The Role of the MYC/miR-150/MYB/ZDHHC11 Network in Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture of Cell Lines and Sorting of GC B-Cells
2.2. DNA Constructs and Viral Transfection
2.3. GFP Competition Assay
2.4. RNA Isolation and RT-qPCR
2.5. AGO2 RNA Immunoprecipitation
2.6. Western Blot
2.7. Analysis of Deposited Microarray and RNA-seq Data
2.8. Statistical Analysis
3. Results
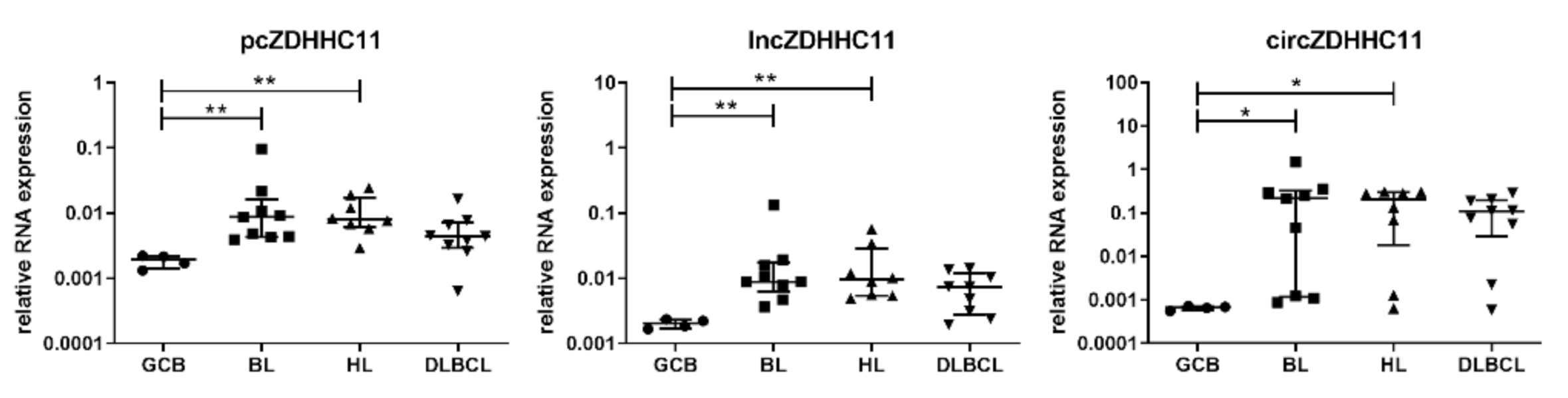
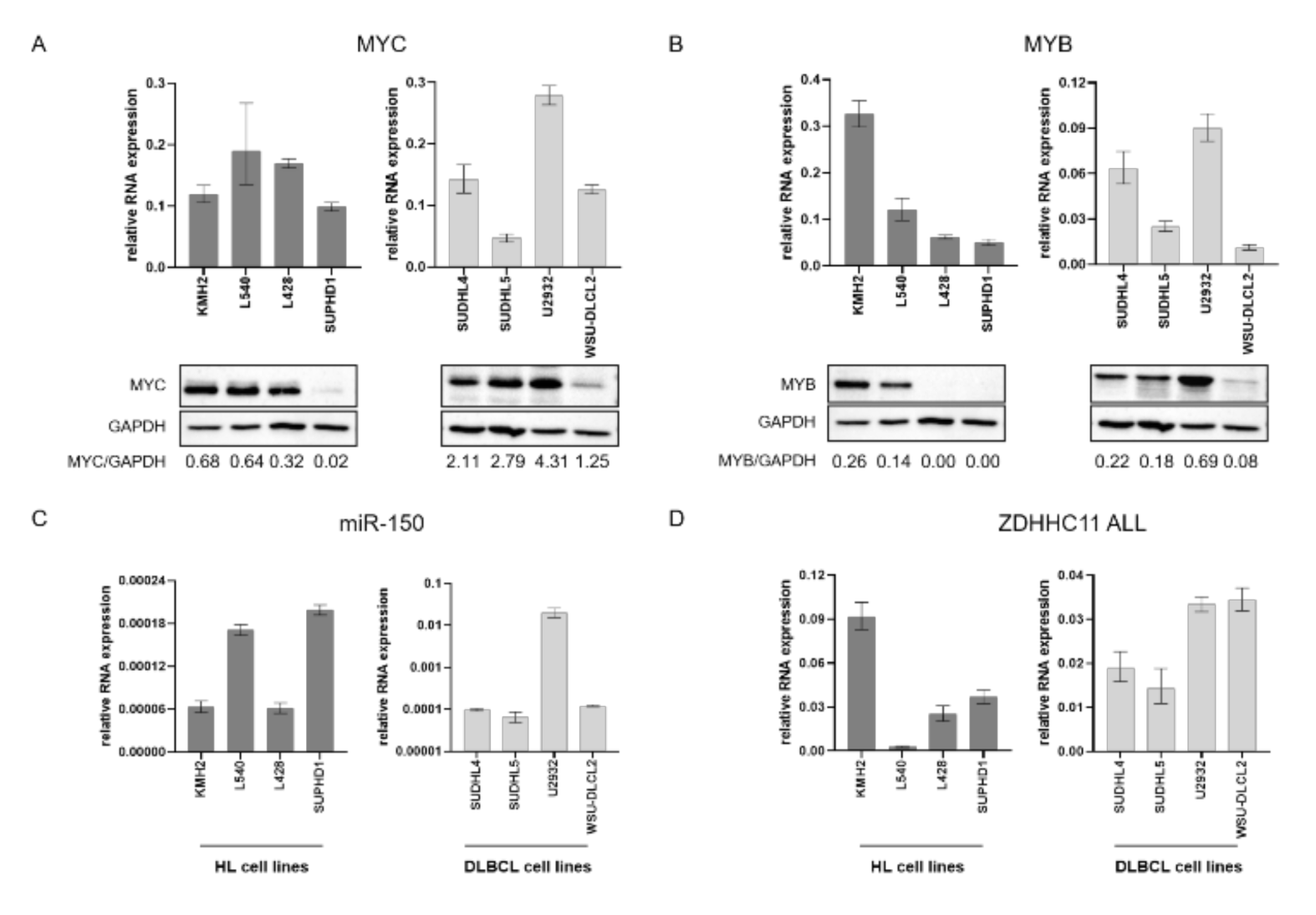
3.1. Expression of Network Components in B-Cell Lymphoma
3.2. Effect of MYC, MYB, ZDHHC11 and miR-150 on Growth of HL and DLBCL Cell Lines
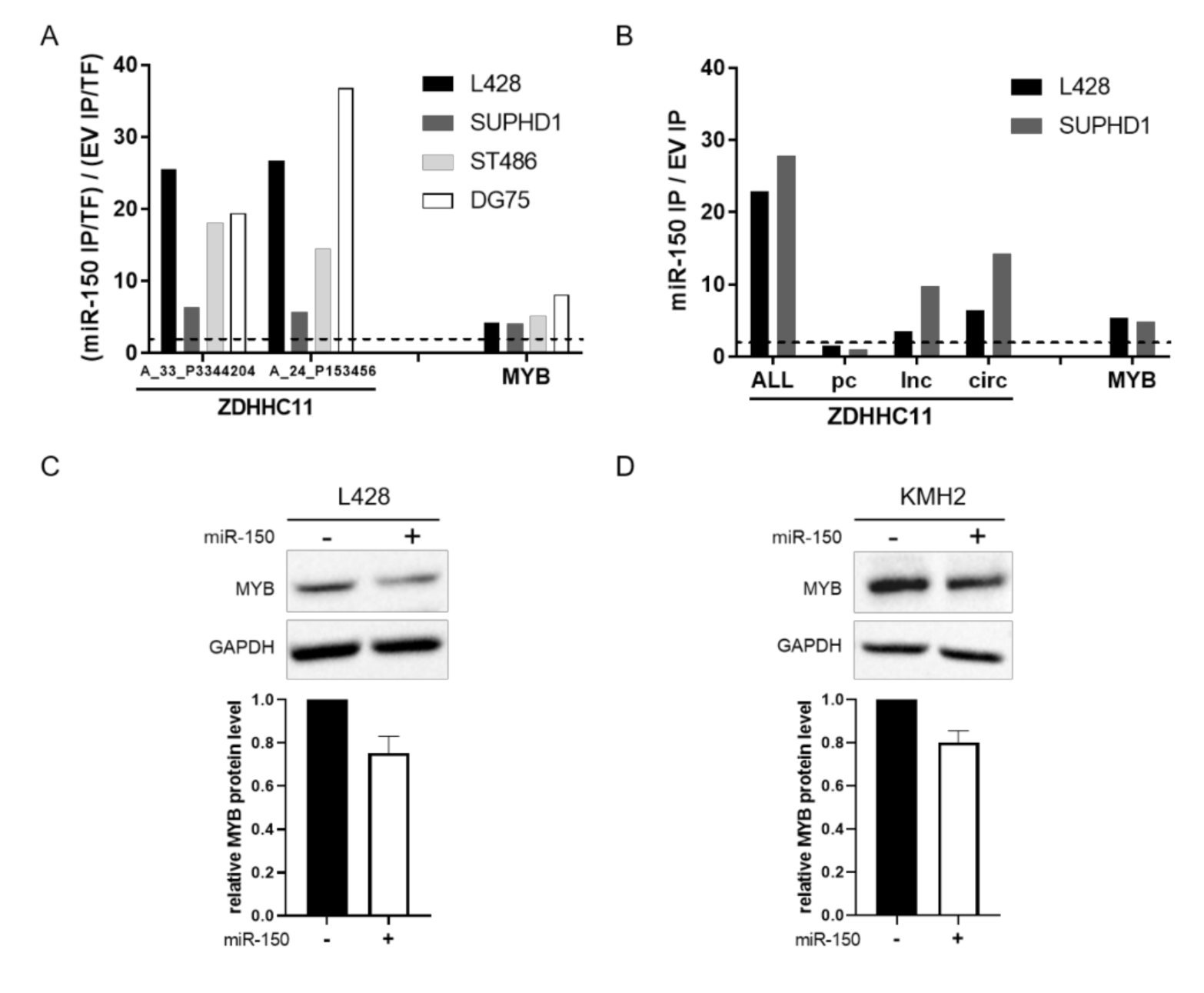
3.3. MiR-150 Targets ZDHHC11 and MYB in HL Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molyneux, E.M.; Rochford, R.; Griffin, B.; Newton, R.; Jackson, G.; Menon, G.; Harrison, C.J.; Israels, T.; Bailey, S. Burkitt’s lymphoma. Lancet 2012, 379, 1234–1244. [Google Scholar] [CrossRef] [Green Version]
- Dang, C.V.; O’Donnell, K.A.; Zeller, K.I.; Nguyen, T.; Osthus, R.C.; Li, F. The c-Myc target gene network. Semin. Cancer Biol. 2006, 16, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.C.; Liu, X.Y.; Teng, L.; Ji, Q.; Wu, Y.; Li, J.M.; Gao, W.; Zhang, Y.Y.; La, T.; Tabatabaee, H.; et al. c-Myc inactivation of p53 through the pan-cancer lncRNA MILIP drives cancer pathogenesis. Nat. Commun. 2020, 11, 4980. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Komiya, M.; Matsumura, K.; Negishi, L.; Suda, S.; Okuno, M.; Yokota, N.; Osada, T.; Nagashima, T.; Hiyoshi, M.; et al. MYU, a Target lncRNA for Wnt/c-Myc Signaling, Mediates Induction of CDK6 to Promote Cell Cycle Progression. Cell Rep. 2016, 16, 2554–2564. [Google Scholar] [CrossRef] [Green Version]
