Interspecific Genetic Differences and Historical Demography in South American Arowanas (Osteoglossiformes, Osteoglossidae, Osteoglossum)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Individuals Sampling
2.2. Mitotic Chromosomal Preparations and Comparative Genomic Hybridization (CGH)
2.3. Chromosomal Analyses and Image Processing
2.4. DArTseq Procedure
2.5. DArT Data Filtering
2.6. Detection of Outlier Markers Putatively under Selection
2.7. Genetic Diversity
2.8. Interspecific Differences Structure
2.9. Demographic Model Selection
2.10. Paleogeographic Modeling
3. Results
3.1. Cytogenetic Data
3.2. Sequencing and Filtering
3.3. Detection of Markers Putatively under Selection
3.4. Genetic Diversity
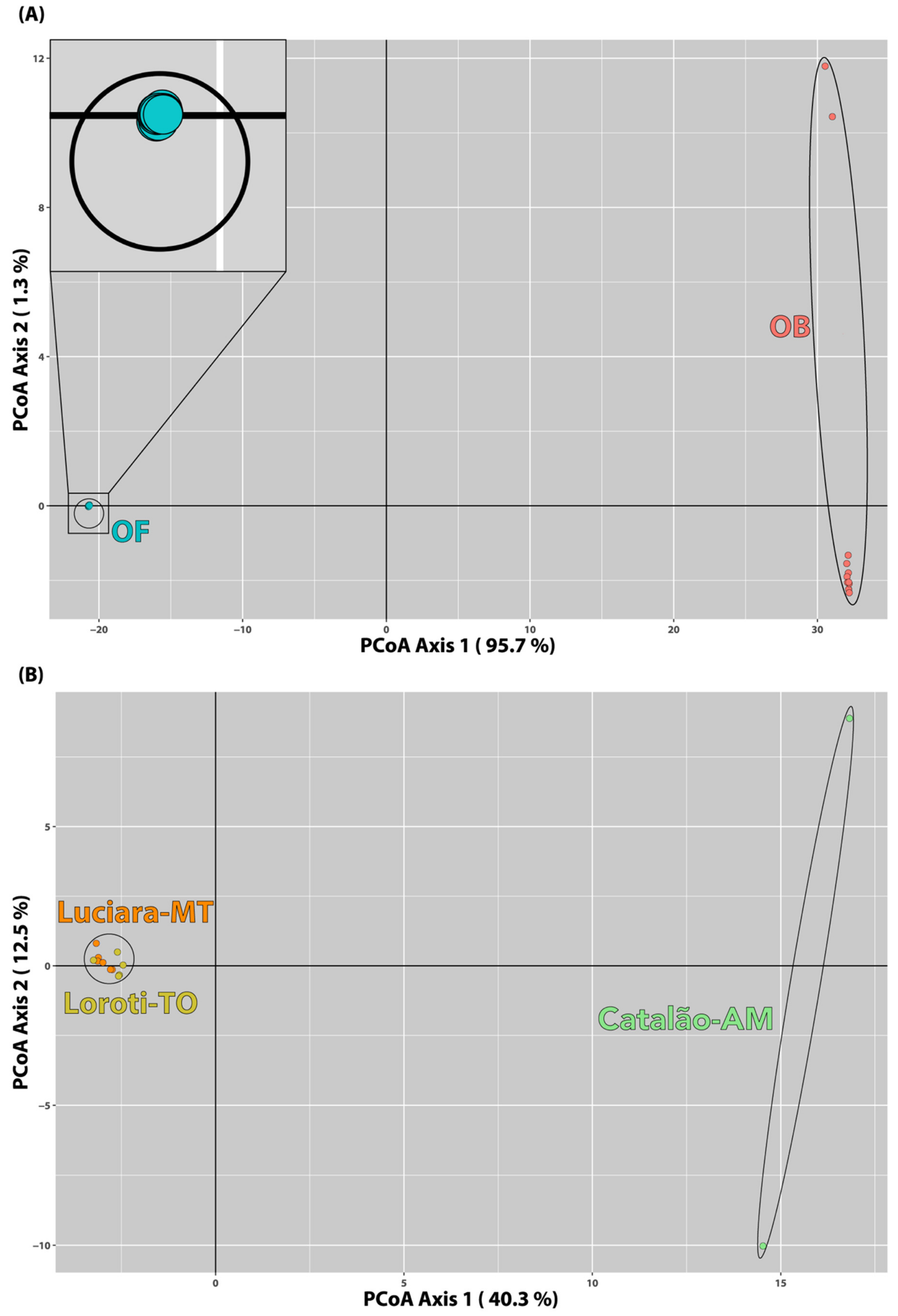
3.5. Population Structure
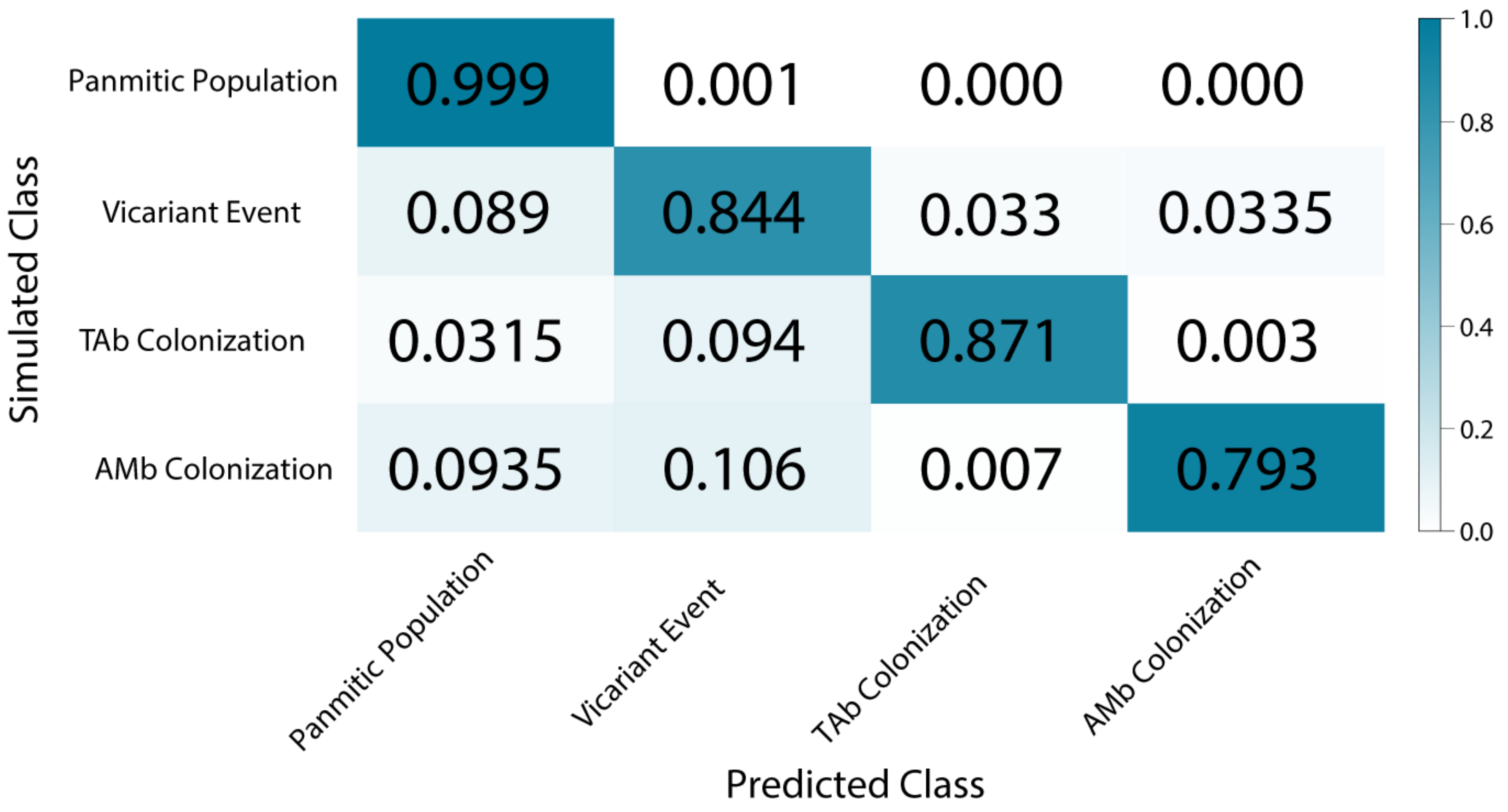
3.6. Demographic Model Selection
3.7. Paleogeographic Modeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antonelli, A.; Zizka, A.; Carvalho, F.A.; Scharn, R.; Bacon, C.D.; Silvestro, D.; Condamine, F.L. Amazonia is the primary source of Neotropical biodiversity. Proc. Natl. Acad. Sci. USA 2018, 115, 6034–6039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, R.E.; Albert, J.S.; Di Dario, F.; Mincarone, M.M.; Petry, P.; Rocha, L.A. Fish biodiversity and conservation in South America. J. Fish Biol. 2016, 89, 12–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubert, N.; Renno, J.F. Historical biogeography of South American freshwater fishes. J. Biogeogr. 2006, 33, 1414–1436. [Google Scholar] [CrossRef]
- Hoorn, C.; Wesselingh, F.P.; ter Steege, H.; Bermudez, M.A.; Mora, A.; Sevink, J.; Sanmartin, I.; Sanchez-Meseguer, A.; Anderson, C.L.; Figueiredo, J.P.; et al. Amazonia Through Time: Andean Uplift, Climate Change, Landscape Evolution, and Biodiversity. Science 2010, 330, 927–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garzon-Orduna, I.J.; Benetti-Longhini, J.E.; Brower, A.V.Z. Timing the diversification of the Amazonian biota: Butterfly divergences are consistent with Pleistocene refugia. J. Biogeogr. 2014, 41, 1631–1638. [Google Scholar] [CrossRef]
- Rull, V. Neotropical diversification: Historical overview and conceptual insights. Peer J. Prepr. 2018. [Google Scholar] [CrossRef]
