Diane-35 and Metformin Induce Autophagy and Apoptosis in Polycystic Ovary Syndrome Women with Early-Stage Endometrial Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Cell Line and Culture
2.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.4. Western Blot Analysis
2.5. CCK-8 Assay
2.6. Colony Formation Assay
2.7. Flow Cytometry
2.8. Immunofluorescence Staining
2.9. Dual-Luciferase Reporter Assay
2.10. Chromatin Immunoprecipitation (ChIP)
2.11. Immunohistochemistry Assay
2.12. Transmissiovn Electron Microscopy Analyses
2.13. Gene Expression Vector Construction
2.14. Lentiviral Transfection
2.15. Construction of PCOS Mouse Model with Early EC
2.16. Statistical Analysis
3. Results
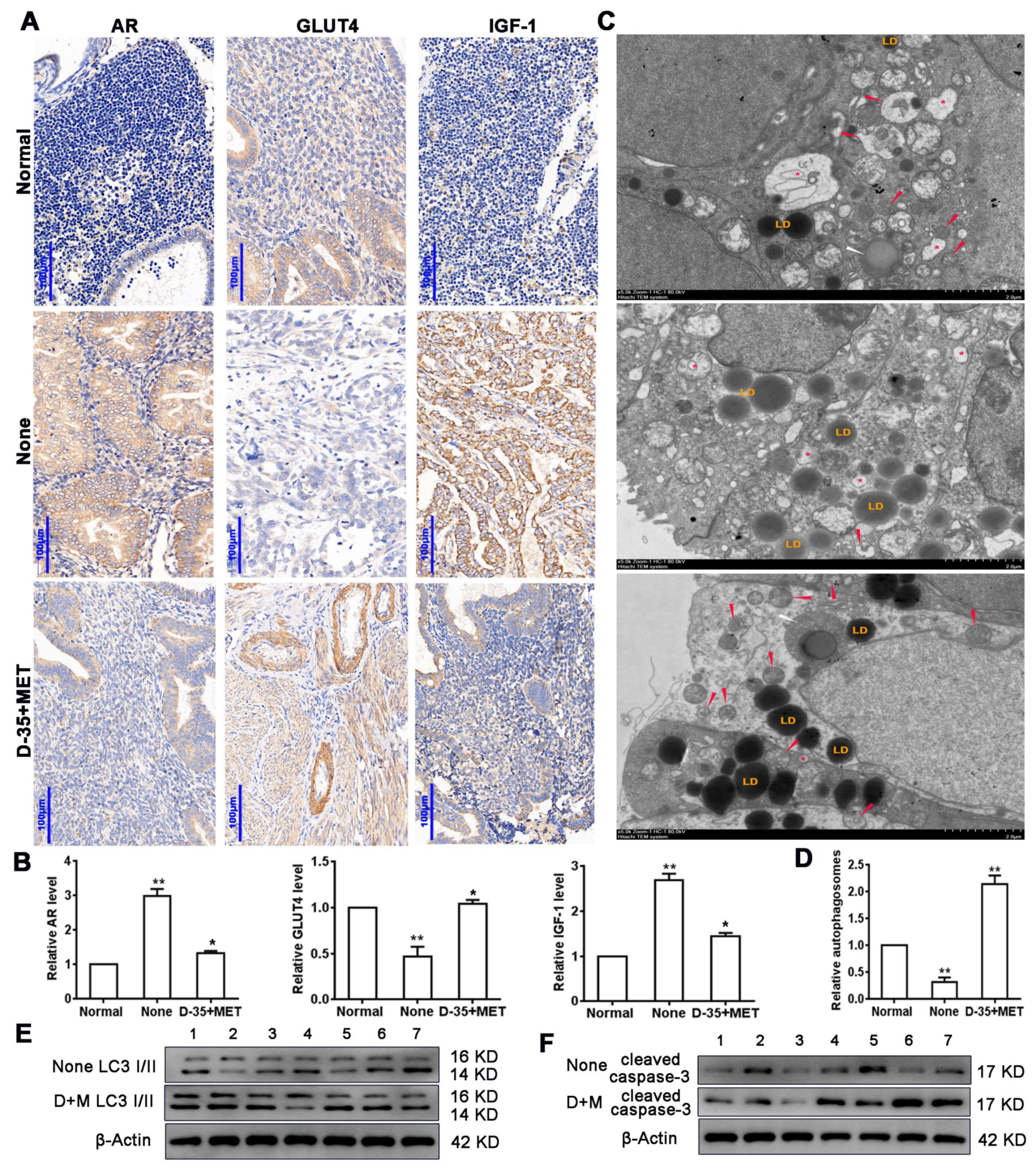
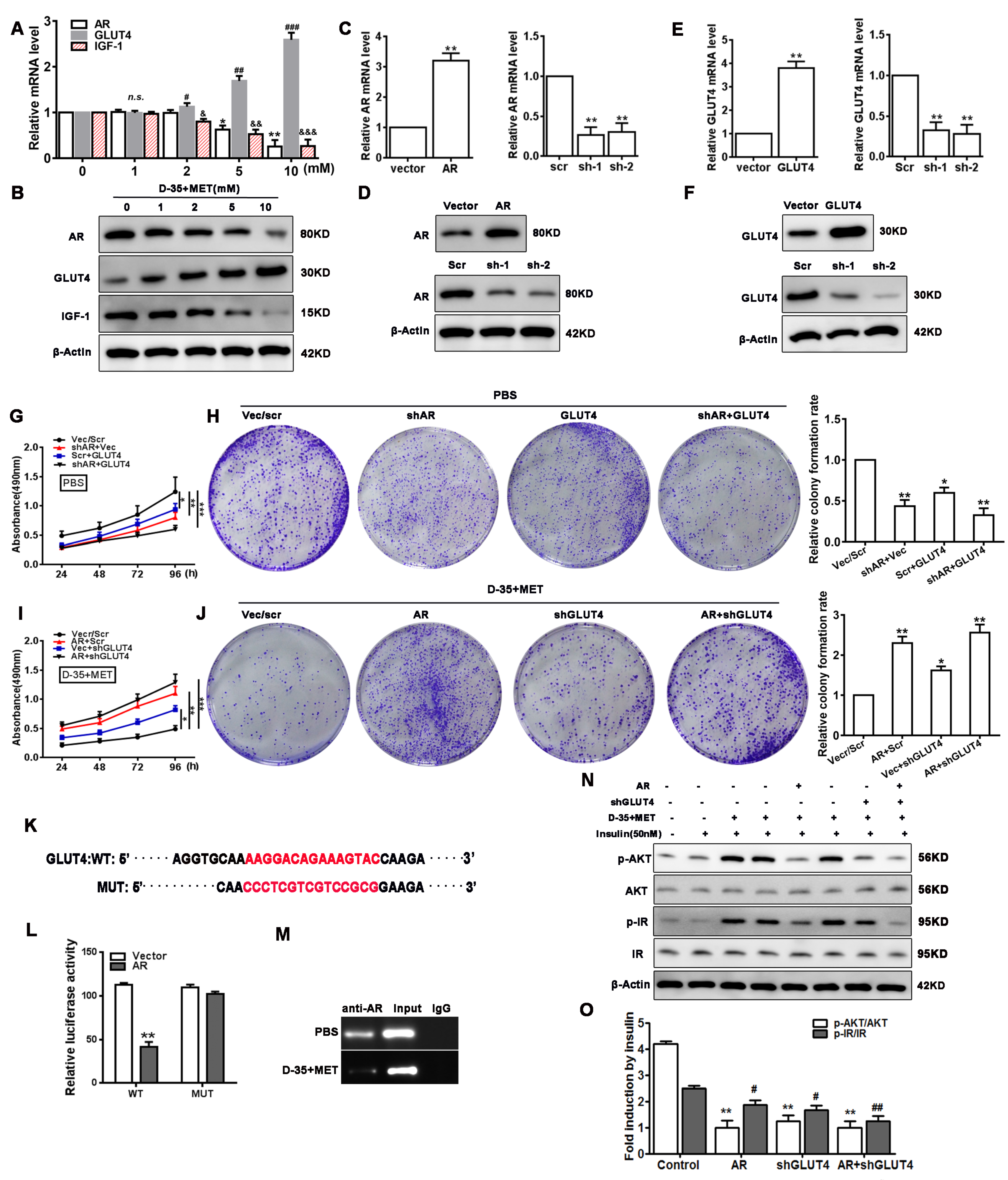
3.1. Diane-35 and Metformin Reduced AR and IGF-1 Expression and Increased GLUT4 Expression in Early Stage Tumor Tissue of PCOS Patients with EC
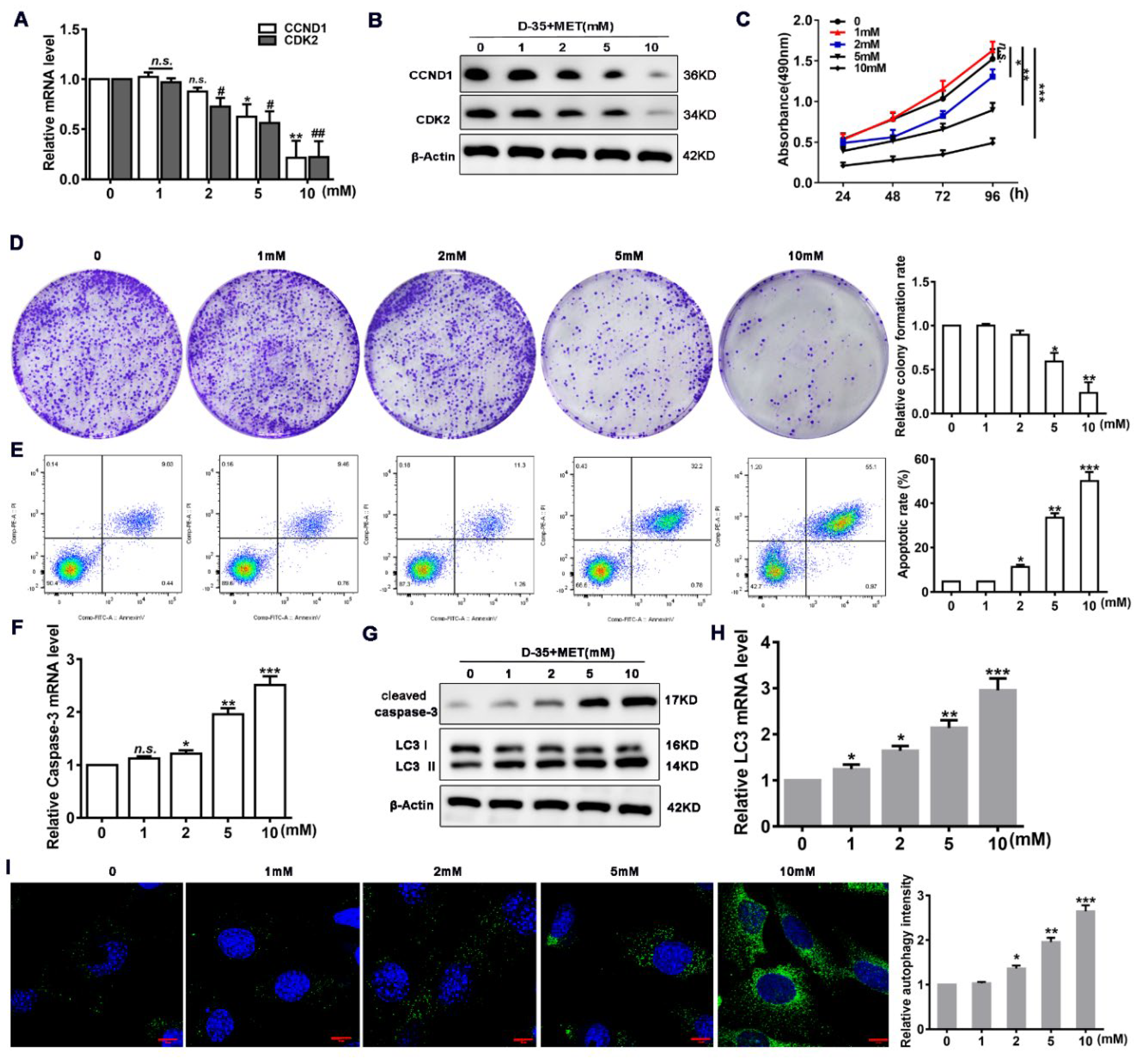
3.2. Diane-35 and Metformin Promote Apoptosis and Autophagy in EC Cells
3.3. Diane-35 and Metformin Repress Apoptotic and Autophagy in Ovarian Granulosa Cells of a PCOS Murine Model
3.4. Cell Proliferation and Insulin Resistance Is Regulated by Diane-35 and Metformin
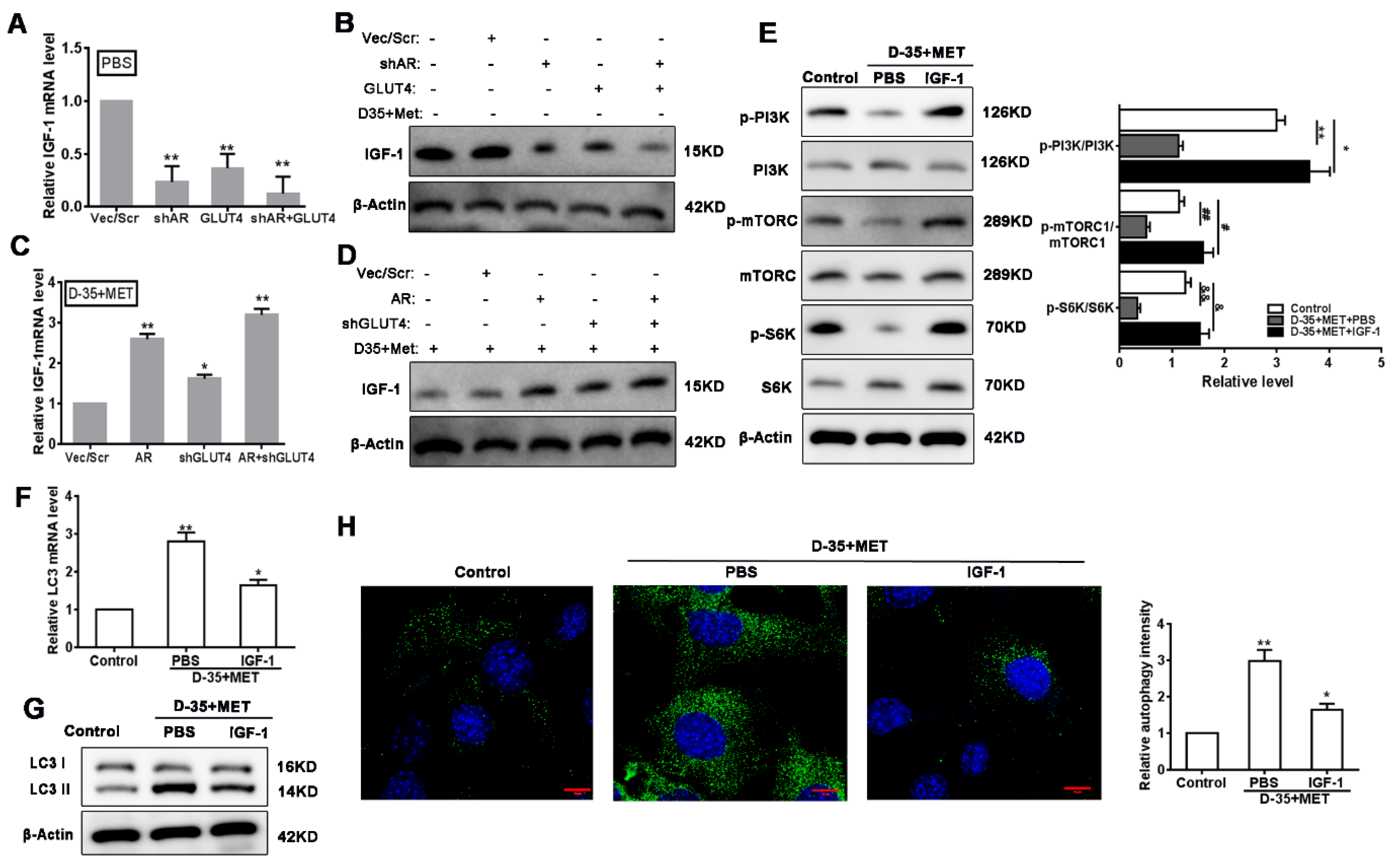
3.5. Autophagy Is Induced by Diane-35 and Metformin via Inhibition of IGF-1
3.6. Verification of PI3K/mTORC1 Pathways on Diane-35 and Metformin-Mediated Autophagy
3.7. Diane-35 and Metformin Administration Ameliorate EC In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCOS | polycystic ovary syndrome |
