Polygenic Models Partially Predict Muscle Size and Strength but Not Low Muscle Mass in Older Women
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Muscle Mass, Size, and Strength Related Phenotypes
2.3. Pre-Sarcopenia/Low Muscle Mass Assessment
2.4. SNPs Selection, DNA Extraction, and Genotyping
2.5. Statistical Analysis
3. Results
3.1. Total Genotype Predisposition Score and Pre-Sarcopenia/Low Muscle Mass
3.2. Data-Driven GPS and Skeletal Muscle Phenotypes
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prior, S.J.; Roth, S.M.; Wang, X.; Kammerer, C.; Miljkovic-Gacic, I.; Bunker, C.H.; Wheeler, V.W.; Patrick, A.L.; Zmuda, J.M. Genetic and environmental influences on skeletal muscle phenotypes as a function of age and sex in large, multigenerational families of African heritage. J. Appl. Physiol. 2007, 103, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Tiainen, K.; Sipila, S.; Alen, M.; Heikkinen, E.; Kaprio, J.; Koskenvuo, M.; Tolvanen, A.; Pajala, S.; Rantanen, T. Heritability of maximal isometric muscle strength in older female twins. J. Appl. Physiol. 2004, 96, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Abney, M.; McPeek, M.S.; Ober, C. Broad and narrow heritabilities of quantitative traits in a founder population. Am. J. Hum. Genet. 2001, 68, 1302–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silventoinen, K.; Magnusson, P.K.; Tynelius, P.; Kaprio, J.; Rasmussen, F. Heritability of body size and muscle strength in young adulthood: A study of one million Swedish men. Genet. Epidemiol. 2008, 32, 341–349. [Google Scholar] [CrossRef]
- Thomis, M.; Beunen, G.; Leemputte, M.V.; Maes, H.; Blimkie, C.; Claessens, A.; Marchal, G.; Willems, E.; Vlietinck, R. Inheritance of static and dynamic arm strength and some of its determinants. Acta Physiol. Scand. 1998, 163, 59–71. [Google Scholar] [CrossRef]
- Bray, M.S.; Hagberg, J.M.; Perusse, L.; Rankinen, T.; Roth, S.M.; Wolfarth, B.; Bouchard, C. The human gene map for performance and health-related fitness phenotypes: The 2006–2007 update. Med. Sci. Sports Exerc. 2009, 41, 34–72. [Google Scholar] [CrossRef]
- Beenakker, K.G.; Koopman, J.J.; van Bodegom, D.; Kuningas, M.; Slagboom, P.E.; Meij, J.J.; Maier, A.B.; Westendorp, R.G. Variants of the IL-10 gene associate with muscle strength in elderly from rural Africa: A candidate gene study. Aging Cell 2014, 13, 862–868. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Zhang, Q.; Chen, J.; Li, Z.; Zhou, Z.; Shen, J.; Wang, D.; Pan, D.; Wang, Z.; Ke, D. Polymorphism of the PPARD Gene and Dynamic Balance Performance in Han Chinese Children. Hereditas 2019, 156, 15. [Google Scholar] [CrossRef]
- Klimentidis, Y.C.; Bea, J.W.; Thompson, P.; Klimecki, W.T.; Hu, C.; Wu, G.; Nicholas, S.; Ryckman, K.K.; Chen, Z. Genetic variant in ACVR2B is associated with lean mass. Med. Sci. Sports Exerc. 2016, 48, 1270. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.-J.; Liu, S.-L.; Lei, S.-F.; Papasian, C.J.; Deng, H.-W. Molecular genetic studies of gene identification for sarcopenia. Hum. Genet. 2012, 131, 1–31. [Google Scholar] [CrossRef]