- Winkle, M.; Berg, A.V.D.; Tayari, M.; Sietzema, J.; Terpstra, M.; Kortman, G.; de Jong, D.; Visser, L.; Diepstra, A.; Kok, K.; et al. Long noncoding RNAs as a novel component of the Myc transcriptional network. FASEB J. 2015, 29, 2338–2346. [Google Scholar] [CrossRef] [Green Version]
- Robertus, J.-L.; Kluiver, J.; Weggemans, C.; Harms, G.; Reijmers, R.M.; Swart, Y.; Kok, K.; Rosati, S.; Schuuring, E.; Van Imhoff, G.; et al. MiRNA profiling in B non-Hodgkin lymphoma: A MYC-related miRNA profile characterizes Burkitt lymphoma. Br. J. Haematol. 2010, 149, 896–899. [Google Scholar] [CrossRef]
- He, Y.; Jiang, X.; Chen, J. The role of miR-150 in normal and malignant hematopoiesis. Oncogene 2013, 33, 3887–3893. [Google Scholar] [CrossRef]
- Watanabe, A.; Tagawa, H.; Yamashita, J.; Teshima, K.; Nara, M.; Iwamoto, K.; Kume, M.; Kameoka, Y.; Takahashi, N.; Nakagawa, T.; et al. The role of microRNA-150 as a tumor suppressor in malignant lymphoma. Leukemia 2011, 25, 1324–1334. [Google Scholar] [CrossRef] [Green Version]
- Xiao, C.; Calado, D.; Galler, G.; Thai, T.-H.; Patterson, H.C.; Wang, J.; Rajewsky, K.; Bender, T.P. MiR-150 Controls B Cell Differentiation by Targeting the Transcription Factor c-Myb. Cell 2007, 131, 146–159. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.C.; Kuo, M.W.; Yu, J.; Kuo, H.H.; Lin, R.J.; Lo, W.L.; Yu, A.L. c-Myb is an evolutionary conserved miR-150 target and miR-150/c-Myb interaction is important for embryonic development. Mol. Biol. Evol. 2008, 25, 2189–2198. [Google Scholar] [CrossRef] [Green Version]
- Dzikiewicz-Krawczyk, A.; Kok, K.; Slezak-Prochazka, I.; Robertus, J.L.; Bruining, J.; Tayari, M.M.; Rutgers, B.; de Jong, D.; Koerts, J.; Seitz, A.; et al. ZDHHC11 and ZDHHC11B are critical novel components of the oncogenic MYC-miR-150-MYB network in Burkitt lymphoma. Leukemia 2017, 31, 1470–1473. [Google Scholar] [CrossRef] [Green Version]
