988–995. [Google Scholar] [CrossRef]
- Jühling, F.; Pütz, J.; Bernt, M.; Donath, A.; Middendorf, M.; Florentz, C.; Stadler, P.F. Improved systematic tRNA gene annotation allows new insights into the evolution of mitochondrial tRNA structures and into the mechanisms of mitochondrial genome rearrangements. Nucleic Acids Res. 2012, 40, 2833–2845. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, E.P.; Eddy, S.R. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics 2013, 29, 2933–2935. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, L. The Grammar of Graphics. (Statistics and Computing); Springer: New York, NY, USA, 2005. [Google Scholar]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Xia, X. DAMBE7: New and improved tools for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2018, 35, 1550–1552. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Ranwez, V.; Douzery, E.J.; Cambon, C.; Chantret, N.; Delsuc, F. MACSE v2: Toolkit for the alignment of coding sequences accounting for frameshifts and stop codons. Mol. Biol. Evol. 2018, 35, 2582–2584. [Google Scholar] [CrossRef]
- Hofacker, I.L.; Stadler, P.F. Memory efficient folding algorithms for circular RNA secondary structures. Bioinformatics 2006, 22, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef]
- Han, H.-Y.; Ro, K.-E. Molecular phylogeny of the superfamily Tephritoidea (Insecta: Diptera) reanalysed based on expanded taxon sampling and sequence data. J. Zool. Syst. Evol. Res. 2016, 54, 276–288. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Shang, J.; Xu, W.; Huang, X.; Zhang, D.; Yan, L.; Pape, T. Comparative Mitogenomics of Flesh Flies: Implications for Phylogeny. Insects 2022, 13, 718. [Google Scholar] [CrossRef]