- Loos, R.J.F.; Hagberg, J.M.; Pérusse, L.; Roth, S.M.; Sarzynski, M.A.; Wolfarth, B.; Rankinen, T.; Bouchard, C. Advances in exercise, fitness, and performance genomics in 2014. Med. Sci. Sports Exerc. 2015, 47, 1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stebbings, G.K.; Morse, C.I.; Williams, A.G.; Day, S.H. Variability and distribution of muscle strength and its determinants in humans. Muscle Nerve 2014, 49, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Liu, D.; Metter, E.J.; Ferrucci, L.; Roth, S.M. ACTN3 genotype is associated with muscle phenotypes in women across the adult age span. J. Appl. Physiol. 2008, 105, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Thomaes, T.; Thomis, M.; Onkelinx, S.; Goetschalckx, K.; Fagard, R.; Lambrechts, D.; Vanhees, L. Genetic predisposition scores associate with muscular strength, size, and trainability. Med. Sci. Sports Exerc. 2013, 45, 1451–1459. [Google Scholar] [CrossRef] [Green Version]
- Degens, H.; Korhonen, M.T. Factors contributing to the variability in muscle ageing. Maturitas 2012, 73, 197–201. [Google Scholar] [CrossRef]
- Leveille, S.G.; Penninx, B.; Melzer, D.; Izmirlian, G.; Guralnik, J.M. Sex differences in the prevalence of mobility disability in old age: The dynamics of incidence, recovery, and mortality. J. Gerontol. Ser. B 2000, 55, S41–S50. [Google Scholar]
- Sallinen, J.; Stenholm, S.; Rantanen, T.; Heliövaara, M.; Sainio, P.; Koskinen, S. Hand-grip strength cut points to screen older persons at risk for mobility limitation. J. Am. Geriatr. Soc. 2010, 58, 1721–1726. [Google Scholar] [CrossRef] [Green Version]
- Kerr, A.; Syddall, H.; Cooper, C.; Turner, G.; Briggs, R.; Sayer, A.A. Does admission grip strength predict length of stay in hospitalised older patients? Age Ageing 2006, 35, 82–84. [Google Scholar] [CrossRef] [Green Version]
- García-Hermoso, A.; Cavero-Redondo, I.; Ramírez-Vélez, R.; Ruiz, J.R.; Ortega, F.B.; Lee, D.-C.; Martínez-Vizcaíno, V. Muscular strength as a predictor of all-cause mortality in an apparently healthy population: A systematic review and meta-analysis of data from approximately 2 million men and women. Arch. Phys. Med. Rehabil. 2018, 99, 2100–2113.e2105. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Janssen, I.; Baumgartner, R.N.; Ross, R.; Rosenberg, I.H.; Roubenoff, R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am. J. Epidemiol. 2004, 159, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.G.; Folland, J.P. Similarity of polygenic profiles limits the potential for elite human physical performance. J. Physiol. 2008, 586, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santiago, C.; Ruiz, J.R.; Muniesa, C.A.; González-Freire, M.; Gómez-Gallego, F.; Lucia, A. Does the polygenic profile determine the potential for becoming a world-class athlete? Insights from the sport of rowing. Scand. J. Med. Sci. Sports 2010, 20, e188–e194. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.R.; Gómez-Gallego, F.; Santiago, C.; González-Freire, M.; Verde, Z.; Foster, C.; Lucia, A. Is there an optimum endurance polygenic profile? J. Physiol. 2009, 587, 1527–1534. [Google Scholar] [CrossRef]
- Massidda, M.; Scorcu, M.; Calò, C.M. New genetic model for predicting phenotype traits in sports. Int. J. Sports Physiol. Perform. 2014, 9, 554–560. [Google Scholar] [CrossRef]