- Lemonidis, K.; Werno, M.W.; Greaves, J.; Diez-Ardanuy, C.; Sanchez-Perez, M.C.; Salaun, C.; Thomson, D.M.; Chamberlain, L.H. The zDHHC family of S-acyltransferases. Biochem. Soc. Trans. 2015, 43, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Kluiver, J.; Koerts, J.; De Jong, D.; Rutgers, B.; Razak, F.R.A.; Terpstra, M.; Plaat, B.; Nolte, I.M.; Diepstra, A.; et al. miR-24-3p Is Overexpressed in Hodgkin Lymphoma and Protects Hodgkin and Reed-Sternberg Cells from Apoptosis. Am. J. Pathol. 2017, 187, 1343–1355. [Google Scholar] [CrossRef] [Green Version]
- Niu, F.; Kazimierska, M.; Nolte, I.M.; Terpstra, M.M.; De Jong, D.; Koerts, J.; Van Der Sluis, T.; Rutgers, B.; O’Connell, R.M.; Kok, K.; et al. The miR-26b-5p/KPNA2 Axis Is an Important Regulator of Burkitt Lymphoma Cell Growth. Cancers 2020, 12, 1464. [Google Scholar] [CrossRef]
- Kluiver, J.; Slezak-Prochazka, I.; van den Berg, A. Studying microRNAs in lymphoma. In Lymphoma; Humana Press: Totowa, NJ, USA, 2013; Volume 971, pp. 265–276. [Google Scholar]
- Ślęzak-Prochazka, I.; Kluiver, J.; De Jong, D.; Kortman, G.; Halsema, N.; Poppema, S.; Kroesen, B.-J.; Berg, A.V.D. Cellular Localization and Processing of Primary Transcripts of Exonic MicroRNAs. PLoS ONE 2013, 8, e76647. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.P.; Seinen, E.; Duns, G.; De Jong, D.; Sibon, O.C.M.; Poppema, S.; Kroesen, B.-J.; Kok, K.; Berg, A.V.D. A high throughput experimental approach to identify miRNA targets in human cells. Nucleic Acids Res. 2009, 37, e137. [Google Scholar] [CrossRef]
- Weniger, M.A.; Tiacci, E.; Schneider, S.; Arnolds, J.; Rüschenbaum, S.; Duppach, J.; Seifert, M.; Döring, C.; Hansmann, M.-L.; Küppers, R. Human CD30+ B cells represent a unique subset related to Hodgkin lymphoma cells. J. Clin. Investig. 2018, 128, 2996–3007. [Google Scholar] [CrossRef] [Green Version]