- Rull, V. Pleistocene speciation is not refuge speciation. J. Biogeogr. 2015, 42, 602–604. [Google Scholar] [CrossRef]
- Val, A.L.; Almeida-Val, V.M.F. The Amazon ichthyofauna. In Fishes of the Amazon and Their Environment; Springer: Berlin/Heidelberg, Germany, 1995; Volume 32, pp. 28–69. [Google Scholar]
- Queiroz, H.L.; Camargo, M. Biologia, Conservação e Manejo dos Aruanãs na Amazônia Brasileira; IDSM: Tefé, Brazil, 2008; 152p. [Google Scholar]
- Duponchelle, F.; Arce, A.R.; Waty, A.; Panfili, J.; Renno, J.-F.; Farfan, F.; Garcia-Vasquez, A.; Koo, F.C.; Davila, C.G.; Vargas, G. Contrasted hydrological systems of the Peruvian Amazon induce differences in growth patterns of the silver arowana, Osteoglossum bicirrhosum. Aquat. Living Resour. 2012, 25, 55–66. [Google Scholar] [CrossRef]
- Duponchelle, F.; Ruiz Arce, A.; Waty, A.; Garcia-Vasquez, A.; Renno, J.; Chu-Koo, F.; Garcia-Davila, C.; Vargas, G.; Tello, S.; Ortiz, A. Variations in reproductive strategy of the silver Arowana, Osteoglossum bicirrhosum Cuvier, 1829 from four sub-basins of the Peruvian Amazon. J. Appl. Ichthyol. 2015, 31, 19–30. [Google Scholar] [CrossRef]
- Leal, M.E.C.; Sant’Anna, V.B. Quantitative analysis of interspecific and ontogenetic variation in Osteoglossum species (Teleostei: Osteoglossiformes: Osteoglossidae). Zootaxa 2006, 1239, 49–68. [Google Scholar] [CrossRef]
- Saint-Paul, U.; Zuanon, J.; Correa, M.A.V.; García, M.; Fabré, N.N.; Berger, U.; Junk, W.J. Fish communities in central Amazonian white-and blackwater floodplains. Environ. Biol. Fishes 2000, 57, 235–250. [Google Scholar] [CrossRef]
- Duncan, W.P.; Fernandes, M.N. Physicochemical characterization of the white, black, and clearwater rivers of the Amazon Basin and its implications on the distribution of freshwater stingrays (Chondrichthyes, Potamotrygonidae). Panam. J. Aquat. Sci. 2010, 5, 454–464. [Google Scholar]
- Moreau, M.-A.; Coomes, O.T. Potential threat of the international aquarium fish trade to silver arawana Osteoglossum bicirrhosum in the Peruvian Amazon. Oryx 2006, 40, 152–160. [Google Scholar] [CrossRef] [Green Version]
- Amaral, E.S.R.; Arantes, C.C. A pesca de aruanãs na região de Tefé. In Biologia, Conservação e Manejo dos Aruanãs na Amazônia Brasileira; Queiroz, H.L., Camargo, M., Eds.; IDSM: Tefé, Brazil, 2008; pp. 61–74. [Google Scholar]