- Junqueira, A.C.M.; Lessinger, A.C.; Torres, T.T.; da Silva, F.R.; Vettore, A.L.; Arruda, P.; Azeredo Espin, A.M.L. The mitochondrial genome of the blowfly Chrysomya chloropyga (Diptera: Calliphoridae). Gene 2004, 339, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ojala, D.; Montoya, J.; Attardi, G. tRNA punctuation model of RNA processing in human mitochondria. Nature 1981, 290, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, B.; He, H.; Xu, X.; Ruan, Y.; Yang, M. Characterization of the Complete Mitochondrial Genome of a Flea Beetle Luperomorpha xanthodera (Coleoptera: Chrysomelidae: Galerucinae) and Phylogenetic Analysis. Genes 2023, 14, 414. [Google Scholar] [CrossRef]
- Kumar, V.; Tyagi, K.; Chakraborty, R.; Prasad, P.; Kundu, S.; Tyagi, I.; Chandra, K. The Complete Mitochondrial Genome of endemic giant tarantula, Lyrognathus crotalus (Araneae: Theraphosidae) and comparative analysis. Sci. Rep. 2020, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- De Mandal, S.; Mazumder, T.H.; Panda, A.K.; Kumar, N.S.; Jin, F. Analysis of synonymous codon usage patterns of HPRT1 gene across twelve mammalian species. Genomics 2020, 112, 304–311. [Google Scholar] [CrossRef]
- Pál, C.; Papp, B.; Lercher, M.J. An integrated view of protein evolution. Nat. Rev. Genet. 2006, 7, 337–348. [Google Scholar] [CrossRef]