- Drozdovska, S.; Dosenko, V.; Ahmetov, I.; Ilyin, V. The association of gene polymorphisms with athlete status in Ukrainians. Biol. Sport 2013, 30, 163. [Google Scholar] [CrossRef] [Green Version]
- Charlier, R.; Caspers, M.; Knaeps, S.; Mertens, E.; Lambrechts, D.; Lefevre, J.; Thomis, M. Limited potential of genetic predisposition scores to predict muscle mass and strength performance in Flemish Caucasians between 19 and 73 years of age. Physiol. Genom. 2016, 49, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Thomaes, T.; Thomis, M.; Onkelinx, S.; Fagard, R.; Matthijs, G.; Buys, R.; Schepers, D.; Cornelissen, V.; Vanhees, L. A genetic predisposition score for muscular endophenotypes predicts the increase in aerobic power after training: The CAREGENE study. BMC Genet. 2011, 12, 84. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Van Roie, E.; Bogaerts, A.; Morse, C.I.; Delecluse, C.; Verschueren, S.; Thomis, M. Genetic predisposition score predicts the increases of knee strength and muscle mass after one-year exercise in healthy elderly. Exp. Gerontol. 2018, 111, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Khanal, P.; He, L.; Herbert, A.J.; Stebbings, G.K.; Onambele-Pearson, G.L.; Degens, H.; Morse, C.I.; Thomis, M.; Williams, A.G. The Association of Multiple Gene Variants with Ageing Skeletal Muscle Phenotypes in Elderly Women. Genes 2020, 11, 1459. [Google Scholar] [CrossRef]
- Khanal, P.; He, L.; Stebbings, G.; Onambele-Pearson, G.L.; Degens, H.; Williams, A.; Thomis, M.; Morse, C.I. Prevalence and association of single nucleotide polymorphisms with sarcopenia in older women depends on definition. Sci. Rep. 2020, 10, 2913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Khanal, P.; Morse, C.I.; Williams, A.; Thomis, M. Associations of combined genetic and epigenetic scores with muscle size and muscle strength: A pilot study in older women. J. Cachexia Sarcopenia Muscle 2020, 11, 1548–1561. [Google Scholar] [CrossRef] [PubMed]
- Villafañe, J.H.; Valdes, K.; Buraschi, R.; Martinelli, M.; Bissolotti, L.; Negrini, S. Reliability of the handgrip strength test in elderly subjects with Parkinson disease. Hand 2016, 11, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Tintle, N.; Gordon, D.; Van Bruggen, D.; Finch, S. The cost effectiveness of duplicate genotyping for testing genetic association. Ann. Hum. Genet. 2009, 73, 370–378. [Google Scholar] [CrossRef]
- Yvert, T.; Miyamoto-Mikami, E.; Murakami, H.; Miyachi, M.; Kawahara, T.; Fuku, N. Lack of replication of associations between multiple genetic polymorphisms and endurance athlete status in Japanese population. Physiol. Rep. 2016, 4, e13003. [Google Scholar] [CrossRef]
- Bouchard, C.; Sarzynski, M.A.; Rice, T.K.; Kraus, W.E.; Church, T.S.; Sung, Y.J.; Rao, D.C.; Rankinen, T. Genomic predictors of the maximal O2 uptake response to standardized exercise training programs. J. Appl. Physiol. 2010, 110, 1160–1170. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-W.; Bae, S.-H.; Jeong, J.-W.; Kim, S.-H.; Kim, K.-W. Hypoxia-inducible factor (HIF-1) α: Its protein stability and biological functions. Exp. Mol. Med. 2004, 36, 1–12. [Google Scholar] [CrossRef]