| EC | endometrial carcinoma |
| AP | androgen receptor |
| AMP | adenosine monophosphate |
| AMPK | adenosine monophosphate-activated protein kinase |
| GLUT4 | Glucose transporter 4 |
| IGF-1 | insulin-like growth factor |
| mTORC | rapamycin complex |
| 3-MA | 3-methyladenine |
References
- Hardiman, P.; Pillay, O.S.; Atiomo, W. Polycystic ovary syndrome and endometrial carcinoma. Lancet 2003, 361, 1810–1812. [Google Scholar] [CrossRef]
- Ali, A.T. Polycystic ovary syndrome and metabolic syndrome. Ceska Gynekol. 2015, 80, 279–289. [Google Scholar]
- Jeanes, Y.; Reeves, S. Metabolic consequences of obesity and insulin resistance in polycystic ovary syndrome: Diagnostic and methodological challenges. Nutr. Res. Rev. 2017, 30, 97–105. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef] [Green Version]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef]
- Zhang, Y.; Ho, K.; Keaton, J.M.; Hartzel, D.N.; Day, F.; Justice, A.E.; Josyula, N.S.; Pendergrass, S.A.; Actkins, K.; Davis, L.K.; et al. A genome-wide association study of polycystic ovary syndrome identified from electronic health records. Am. J. Obstet. Gynecol. 2020, 223, 559.e1–559.e21. [Google Scholar] [CrossRef]
- Barry, J.; Azizia, M.M.; Hardiman, P.J. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Updat. 2014, 20, 748–758. [Google Scholar] [CrossRef]
- Zeina, H.; Maisa, S.; William, A. Evaluating the association between endometrial cancer and polycystic ovary syndrome. Hum. Reprod. 2012, 27, 1327–1331. [Google Scholar]