- Sharp, P.M.; Li, W.-H. An evolutionary perspective on synonymous codon usage in unicellular organisms. J. Mol. Evol. 1986, 24, 28–38. [Google Scholar] [CrossRef]
- Ye, F.; Liu, T.; King, S.D.; You, P. Mitochondrial genomes of two phlebotomine sand flies, Phlebotomus chinensis and Phlebotomus papatasi (Diptera: Nematocera), the first representatives from the family Psychodidae. Parasites Vectors 2015, 8, 1–13. [Google Scholar] [CrossRef]
- Gong, Y.-J.; Shi, B.-C.; Kang, Z.-J.; Zhang, F.; Wei, S.-J. The complete mitochondrial genome of the oriental fruit moth Grapholita molesta (Busck) (Lepidoptera: Tortricidae). Mol. Biol. Rep. 2012, 39, 2893–2900. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, H.; Ge, X.; Yang, G.; Xie, G.; Yang, Y. A Mitochondrial Genome Phylogeny of Cleridae (Coleoptera, Cleroidea). Insects 2022, 13, 118. [Google Scholar] [CrossRef]
- Li, R.; Shu, X.; Li, X.; Meng, L.; Li, B. Comparative mitogenome analysis of three species and monophyletic inference of Catantopinae (Orthoptera: Acridoidea). Genomics 2019, 111, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-T.; Zhao, M.-Y.; Xu, C.; Du, Y.-Z. Molecular phylogeny of Systellognatha (Plecoptera: Arctoperlaria) inferred from mitochondrial genome sequences. Int. J. Biol. Macromol. 2018, 111, 542–547. [Google Scholar] [CrossRef]