- Stebbings, G.K.; Williams, A.G.; Morse, C.I.; Day, S.H. Polymorphisms in PTK2 are associated with skeletal muscle specific force: An independent replication study. Eur. J. Appl. Physiol. 2017, 117, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Erskine, R.M.; Williams, A.G.; Jones, D.A.; Stewart, C.E.; Degens, H. Do PTK2 gene polymorphisms contribute to the interindividual variability in muscle strength and the response to resistance training? A preliminary report. J. Appl. Physiol. 2012, 112, 1329–1334. [Google Scholar] [CrossRef] [Green Version]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.B.; Elliott, K.S.; Lango, H.; Rayner, N.W. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livingstone, K.M.; Celis-Morales, C.; Papandonatos, G.D.; Erar, B.; Florez, J.C.; Jablonski, K.A.; Razquin, C.; Marti, A.; Heianza, Y.; Huang, T. FTO genotype and weight loss: Systematic review and meta-analysis of 9563 individual participant data from eight randomised controlled trials. BMJ 2016, 354, i4707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Serri, A.; Al-Bustan, S.A.; Kamkar, M.; Thomas, D.; Alsmadi, O.; Al-Temaimi, R.; Mojiminiyi, O.A.; Abdella, N.A. Association of FTO rs9939609 with Obesity in the Kuwaiti Population: A Public Health Concern? Med. Princ. Pract. 2018, 27, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonestedt, E.; Gullberg, B.; Ericson, U.; Wirfält, E.; Hedblad, B.; Orho-Melander, M. Association between fat intake, physical activity and mortality depending on genetic variation in FTO. Int. J. Obes. 2011, 35, 1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livshits, G.; Malkin, I.; Moayyeri, A.; Spector, T.D.; Hammond, C.J. Association of FTO gene variants with body composition in UK twins. Ann. Hum. Genet. 2012, 76, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, S.M.; Schrager, M.A.; Ferrell, R.E.; Riechman, S.E.; Metter, E.J.; Lynch, N.A.; Lindle, R.S.; Hurley, B.F. CNTF genotype is associated with muscular strength and quality in humans across the adult age span. J. Appl. Physiol. 2001, 90, 1205–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, S.; Kelsey, B.K.; Angelopoulos, T.J.; Clarkson, P.M.; Gordon, P.M.; Moyna, N.M.; Visich, P.S.; Zoeller, R.F.; Seip, R.L.; Bilbie, S. CNTF 1357 G→ A polymorphism and the muscle strength response to resistance training. J. Appl. Physiol. 2009, 107, 1235–1240. [Google Scholar] [CrossRef]
- Arking, D.E.; Fallin, D.M.; Fried, L.P.; Li, T.; Beamer, B.A.; Xue, Q.L.; Chakravarti, A.; Walston, J. Variation in the ciliary neurotrophic factor gene and muscle strength in older Caucasian women. J. Am. Geriatr. Soc. 2006, 54, 823–826. [Google Scholar] [CrossRef]
- De Mars, G.; Windelinckx, A.; Beunen, G.; Delecluse, C.; Lefevre, J.; Thomis, M.A.I. Polymorphisms in the CNTF and CNTF receptor genes are associated with muscle strength in men and women. J. Appl. Physiol. 2007, 102, 1824–1831. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-G.; Tan, L.-J.; Lei, S.-F.; Liu, Y.-J.; Shen, H.; Wang, L.; Yan, H.; Guo, Y.-F.; Xiong, D.-H.; Chen, X.-D. Genome-wide association and replication studies identified TRHR as an important gene for lean body mass. Am. J. Hum. Genet. 2009, 84, 418–423. [Google Scholar] [CrossRef] [Green Version]