- Tiacci, E.; Doring, C.; Brune, V.; Noesel, C.J.M.; Klapper, W.; Mechtersheimer, G.; Falini, B.; Küppers, R.; Hansmann, M.-L. Analyzing primary Hodgkin and Reed-Sternberg cells to capture the molecular and cellular pathogenesis of classical Hodgkin lymphoma. Blood 2012, 120, 4609–4620. [Google Scholar] [CrossRef] [Green Version]
- Steidl, C.; Diepstra, A.; Lee, T.; Chan, F.C.; Farinha, P.; Tan, K.; Telenius, A.; Barclay, L.; Shah, S.P.; Connors, J.M.; et al. Gene expression profiling of microdissected Hodgkin Reed-Sternberg cells correlates with treatment outcome in classical Hodgkin lymphoma. Blood 2012, 120, 3530–3540. [Google Scholar] [CrossRef] [Green Version]
- Scott, D.W.; Wright, G.W.; Williams, P.M.; Lih, C.G.; Walsh, W.; Jaffe, E.S.; Rosenwald, A.; Campo, E.; Chan, W.C.; Connors, J.M.; et al. Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraf-fin-embedded tissue. Blood 2014, 123, 1214–1217. [Google Scholar] [CrossRef]
- Ennishi, H.; Mottok, A.; Ben-Neriah, S.; Shulha, H.P.; Farinha, P.; Chan, F.C.; Meissner, B.; Boyle, M.; Hother, C.; Kridel, R.; et al. Genetic profiling of MYC and BCL2 in diffuse large B-cell lymphoma determines cell-of-origin-specific clinical impact. Blood 2017, 129, 2760–2770. [Google Scholar] [CrossRef] [Green Version]
- Visco, C.; Li, Y.; Xu-Monette, Z.Y.; Miranda, R.N.; Green, T.M.; Li, Y.; Tzankov, A.; Wen, W.; Liu, W.; Kahl, B.S.; et al. Comprehensive gene expression profiling and immunohistochemical studies support application of immunophenotypic algorithm for molecular subtype classification in diffuse large B-cell lymphoma: A report from the International DLBCL Rituximab—CHOP Consortium Program Study. Leukemia 2012, 26, 2103–2113. [Google Scholar] [PubMed]