- Huang, X.; Chen, B.; Wei, Z.; Shi, A. First Report of Complete Mitochondrial Genome in the Tribes Coomaniellini and Dicercini (Coleoptera: Buprestidae) and Phylogenetic Implications. Genes 2022, 13, 1074. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.-S.; Chua, K.-O.; Song, S.-L.; Liew, Y.J.-M.; Eamsobhana, P.; Chan, K.-G. Complete mitochondrial genome of Dacus vijaysegarani and phylogenetic relationships with congeners and other tephritid fruit flies (Insecta: Diptera). Mol. Biol. Rep. 2021, 48, 6047–6056. [Google Scholar] [CrossRef]
- Sugimoto, N.; Takahashi, A.; Ihara, R.; Itoh, Y.; Jouraku, A.; Van Leeuwen, T.; Osakabe, M. QTL mapping using microsatellite linkage reveals target-site mutations associated with high levels of resistance against three mitochondrial complex II inhibitors in Tetranychus urticae. Insect Biochem. Mol. Biol. 2020, 123, 103410. [Google Scholar] [CrossRef]
- Motyka, M.; Kusy, D.; Háva, J.; Jahodářová, E.; Bílková, R.; Vogler, A.P.; Bocak, L. Mitogenomic data elucidate the phylogeny and evolution of life strategies in Dermestidae (Coleoptera). Syst. Entomol. 2022, 47, 82–93. [Google Scholar] [CrossRef]
- Sarkar, I.; Rathore, S.S.; Singh, G.D.; Singh, R.P. Whole-genome and mitogenome based in silico analysis of select Plasmodium and identification of a novel drug molecule against the malaria parasite. bioRxiv 2022. [Google Scholar] [CrossRef]

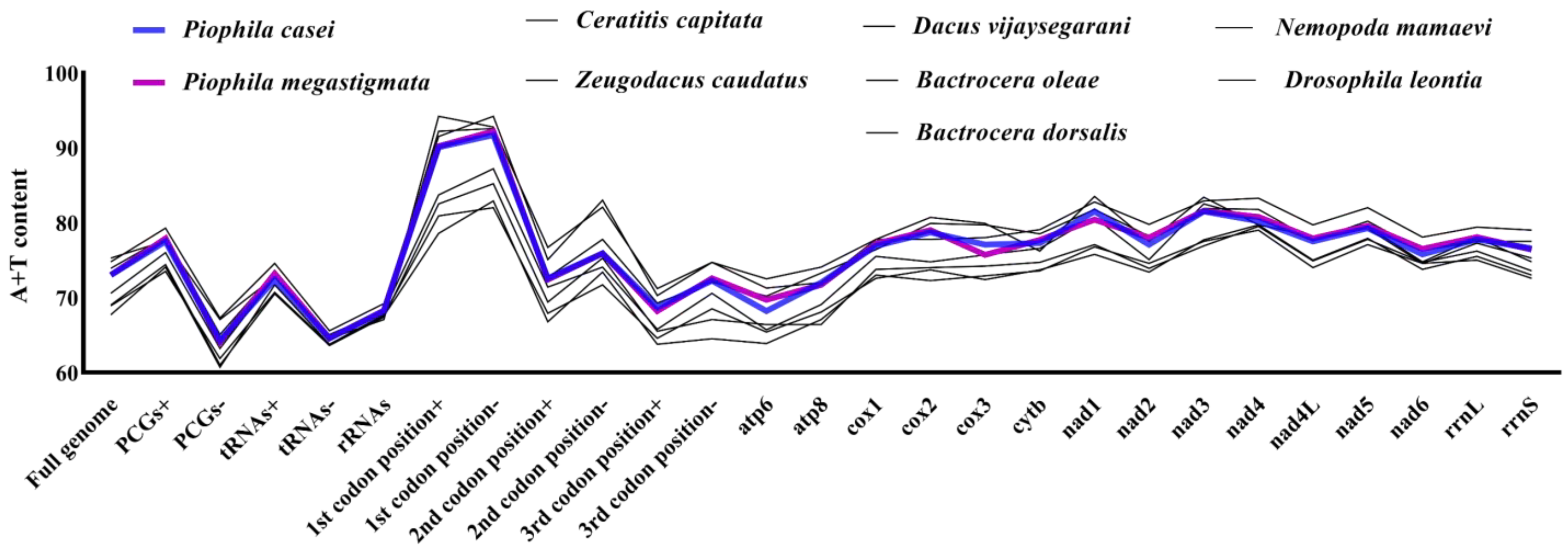

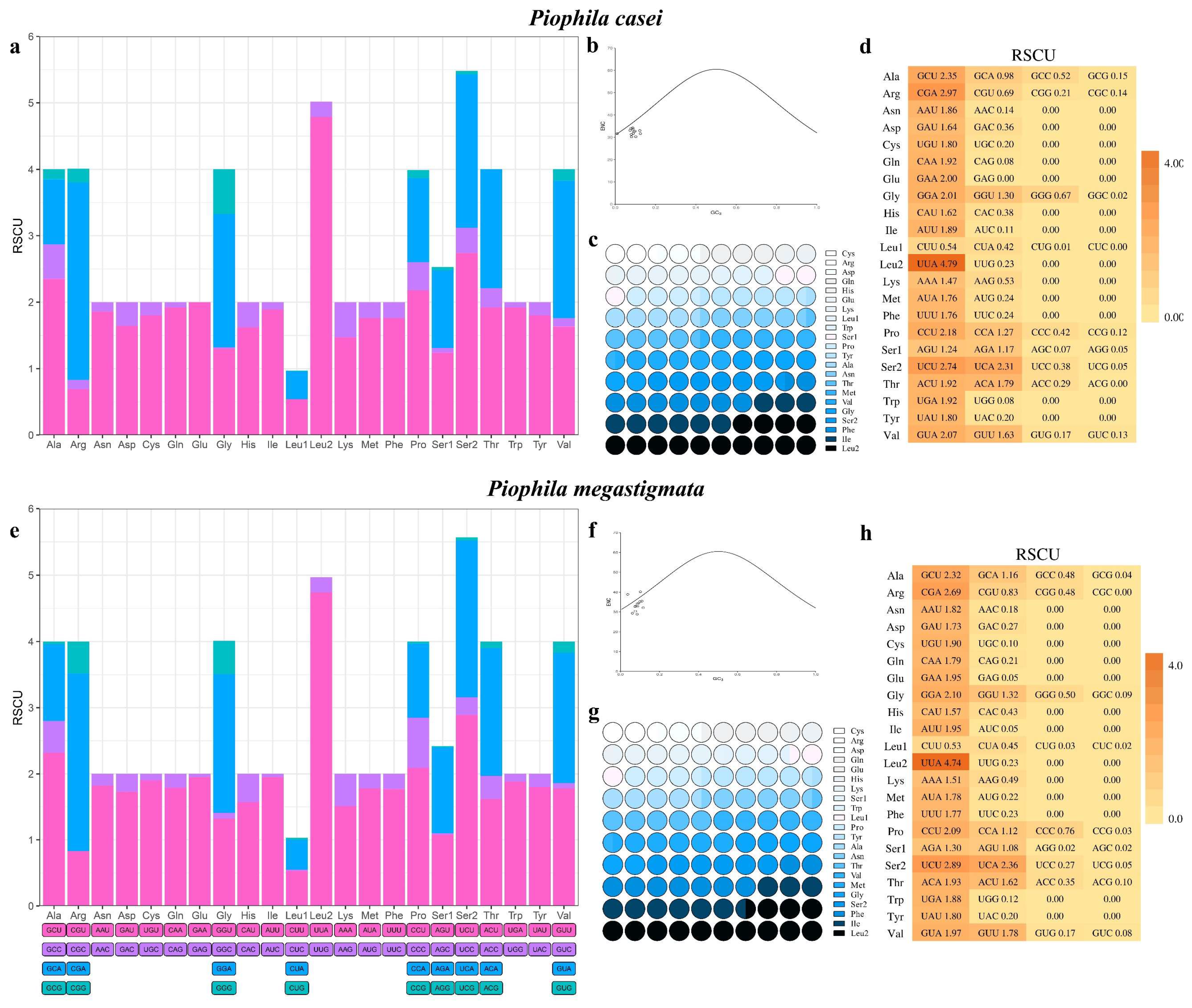
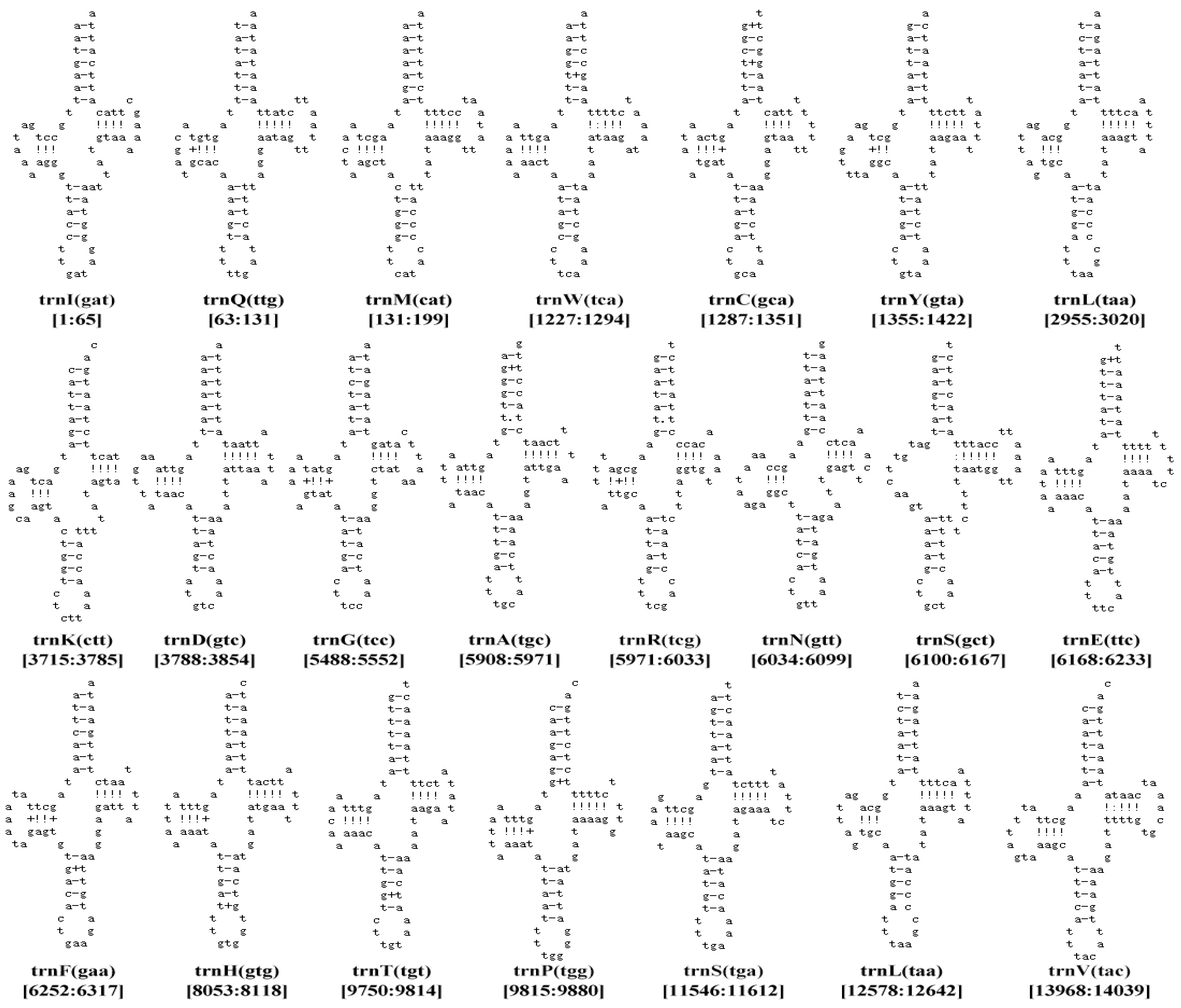
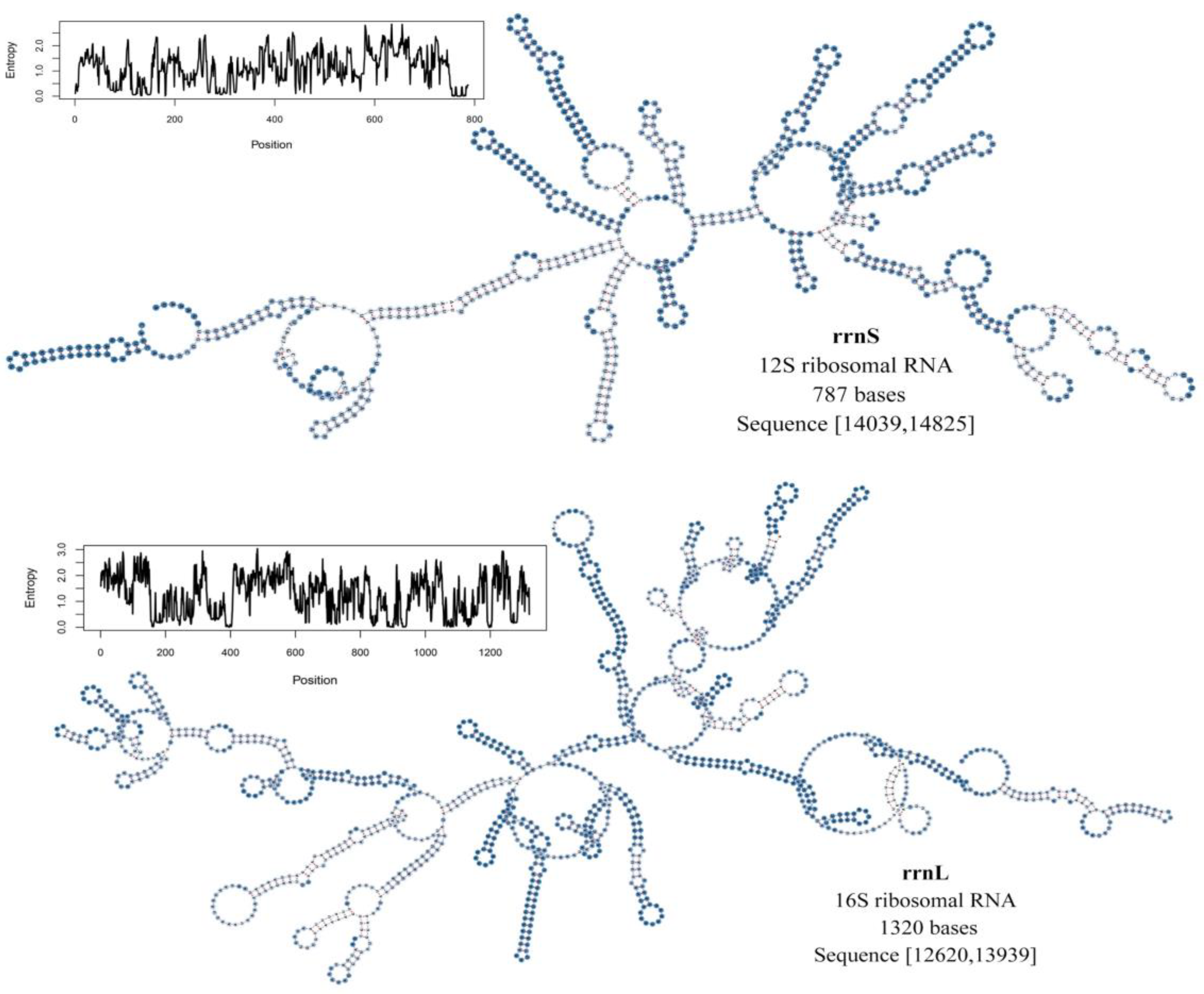


| (P. casei) Region | Size (bp) | A% | T% | AT-skew | C% | G% | CG-skew |
| Mitogenome | 15,785 | 39.5 | 37.1 | 0.03 | 13.8 | 9.6 | −0.18 |
| Protein-coding genes | 11,206 | 31.2 | 43.6 | −0.17 | 12.3 | 12.9 | 0.02 |