- Okamura, Y.; Saito, F.; Takaishi, K.; Motohara, T.; Honda, R.; Ohba, T.; Katabuchi, H. Polycystic ovary syndrome: Early diagnosis and intervention are necessary for fertility preservation in young women with endometrial cancer under 35 years of age. Reprod. Med. Biol. 2017, 16, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Hussein, Y.R.; Soslow, R.A. Molecular insights into the classification of high-grade endometrial carcinoma. Pathology 2018, 50, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Braun, M.M.; Overbeek-Wager, E.A.; Grumbo, R.J. Diagnosis and Management of Endometrial Cancer. Am. Fam. Phys. 2016, 93, 468–474. [Google Scholar]
- Shafiee, M.N.; Khan, G.; Ariffin, R.; Abu, J.; Chapman, C.; Deen, S.; Nunns, D.; Barrett, D.; Seedhouse, C.; Atiomo, W. Preventing endometrial cancer risk in polycystic ovarian syndrome (PCOS) women: Could metformin help? Gynecol. Oncol. 2014, 132, 248–253. [Google Scholar] [CrossRef]
- Li, X.; Guo, Y.-R.; Lin, J.-F.; Feng, Y.; Billig, H.; Shao, R. Combination of Diane-35 and Metformin to Treat Early Endometrial Carcinoma in PCOS Women with Insulin Resistance. J. Cancer 2014, 5, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.-M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; Macdonald, M.J.; et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 2014, 510, 542–546. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Tu, H.; Yao, J.; Le, J.; Jiang, Z.; Tang, Q.; Zhang, R.; Huo, P.; Lei, X. Combined use of Diane-35 and metformin improves the ovulation in the PCOS rat model possibly via regulating glycolysis pathway. Reprod. Biol. Endocrinol. 2020, 18, 58. [Google Scholar] [CrossRef]