- Razak, F.A.; Diepstra, A.; Jong, D.; Koerts, J.; Rutgers, B.; Kluiver, J.; Visser, L.; van den Berg, A. Mutations in Cd58 and Myb in Hodgkin Lymphoma. Haematologica 2016, 101, 4–5. [Google Scholar]
- Jiang, X.; Huang, H.; Li, Z.; Li, Y.; Wang, X.; Gurbuxani, S.; Chen, P.; He, C.; You, D.; Zhang, S.; et al. Blockade of Mir-150 Maturation by MLL-Fusion/MYC/Lin-28 Is Required for MLL-Associated Leukemia. Blood 2012, 120, 3499. [Google Scholar] [CrossRef]
- Bousquet, M.; Zhuang, G.; Meng, C.; Ying, W.; Cheruku, P.S.; Shie, A.T.; Wang, S.; Ge, G.; Wong, P.; Wang, G.; et al. miR-150 Blocks MLL-AF9–Associated Leukemia through Oncogene Repression. Mol. Cancer Res. 2013, 11, 912–922. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziel-Swier, L.J.Y.M.; Liu, Y.; Seitz, A.; de Jong, D.; Koerts, J.; Rutgers, B.; Veenstra, R.; Razak, F.R.A.; Dzikiewicz-Krawczyk, A.; van den Berg, A.; et al. The Role of the MYC/miR-150/MYB/ZDHHC11 Network in Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma. Genes 2022, 13, 227. https://doi.org/10.3390/genes13020227
Ziel-Swier LJYM, Liu Y, Seitz A, de Jong D, Koerts J, Rutgers B, Veenstra R, Razak FRA, Dzikiewicz-Krawczyk A, van den Berg A, et al. The Role of the MYC/miR-150/MYB/ZDHHC11 Network in Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma. Genes. 2022; 13(2):227. https://doi.org/10.3390/genes13020227
Chicago/Turabian StyleZiel-Swier, Lotteke J. Y. M., Yichen Liu, Annika Seitz, Debora de Jong, Jasper Koerts, Bea Rutgers, Rianne Veenstra, Fazlyn R. Abdul Razak, Agnieszka Dzikiewicz-Krawczyk, Anke van den Berg, and et al. 2022. "The Role of the MYC/miR-150/MYB/ZDHHC11 Network in Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma" Genes 13, no. 2: 227. https://doi.org/10.3390/genes13020227