| tRNA | 1467 | 37.7 | 38.9 | −0.02 | 10.2 | 13.2 | 0.13 |
| rRNA | 2107 | 38.2 | 41.0 | −0.04 | 7.4 | 13.4 | 0.29 |
| (P. megastigmata) Region | Size (bp) | A% | T% | AT-skew | C% | G% | CG-skew |
| Mitogenome | 15,410 | 39.2 | 37.2 | 0.03 | 14.1 | 9.5 | −0.2 |
| Protein-coding genes | 11,207 | 31.3 | 43.6 | −0.16 | 12.4 | 12.7 | 0.01 |
| tRNA | 1463 | 38.3 | 38.8 | −0.01 | 10.0 | 12.9 | 0.13 |
| rRNA | 2111 | 37.9 | 41.8 | −0.05 | 7.1 | 13.2 | 0.3 |
| Gene | Type | Strand | Position (Start–End) | Length (bp) | Intergenic Spacer |
|---|---|---|---|---|---|
| trnl(gat) | tRNA | J | 1–65 | 65 | − |
| trnQ(ttq) | tRNA | N | 63–131 | 69 | −3 |
| trnM(cat) | tRNA | J | 131–199 | 69 | −1 |
| nad2 | CDS | J | 200–1228 | 1029 | 0 |
| trnW(tca) | tRNA | J | 1227–1294 | 68 | −2 |
| trnC(gca) | tRNA | N | 1287–1351 | 65 | −8 |
| trnY(gta) | tRNA | N | 1355–1422 | 68 | 3 |
| cox1 | CDS | J | 1424–2954 | 1531 | 1 |
| trnL(taa) | tRNA | J | 2955–3020 | 66 | 0 |
| cix2 | CDS | J | 3027–3714 | 688 | 6 |
| trnK(ctt) | tRNA | J | 3715–3785 | 71 | 0 |
| trnD(gtc) | tRNA | J | 3788–3854 | 67 | 2 |
| atp8 | CDS | J | 3855–4016 | 162 | 0 |
| atp6 | CDS | J | 4010–4687 | 678 | −7 |
| cox3 | CDS | J | 4691–5479 | 789 | 3 |
| trnG(tcc) | tRNA | J | 5488–5552 | 65 | 8 |
| nad3 | CDS | J | 5553–5906 | 63 | 0 |
| trnA(tgc) | tRNA | J | 5908–5971 | 66 | 1 |
| trnR(tcg) | tRNA | J | 5971–6033 | 68 | −1 |
| trnN(gtt) | tRNA | J | 6034–6099 | 66 | 0 |
| trnS(qct) | tRNA | J | 6100–6167 | 68 | 0 |