- Kostek, M.A.; Angelopoulos, T.J.; Clarkson, P.M.; Gordon, P.M.; Moyna, N.M.; Visich, P.S.; Zoeller, R.F.; Price, T.B.; Seip, R.L.; Thompson, P.D. Myostatin and follistatin polymorphisms interact with muscle phenotypes and ethnicity. Med. Sci. Sports Exerc. 2009, 41, 1063. [Google Scholar] [CrossRef] [Green Version]
- Santiago, C.; Ruiz, J.R.; Rodríguez-Romo, G.; Fiuza-Luces, C.; Yvert, T.; Gonzalez-Freire, M.; Gómez-Gallego, F.; Morán, M.; Lucia, A. The K153R polymorphism in the myostatin gene and muscle power phenotypes in young, non-athletic men. PLoS ONE 2011, 6, e16323. [Google Scholar] [CrossRef] [PubMed]
- Seibert, M.J.; Xue, Q.L.; Fried, L.P.; Walston, J.D. Polymorphic variation in the human myostatin (GDF-8) gene and association with strength measures in the Women’s Health and Aging Study II cohort. J. Am. Geriatr. Soc. 2001, 49, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Stebbings, G.; Williams, A.; Herbert, A.J.; Lockey, S.; Heffernan, S.; Erskine, R.; Morse, C.; Day, S. TTN genotype is associated with fascicle length and marathon running performance. Scand. J. Med. Sci. Sports 2018, 28, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, S.M.; Stebbings, G.; Kilduff, L.P.; Erskine, R.; Day, S.H.; Morse, C.; McPhee, J.; Cook, C.; Vance, B.; Ribbans, W.J. Fat mass and obesity associated (FTO) gene influences skeletal muscle phenotypes in non-resistance trained males and elite rugby playing position. BMC Genet. 2017, 18, 4. [Google Scholar] [CrossRef] [Green Version]
- Ahisar, Y.; Thanassoulis, G.; Huang, K.; Ohayon, S.; Afilalo, J. Intersecting Genetics of Frailty and Cardiovascular Disease. J. Nutr. Health Aging 2021, 25, 1023–1027. [Google Scholar] [CrossRef]
- Cordero, A.I.H.; Gonzales, N.M.; Parker, C.C.; Sokolof, G.; Vandenbergh, D.J.; Cheng, R.; Abney, M.; Sko, A.; Douglas, A.; Palmer, A.A. Genome-wide associations reveal human-mouse genetic convergence and modifiers of myogenesis, CPNE1 and STC2. Am. J. Hum. Genet. 2019, 105, 1222–1236. [Google Scholar] [CrossRef] [Green Version]
- Karasik, D.; Zillikens, M.C.; Hsu, Y.-H.; Aghdassi, A.; Akesson, K.; Amin, N.; Barroso, I.; Bennett, D.A.; Bertram, L.; Bochud, M. Disentangling the genetics of lean mass. Am. J. Clin. Nutr. 2019, 109, 276–287. [Google Scholar] [CrossRef]
- Ahmetov, I.I.; Hakimullina, A.M.; Lyubaeva, E.V.; Vinogradova, O.L.; Rogozkin, V.A. Effect of HIF1A gene polymorphism on human muscle performance. Bull. Exp. Biol. Med. 2008, 146, 351–353. [Google Scholar] [CrossRef]
- Cenikli, A.; Nursal, A.; Tural, E.; Polat, Y.; Tasmektepligil, M.; Yigit, S. The Correlation Between Rs1800795 Variant of IL-6 and sports performance among turkish elite athletes. Int. J. Humanit. Soc. Sci. Educ. 2016, 3, 1–5. [Google Scholar]
- Charbonneau, D.E.; Hanson, E.D.; Ludlow, A.T.; Delmonico, M.J.; Hurley, B.F.; Roth, S.M. ACE genotype and the muscle hypertrophic and strength responses to strength training. Med. Sci. Sports Exerc. 2008, 40, 677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Zhang, B.; Li, Y.; Liang, T. Association Between rs1815739 Polymorphism of ACTN3 Gene and Athletic Ability in Chinese Sprinters. J. Sci. Sport Exerc. 2020, 2, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.; Lee, I.; Kang, H. ACTN3 gene and susceptibility to sarcopenia and osteoporotic status in older Korean adults. BioMed Res. Int. 2017, 2017, 4239648. [Google Scholar] [CrossRef] [PubMed]