- Rocha, P.; Ramíres, P.F. Contribución a la Gestión Sostenible y al Conocimiento Biológico y Socio Económico de la Cadena de Valor de Peces Ornamentales de Puerto Carreño-Reserva de Biósfera el Tuparro (Vichada-Colombia); Fundación Omacha; Fundación Horizonte Verde: Bogotá, Colômbia, 2017. [Google Scholar]
- Moreau, M.-A.; Coomes, O.T. Aquarium fish exploitation in western Amazonia: Conservation issues in Peru. Environ. Conserv. 2007, 34, 12–22. [Google Scholar] [CrossRef]
- Reis, R.; Lima, F. Osteoglossum ferreirai. The IUCN Red List of Threatened Species 2009, 2009. Available online: https://www.iucnredlist.org/species/167687/6367885. [CrossRef]
- Mojica, J.I.; Usma, J.S.; Álvarez-León, R.; Lasso, C.A. Libro rojo de peces Dulceacuícolas de Colombia 2012. In Instituto de Investigación de Recursos Biológicos Alexander von Humboldt; Instituto de Ciencias Naturales de la Universidad Nacional de Colombia; WWF Colombia y Universidad de Manizales: Bogotá, Colômbia, 2012. [Google Scholar]
- Escobar L., M.D.; Farias, I.P.; Taphorn B., D.C.; Landines, M.; Hrbek, T. Molecular diagnosis of the arowanas Osteoglossum ferreirai Kanazawa, 1966 and O. bicirrhossum (Cuvier, 1829) from the Orinoco and Amazon River basins. Neotrop. Ichthyol. 2013, 11, 335–340. [Google Scholar]
- Olivares, A.M.; Hrbek, T.; Escobar, M.D.; Caballero, S. Population structure of the black arowana (Osteoglossum ferreirai) in Brazil and Colombia: Implications for its management. Conserv. Genet. 2013, 14, 695–703. [Google Scholar] [CrossRef]
- Ekblom, R.; Galindo, J. Applications of next generation sequencing in molecular ecology of non-model organisms. Heredity 2011, 107, 1. [Google Scholar] [CrossRef] [PubMed]
- Garrick, R.C.; Bonatelli, I.A.S.; Hyseni, C.; Morales, A.; Pelletier, T.A.; Perez, M.F.; Rice, E.; Satler, J.D.; Symula, R.E.; Thomé, M.T.C.; et al. The evolution of phylogeographic data sets. Mol. Ecol. 2015, 24, 1164–1171. [Google Scholar] [CrossRef] [Green Version]
- Barby, F.F.; Bertollo, L.A.C.; de Oliveira, E.A.; Yano, C.F.; Hatanaka, T.; Ráb, P.; Sember, A.; Ezaz, T.; Artoni, R.F.; Liehr, T.; et al. Emerging patterns of genome organization in Notopteridae species (Teleostei, Osteoglossiformes) as revealed by Zoo-FISH and Comparative Genomic Hybridization (CGH). Sci. Rep. 2019, 9, 1112. [Google Scholar] [CrossRef] [PubMed]