- Wang, J.; Wu, D.; Guo, H.; Li, M. Hyperandrogenemia and insulin resistance: The chief culprit of polycystic ovary syndrome. Life Sci. 2019, 236, 116940. [Google Scholar] [CrossRef]
- Zhai, J.; Liu, C.-X.; Tian, Z.-R.; Jiang, Q.-H.; Sun, Y.-P.; Losa-Ward, S.M.; Todd, K.L.; McCaffrey, K.A.; Tsutsui, K.; Patisaul, H.B. Effects of Metformin on the Expression of GLUT4 in Endometrium of Obese Women with Polycystic Ovary Syndrome1. Biol. Reprod. 2012, 87, 29. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cui, P.; Jiang, H.-Y.; Guo, Y.-R.; Pishdari, B.; Hu, M.; Feng, Y.; Billig, H.; Shao, R. Reversing the reduced level of endometrial GLUT4 expression in polycystic ovary syndrome: A mechanistic study of metformin action. Am. J. Transl. Res. 2015, 7, 574–586. [Google Scholar]
- Shafiee, M.N.; Seedhouse, C.; Mongan, N.; Chapman, C.; Deen, S.; Abu, J.; Atiomo, W. Up-regulation of genes involved in the insulin signalling pathway (IGF1, PTEN and IGFBP1) in the endometrium may link polycystic ovarian syndrome and endometrial cancer. Mol. Cell. Endocrinol. 2016, 424, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.; Li, N.; Song, G.; Yang, Y.; Ning, X. Insulin-like growth factor 1 regulates growth of endometrial carcinoma through PI3k signaling pathway in insulin-resistant type 2 diabetes. Am. J. Transl. Res. 2016, 8, 3329–3336. [Google Scholar]