- Cięszczyk, P.; Zarębska, A.; Jastrzębski, Z.; Sawczyn, M.; Kozakiewicz-Drobnik, I.; Leońska-Duniec, A.; Kaczmarczyk, M.; Maciejewska-Skrendo, A.; Żmijewski, P.; Trybek, G. Does the MTHFR A1298C Polymorphism Modulate the Cardiorespiratory Response to Training? J. Hum. Kinet. 2016, 54, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Corsi, A.M.; Ferrucci, L.; Gozzini, A.; Tanini, A.; Brandi, M.L. Myostatin polymorphisms and age-related sarcopenia in the Italian population. J. Am. Geriatr. Soc. 2002, 50, 1463. [Google Scholar] [CrossRef]
- Eider, J.; Ficek, K.; Kaczmarczyk, M.; Maciejewska-Karłowska, A.; Sawczuk, M.; Cięszczyk, P. Endothelial nitric oxide synthase g894t (rs1799983) gene polymorphism in polish athletes. Open Life Sci. 2014, 9, 260–267. [Google Scholar] [CrossRef]
- Gómez-Gallego, F.; Ruiz, J.R.; Buxens, A.; Artieda, M.; Arteta, D.; Santiago, C.; Rodríguez-Romo, G.; Lao, J.I.; Lucia, A. The− 786 T/C polymorphism of the NOS3 gene is associated with elite performance in power sports. Eur. J. Appl. Physiol. 2009, 107, 565–569. [Google Scholar] [CrossRef]
- González-Freire, M.; Rodríguez-Romo, G.; Santiago, C.; Bustamante-Ara, N.; Yvert, T.; Gómez-Gallego, F.; Rexach, J.A.S.; Ruiz, J.R.; Lucia, A. The K153R variant in the myostatin gene and sarcopenia at the end of the human lifespan. Age 2010, 32, 405–409. [Google Scholar] [CrossRef] [Green Version]
- Hand, B.D.; Mccole, S.D.; Brown, M.D.; Park, J.J.; Ferrell, R.E.; Huberty, A.; Douglass, L.W.; Hagberg, J.M. NOS3 gene polymorphisms and exercise hemodynamics in postmenopausal women. Int. J. Sports Med. 2006, 27, 951–958. [Google Scholar] [CrossRef]
- Hopkinson, N.S.; Li, K.W.; Kehoe, A.; Humphries, S.E.; Roughton, M.; Moxham, J.; Montgomery, H.; Polkey, M.I. Vitamin D receptor genotypes influence quadriceps strength in chronic obstructive pulmonary disease. Am. J. Clin. Nutr. 2008, 87, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Kostek, M.C.; Devaney, J.M.; Gordish-Dressman, H.; Harris, T.B.; Thompson, P.D.; Clarkson, P.M.; Angelopoulos, T.J.; Gordon, P.M.; Moyna, N.M.; Pescatello, L.S. A polymorphism near IGF1 is associated with body composition and muscle function in women from the Health, Aging, and Body Composition Study. Eur. J. Appl. Physiol. 2010, 110, 315–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunardi, C.C.; Lima, R.M.; Pereira, R.W.; Leite, T.K.M.; Siqueira, A.B.M.; Oliveira, R.J. Association between polymorphisms in the TRHR gene, fat-free mass, and muscle strength in older women. Age 2013, 35, 2477–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prior, S.J.; Hagberg, J.M.; Phares, D.A.; Brown, M.D.; Fairfull, L.; Ferrell, R.E.; Roth, S.M. Sequence variation in hypoxia-inducible factor 1α (HIF1A): Association with maximal oxygen consumption. Physiol. Genom. 2003, 15, 20–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, S.M.; Zmuda, J.M.; Cauley, J.A.; Shea, P.R.; Ferrell, R.E. Vitamin D receptor genotype is associated with fat-free mass and sarcopenia in elderly men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, B10–B15. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.R.; Buxens, A.; Artieda, M.; Arteta, D.; Santiago, C.; Rodríguez-Romo, G.; Lao, J.I.; Gómez-Gallego, F.; Lucia, A. The −174 G/C polymorphism of the IL6 gene is associated with elite power performance. J. Sci. Med. Sport 2010, 13, 549–553. [Google Scholar] [CrossRef]