| trnE(ttc) | tRNA | J | 6168–6233 | 66 | 0 |
| trnF(gaa) | tRNA | N | 6252–6317 | 66 | 18 |
| nad5 | CDS | N | 6318–8052 | 1753 | 0 |
| trnH(gtg) | tRNA | N | 8053–8118 | 66 | 0 |
| nad4 | CDS | N | 8119–9457 | 1339 | 0 |
| nad4l | CDS | N | 9451–9747 | 297 | −7 |
| trnT(tgt) | tRNA | J | 9750–9814 | 65 | 2 |
| trnP(tgg) | tRNA | N | 9815–9880 | 66 | 0 |
| nad6 | CDS | J | 9883–10,407 | 525 | 2 |
| cytb | CDS | J | 10,411–11,547 | 1137 | 3 |
| trnS(tga) | tRNA | J | 11,546–11,612 | 67 | −2 |
| nad1 | CDS | N | 11,628–12,576 | 949 | 5 |
| trnL(tag) | tRNA | N | 12,578–12,642 | 65 | 1 |
| rrn16 | rRNA | N | 12,643–13,939 | 1297 | 0 |
| trnV(tac) | tRNA | N | 13,968–14,039 | 72 | −2 |
| rrn12 | rRNA | N | 14,039–14,825 | 787 | −1 |
| control region | D-loop | J | 15,042–15,785 | 744 | 16 |
| Gene | ENc | GC3s | L_sym | L_aa | Molecular Weight (Da) | Isoelectric Points | Instability Index | Aliphatic Index | GRAVY |
|---|---|---|---|---|---|---|---|---|---|
| atp6 | 33.95 | 0.083 | 218 | 220 | 25,145.94 | 7.09 | 41.84 | 123.91 | 0.884 |
| atp8 | 32.74 | 0.08 | 50 | 50 | 6118.28 | 9.4 | 44.61 | 93.77 | 0.477 |
| cytb | 32.52 | 0.083 | 363 | 365 | 43,057.3 | 8.33 | 29 | 126.64 | 0.781 |
| cox1 | 33.09 | 0.101 | 496 | 496 | 56,373.37 | 5.97 | 26.74 | 110.69 | 0.732 |
| cox2 | 34.23 | 0.077 | 221 | 222 | 26,113.28 | 4.92 | 31.38 | 113.64 | 0.346 |
| cox3 | 33.01 | 0.114 | 246 | 250 | 30,025.75 | 6.2 | 32.14 | 98.21 | 0.516 |
| nad1 | 31.27 | 0.082 | 305 | 308 | 36,067.56 | 8.45 | 32.83 | 127.06 | 1.085 |