- Feng, Y.; Ke, C.; Tang, Q.; Dong, H.; Zheng, X.; Lin, W.; Ke, J.; Huang, J.; Yeung, S.-C.J.; Zhang, H. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis. 2014, 5, e1088. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, W.; Yan, Z.; Zhao, W.; Mi, J.; Li, J.; Yan, H. Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J. Exp. Clin. Cancer Res. 2018, 37, 63. [Google Scholar] [CrossRef]
- Puustinen, P.; Rytter, A.; Mortensen, M.; Kohonen, P.; Moreira, J.; Jäättelä, M. CIP2A oncoprotein controls cell growth and autophagy through mTORC1 activation. J. Cell Biol. 2014, 204, 713–727. [Google Scholar] [CrossRef]
- Yu, N.; Zhang, T.; Zhao, D.; Cao, Z.; Du, J.; Zhang, Q. CIP2A is overexpressed in human endometrioid adenocarcinoma and regulates cell proliferation, invasion and apoptosis. Pathol. -Res. Pract. 2018, 214, 233–239. [Google Scholar] [CrossRef]
- Feng, W.; Jia, Y.-Y.; Zhang, D.-Y.; Shi, H.-R. Management of polycystic ovarian syndrome with Diane-35 or Diane-35 plus metformin. Gynecol. Endocrinol. 2016, 32, 147–150. [Google Scholar] [CrossRef]
- Orfanelli, T.; Jeong, J.; Doulaveris, G.; Holcomb, K.; Witkin, S. Involvement of autophagy in cervical, endometrial and ovarian cancer. Int. J. Cancer 2013, 135, 519–528. [Google Scholar] [CrossRef] [Green Version]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in Mammalian Autophagy Research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef] [Green Version]
- Hang, Z.; Wei, L.; Chao, Z.; Yu, Z.; Nie, C. PP2A mediates apoptosis or autophagic cell death in multiple myeloma cell lines. Oncotarget 2017, 8, 80770–80789. [Google Scholar]
- Navaratnarajah, R.; Pillay, O.; Hardiman, P. Polycystic Ovary Syndrome and Endometrial Cancer. Semin. Reprod. Med. 2008, 26, 62–71. [Google Scholar] [CrossRef]