- Jaccoud, D.; Peng, K.; Feinstein, D.; Kilian, A. Diversity arrays: A solid state technology for sequence information independent genotyping. Nucleic Acids Res. 2001, 29, e25. [Google Scholar] [CrossRef]
- Kilian, A.; Wenzl, P.; Huttner, E.; Carling, J.; Xia, L.; Blois, H.; Caig, V.; Heller-Uszynska, K.; Jaccoud, D.; Hopper, C. Diversity arrays technology: A generic genome profiling technology on open platforms. In Data Production and Analysis in Population Genomics; Pompanon, F., Bonin, A., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 67–89. [Google Scholar]
- Cioffi, M.D.B.; Bertollo, L.A.C. Chromosomal distribution and evolution of repetitive DNAs in fish. In Genome Dynamics; v. 7; Garrido-Ramos, M.A., Ed.; Karger: Basel, Switzerland, 2012; pp. 197–221. [Google Scholar]
- Moraes, R.L.R.; Bertollo, L.A.C.; Marinho, M.M.F.; Yano, C.F.; Hatanaka, T.; Barby, F.F.; Troy, W.P.; Cioffi, M.B. Evolutionary relationships and cytotaxonomy considerations in the genus Pyrrhulina (Characiformes, Lebiasinidae). Zebrafish 2017, 14, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Sember, A.; Bertollo, L.A.C.; Ráb, P.; Yano, C.F.; Hatanaka, T.; de Oliveira, E.A.; Cioffi, M.D.B. Sex Chromosome Evolution and Genomic Divergence in the Fish Hoplias malabaricus (Characiformes, Erythrinidae). Front. Genet. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.A.; Sember, A.; Bertollo, L.A.C.; Yano, C.F.; Ezaz, T.; Moreira-Filho, O.; Hatanaka, T.; Trifonov, V.; Liehr, T.; Al-Rikabi, A.B.H.; et al. Tracking the evolutionary pathway of sex chromosomes among fishes: Characterizing the unique XX/XY1Y2 system in Hoplias malabaricus (Teleostei, Characiformes). Chromosoma 2018, 127, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Symonová, R.; Majtánová, Z.; Sember, A.; Staaks, G.B.O.; Bohlen, J.; Freyhof, J. Genome differentiation in a species pair of coregonine fishes: An extremely rapid speciation driven by stress—Activated retrotransposons mediating extensive ribosomal DNA multiplications. BMC Evol. Biol. 2013, 13, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Bertollo, L.A.C.; Cioffi, M.B.; Moreira-Filho, O. Direct chromosome preparation from Freshwater Teleost Fishes. In Fish