- Sessa, F.; Chetta, M.; Petito, A.; Franzetti, M.; Bafunno, V.; Pisanelli, D.; Sarno, M.; Iuso, S.; Margaglione, M. Gene polymorphisms and sport attitude in Italian athletes. Genet. Test. Mol. Biomark. 2011, 15, 285–290. [Google Scholar] [CrossRef]
- Svendstrup, M.; Appel, E.V.R.; Sandholt, C.H.; Ahluwalia, T.S.; Ängquist, L.H.; Thuesen, B.H.; Jørgensen, M.E.; Pedersen, O.; Grarup, N.; Hansen, T. Prospective studies exploring the possible impact of an ID3 polymorphism on changes in obesity measures. Obesity 2018, 26, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Van Pottelbergh, I.; Goemaere, S.; Nuytinck, L.; De Paepe, A.; Kaufman, J.M. Association of the type I collagen alpha1 Sp1 polymorphism, bone density and upper limb muscle strength in community-dwelling elderly men. Osteoporos. Int. 2001, 12, 895–901. [Google Scholar] [CrossRef]
- Voisin, S.; Guilherme, J.P.F.L.; Yan, X.; Pushkarev, V.P.; Cieszczyk, P.; Massidda, M.; Calò, C.M.; Dyatlov, D.A.; Kolupaev, V.A.; Pushkareva, Y.E. ACVR1B rs2854464 is associated with sprint/power athletic status in a large cohort of Europeans but not Brazilians. PLoS ONE. 2016, 11, e0156316. [Google Scholar] [CrossRef] [Green Version]
- Windelinckx, A.; De Mars, G.; Beunen, G.; Aerssens, J.; Delecluse, C.; Lefevre, J.; Thomis, M.A.I. Polymorphisms in the vitamin D receptor gene are associated with muscle strength in men and women. Osteoporos. Int. 2007, 18, 1235–1242. [Google Scholar] [CrossRef]
- Windelinckx, A.; De Mars, G.; Huygens, W.; Peeters, M.W.; Vincent, B.; Wijmenga, C.; Lambrechts, D.; Delecluse, C.; Roth, S.M.; Metter, E.J. Comprehensive fine mapping of chr12q12-14 and follow-up replication identify activin receptor 1B (ACVR1B) as a muscle strength gene. Eur. J. Hum. Genet. 2011, 19, 208. [Google Scholar] [CrossRef] [PubMed]
- Zarebska, A.; Ahmetov, I.I.; Sawczyn, S.; Weiner, A.S.; Kaczmarczyk, M.; Ficek, K.; Maciejewska-Karlowska, A.; Sawczuk, M.; Leonska-Duniec, A.; Klocek, T. Association of the MTHFR 1298A> C (rs1801131) polymorphism with speed and strength sports in Russian and Polish athletes. J. Sports Sci. 2014, 32, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Zempo, H.; Tanabe, K.; Murakami, H.; Iemitsu, M.; Maeda, S.; Kuno, S. ACTN3 polymorphism affects thigh muscle area. Int. J. Sports Med. 2010, 31, 138–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zmijewski, P.; Cieszczyk, P.; Ahmetov, I.I. The NOS3 G894T (rs1799983) and-786T/C (rs2070744) polymorphisms are associated with elite swimmer status. Biol. Sport 2018, 35, 313–319. [Google Scholar] [CrossRef]
| SMI Threshold | %SMMr Threshold | ||||
|---|---|---|---|---|---|
| All (n = 300) | Low (n = 181) | High (n = 119) | Low (n = 41) | High (n = 259) | |
| Age (years) | 70.7 ± 5.7 | 71.0 ± 5.2 | 70.3 ± 6.3 | 71.8 ± 5.8 | 70.6 ± 5.6 |