- Jia, X.; Yang, L.; Xu, P.; Li, N.; Chen, C.; Wang, H. Endometrial cancer combined with polycystic ovary syndrome in 9 women under 40-years old: A case report. Biomed. Rep. 2020, 13, 50. [Google Scholar] [CrossRef]
- Kempegowda, P.; Melson, E.; Manolopoulos, K.N.; Arlt, W.; O’Reilly, M.W. Implicating androgen excess in propagating metabolic disease in polycystic ovary syndrome. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820934319. [Google Scholar] [CrossRef]
- Eritja, N.; Chen, B.-J.; Rodriguez-Barrueco, R.; Santacana, M.; Gatius, S.; Vidal, A.; Martí, M.D.; Ponce, J.; Bergadà, L.; Yeramian, A.; et al. Autophagy orchestrates adaptive responses to targeted therapy in endometrial cancer. Autophagy 2017, 13, 608–624. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, Z.; Wang, A.; Yu, H. Metformin targeting autophagy overcomes progesterone resistance in endometrial carcinoma. Arch. Gynecol. Obstet. 2016, 294, 1055–1061. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chen, H.-Y.; Wang, C.-W.; Shieh, T.-M.; Huang, T.-C.; Lin, L.-C.; Wang, K.-L.; Hsia, S.-M. Isoliquiritigenin induces apoptosis and autophagy and inhibits endometrial cancer growth in mice. Oncotarget 2016, 7, 73432–73447. [Google Scholar] [CrossRef] [Green Version]
- Mitsuhashi, A.; Kiyokawa, T.; Sato, Y.; Shozu, M. Effects of metformin on endometrial cancer cell growth in vivo: A preoperative prospective trial. Cancer 2014, 120, 2986–2995. [Google Scholar] [CrossRef]