| nad2 | 33.72 | 0.127 | 332 | 333 | 39,520.5 | 9.1 | 39.9 | 121.78 | 0.924 |
| nad3 | 31.66 | 0.105 | 114 | 114 | 13,510.31 | 5.66 | 45.26 | 134.19 | 0.998 |
| nad4 | 30.26 | 0.061 | 426 | 436 | 51,075.7 | 8.3 | 38.68 | 127.65 | 1.068 |
| nad4l | 30.22 | 0.000 | 96 | 97 | 11,502.86 | 5.99 | 24.97 | 121.22 | 1.093 |
| nad5 | 31.64 | 0.089 | 560 | 564 | 65,550.86 | 6.5 | 33.48 | 122.94 | 0.972 |
| nad6 | 31.64 | 0.076 | 172 | 173 | 20,277.72 | 8.64 | 26.59 | 132.82 | 1.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, S.; Song, Y.; Liu, L.; Wan, J.; Zhou, Y.; Zhu, Q.; Liu, J. Complete Mitochondrial Genome of Piophila casei (Diptera: Piophilidae): Genome Description and Phylogenetic Implications. Genes 2023, 14, 883. https://doi.org/10.3390/genes14040883
Bi S, Song Y, Liu L, Wan J, Zhou Y, Zhu Q, Liu J. Complete Mitochondrial Genome of Piophila casei (Diptera: Piophilidae): Genome Description and Phylogenetic Implications. Genes. 2023; 14(4):883. https://doi.org/10.3390/genes14040883
Chicago/Turabian StyleBi, Shenghui, Yanfei Song, Linggao Liu, Jing Wan, Ying Zhou, Qiujin Zhu, and Jianfeng Liu. 2023. "Complete Mitochondrial Genome of Piophila casei (Diptera: Piophilidae): Genome Description and Phylogenetic Implications" Genes 14, no. 4: 883. https://doi.org/10.3390/genes14040883
APA StyleBi, S., Song, Y., Liu, L., Wan, J., Zhou, Y., Zhu, Q., & Liu, J. (2023). Complete Mitochondrial Genome of Piophila casei (Diptera: Piophilidae): Genome Description and Phylogenetic Implications. Genes, 14(4), 883. https://doi.org/10.3390/genes14040883