| Body mass (kg) | 66.3 ± 11.3 | 63.3 ± 9.2 | 70.8 ± 12.6 | 77.6 ± 13.3 * | 64.5 ± 9.8 |
| Height (kg/m2) | 1.60 ± 0.06 | 1.60 ± 0.06 | 1.59 ± 0.05 | 1.60 ± 0.05 | 1.60 ± 0.06 |
| BMI (kg/m2) | 25.9 ± 4.2 | 24.6 ± 3.2 * | 27.8 ± 4.6 | 30.3 ± 5.5 * | 25.2 ± 3.4 |
| SMI (kg/m2) | 6.56 ± 0.82 | 6.04 ± 0.51 * | 7.34 ± 0.53 | 6.01 ± 0.95 * | 6.64 ± 0.76 |
| %SMMr | 25.7 ± 3.8 | 24.9 ± 3.3 | 26.9 ± 4.2 | 20.0 ± 1.3 | 26.6 ± 3.3 |
| BB thickness (cm) | 1.77 ± 0.32 | 1.73 ± 0.32 * | 1.82 ± 0.31 | 1.85 ± 0.36 | 1.76 ± 0.31 |
| VLACSA (cm2) | 16.3 ± 3.4 | 15.4 ± 3.1 * | 17.6 ± 3.3 | 16.9 ± 3.9 | 16.2 ± 3.3 |
| HGS (kg) | 30.0 ± 5.0 | 29.2 ± 4.3 * | 31.1 ± 5.6 | 28.7 ± 4.9 | 30.2 ± 5.0 |
| MVCEF (N·m) | 24.8 ± 5.8 | 23.8 ± 5.5 * | 26.3 ± 6.0 | 23.3 ± 5.0 | 25.0 ± 5.9 |
| MVCKE (N·m) | 55.2 ± 18.3 | 53.2 ± 17.3 * | 58.2 ± 19.3 | 49.7 ± 19.3 * | 56.0 ± 18.0 |
| GPSTOTAL | 21.5 ± 2.8 | 21.5 ± 2.7 | 21.5 ± 2.9 | 21.0 ± 2.4 | 21.6 ± 2.8 |
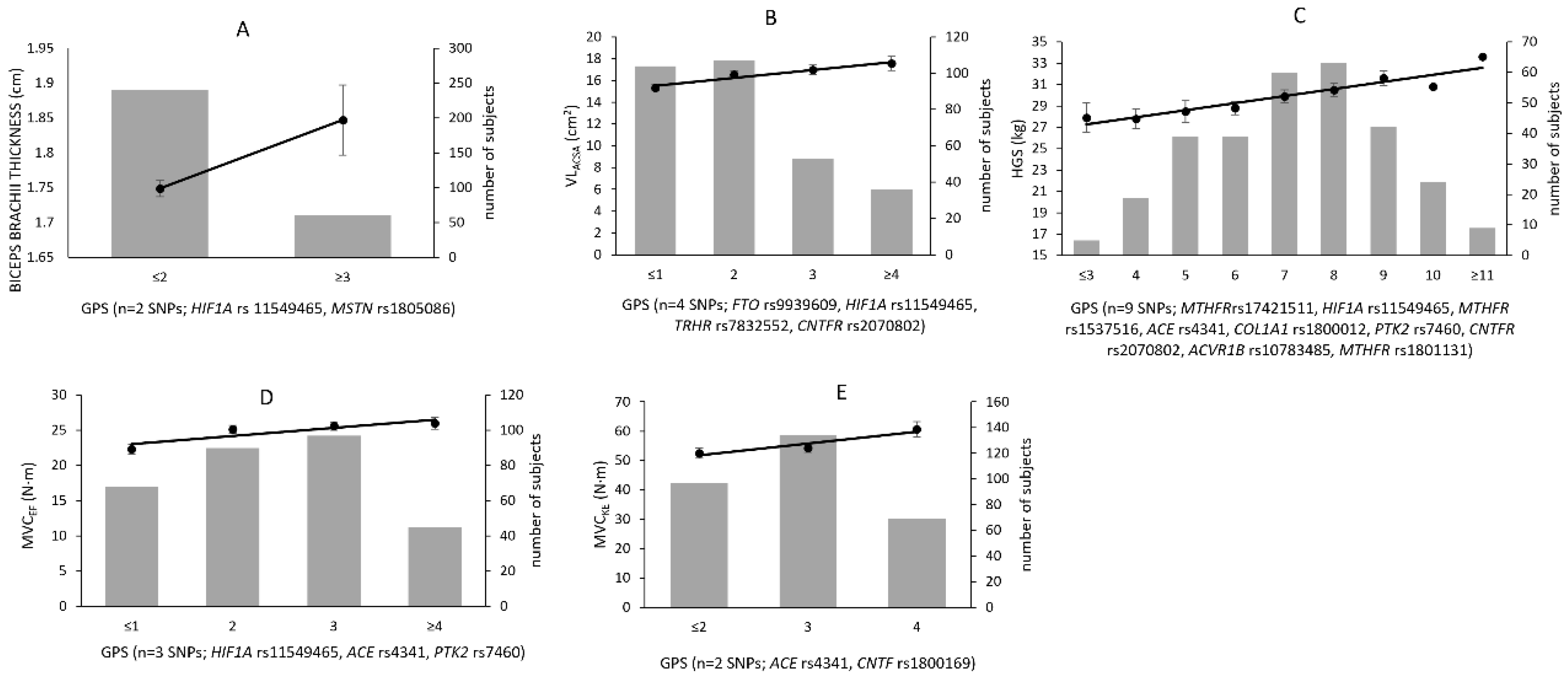
| Phenotypes | GPSDD | Age | Adj r2 | Associated SNPs (Predisposing Allele) | |
|---|---|---|---|---|---|
| Biceps brachii thickness (cm) | estimate | 0.101 | −0.003 | HIF1A rs11549465 (T), MSTN rs1805086 (T) | |
| β value | 0.146 | −0.058 | 1.7% | ||
| partial r | 0.146 | −0.058 | |||
| p | 0.014 | 0.327 | |||
| VLACSA (cm2) | estimate | 0.710 | −0.172 | FTO rs9939609 (A), HIF1A rs11549465 (T), TRHR rs7832552 (T), CNTFR rs2070802 (T) | |
| β value | 0.251 | −0.287 | 12.5% | ||
| partial r | 0.258 | −0.293 | |||
| p | <0.001 | <0.001 | |||
| HGS (kg) | estimate | 0.685 | −0.323 | MTHFR rs17421511 (A), HIF1A rs11549465 (T), MTHFR rs1537516 (A), ACE rs4341 (G), COL1A1 rs1800012 (A), PTK2 rs7460 (A), CNTFR rs2070802 (T), ACVR1B rs10783485 (G), MTHFR rs1801131 (T) | |
| β value | 0.260 | −0.368 | 19.0% | ||
| partial r | 0.278 | −0.380 | |||
| p | <0.001 | <0.001 | |||
| MVCEF (N·m) | estimate | 1.066 | −0.220 | HIF1A rs11549465 (T), ACE rs4341 (G), PTK2 rs7460 (A) | |
| β value | 0.203 | −0.213 | 8.2% | ||
| partial r | 0.208 | −0.218 | |||
| p | <0.001 | <0.001 | |||
| MVCKE (N·m) | estimate | 3.743 | −0.803 | ACE rs4341 (G), CNTF rs1800169 (G) | |
| β value | 0.172 | −0.258 | 9.6% | ||
| partial r | 0.178 | −0.252 | |||
| p | 0.002 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanal, P.; Morse, C.I.; He, L.; Herbert, A.J.; Onambélé-Pearson, G.L.; Degens, H.; Thomis, M.; Williams, A.G.; Stebbings, G.K. Polygenic Models Partially Predict Muscle Size and Strength but Not Low Muscle Mass in Older Women. Genes 2022, 13, 982. https://doi.org/10.3390/genes13060982
Khanal P, Morse CI, He L, Herbert AJ, Onambélé-Pearson GL, Degens H, Thomis M, Williams AG, Stebbings GK. Polygenic Models Partially Predict Muscle Size and Strength but Not Low Muscle Mass in Older Women. Genes. 2022; 13(6):982. https://doi.org/10.3390/genes13060982
Chicago/Turabian StyleKhanal, Praval, Christopher I. Morse, Lingxiao He, Adam J. Herbert, Gladys L. Onambélé-Pearson, Hans Degens, Martine Thomis, Alun G. Williams, and Georgina K. Stebbings. 2022. "Polygenic Models Partially Predict Muscle Size and Strength but Not Low Muscle Mass in Older Women" Genes 13, no. 6: 982. https://doi.org/10.3390/genes13060982