- Shan, Y.; Guan, F.; Zhao, X.; Wang, M.; Chen, Y.; Wang, Q.; Feng, X. Macranthoside B Induces Apoptosis and Autophagy Via Reactive Oxygen Species Accumulation in Human Ovarian Cancer A2780 Cells. Nutr. Cancer 2016, 68, 280–289. [Google Scholar] [CrossRef]
- Wu, Y.; Gu, T.T.; Zheng, P.S. CIP2A cooperates with H-Ras to promote epithelial-mesenchymal transition in cervical-cancer progression. Cancer Lett. 2015, 356, 646–655. [Google Scholar] [CrossRef]
- Puustinen, P.; Jäättelä, M. KIAA1524/CIP2A promotes cancer growth by coordinating the activities of MTORC1 and MYC. Autophagy 2014, 10, 1352–1354. [Google Scholar] [CrossRef] [PubMed]
- De Santi, M.; Baldelli, G.; Diotallevi, A.; Galluzzi, L.; Schiavano, G.F.; Brandi, G. Metformin prevents cell tumorigenesis through autophagy-related cell death. Sci. Rep. 2019, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Ruan, X.; Jin, J.; Mueck, A.O. Metabolic profile of Diane-35 versus Diane-35 plus metformin in Chinese PCOS women under standardized life-style changes. Gynecol. Endocrinol. 2015, 31, 548–551. [Google Scholar] [CrossRef]
- Shao, R.; Li, X.; Billig, H. Promising clinical practices of metformin in women with PCOS and early-stage endometrial cancer. BBA Clin. 2014, 2, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Jing, Z.; Liang-Zhi, X.; Tai-Xiang, W.; Ying, T.; Yu-Jian, J. The effects of Diane-35 and metformin in treatment of polycystic ovary syndrome: An updated systematic review. Gynecol. Endocrinol. 2008, 24, 590–600. [Google Scholar] [CrossRef]



| AR | Forward: 5′ AGGATGCTCTACTTCGCCCC Reverse: 5′ CTGGCTGTACATCCGGGAC |
| GLUT4 | Forward: 5′ CAGCTCTCAGGCATCAAT Reverse: 5′ TCTACTAAGAGCACCGAG |
| IGF-1 | Forward: 5′ CCATAGAAAGAGGAATAACAGCAG Reverse: 5′ TACCTCCCATTCATCAGGCA |
| CIP2A | Forward: 5′ GAACAGATAAGAAAAGAGTTGAGCATT Reverse: 5′ CGACCTTCTAATTGTGCCTTTT |
| PP2A | Forward: 5′ CACACGGACCAAAAGATGTGCC Reverse: 5′ CAGCACCAGTCGTGCCCACTG |
| LC3 | Forward: 5′ AGCAGCATCCAACCAAAATC Reverse: 5′ CTGTGTCCGTTCACCAACAG |
| Caspase-3 | Forward: 5′ CAGTGGAGGCCGACTTCTTG Reverse: 5′ TGGCACAAAGCGACTGGAT |
| CDK2 | Forward: 5′ CCAGGAGTTACTTCTATGCCTGA Reverse: 5′ TTCATCCAGGGGAGGTACAAC |
| CCND1 | Forward: 5′ GCTGCGAAGTGGAAACCATC Reverse: 5′ CCTCCTTCTGCACACATTTGAA |
| GAPDN | Forward: 5′ CGGGGCTGGCATTGCTCTC Reverse: 5′ GGGGCCGACTTGGGATAGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, Y.; Yao, D.; Chen, X.; Zhang, F.; Feng, Y.; Li, X. Diane-35 and Metformin Induce Autophagy and Apoptosis in Polycystic Ovary Syndrome Women with Early-Stage Endometrial Carcinoma. Genes 2022, 13, 131. https://doi.org/10.3390/genes13010131
Liu Y, Wang Y, Yao D, Chen X, Zhang F, Feng Y, Li X. Diane-35 and Metformin Induce Autophagy and Apoptosis in Polycystic Ovary Syndrome Women with Early-Stage Endometrial Carcinoma. Genes. 2022; 13(1):131. https://doi.org/10.3390/genes13010131
Chicago/Turabian StyleLiu, Yanjun, Yang Wang, Dan Yao, Xing Chen, Feifei Zhang, Yi Feng, and Xin Li. 2022. "Diane-35 and Metformin Induce Autophagy and Apoptosis in Polycystic Ovary Syndrome Women with Early-Stage Endometrial Carcinoma" Genes 13, no. 1: 131. https://doi.org/10.3390/genes13010131