Cytogenetic Techniques (Chondrichthyans and Teleosts); Ozouf-Costaz, C., Pisano, E., Foresti, F., Almeida Toledo, L.F., Eds.; CRC Press: Enfield, CT, USA, 2015; pp. 21–26. [Google Scholar]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Zwick, M.S.; Hanson, R.E.; Mcknight, T.D.; Islam-Faridi, M.H.; Stelly, D.M.; Wing, R.A.; Price, H.J. A rapid procedure for the isolation of C 0 t-1 DNA from plants. Genome 1997, 40, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Symonová, R.; Sember, A.; Majtánová, Z.; Ráb, P. Characterization of fish genomes by GISH and CGH. In Fish Cytogenet. Tech. Ray-Fin Fishes Chondrichthyans; CCR Press: Boca Raton, FL, USA, 2015; pp. 118–131. [Google Scholar]
- Akbari, M.; Wenzl, P.; Caig, V.; Carling, J.; Xia, L.; Yang, S.; Uszynski, G.; Mohler, V.; Lehmensiek, A.; Kuchel, H. Diversity arrays technology (DArT) for high-throughput profiling of the hexaploid wheat genome. Theor. Appl. Genet. 2006, 113, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Wenzl, P.; Li, H.; Carling, J.; Zhou, M.; Raman, H.; Paul, E.; Hearnden, P.; Maier, C.; Xia, L.; Caig, V.; et al. A high-density consensus map of barley linking DArT markers to SSR, RFLP and STS loci and agricultural traits. BMC Genom. 2006, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.A.R.; Overcast, I. ipyrad v. 0.7.28 2017. Available online: https://github.com/dereneaton/ipyrad (accessed on 12 March 2019).
- Foll, M.; Gaggiotti, O. A genome-scan method to identify selected loci appropriate for both dominant and codominant markers: A Bayesian perspective. Genetics 2008, 180, 977–993. [Google Scholar] [CrossRef]
- Meirmans, P.G.; van Tienderen, P.H. genotype and genodive: Two programs for the analysis of genetic diversity of asexual organisms. Mol. Ecol. Notes 2004, 4, 792–794. [Google Scholar] [CrossRef]
- Gruber, B.; Georges, A.; Berry, O.; Unmack, P. dartR: Importing and Analysing SNP and Silicodart Data Generated by Genome-Wide Restriction Fragment Analysis, R package version 1.0.5.; The R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Raj, A.; Stephens, M.; Pritchard, J.K. fastSTRUCTURE: Variational Inference of Population Structure in Large SNP Data Sets. Genetics 2014, 197, 573–589. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [PubMed]
- Melville, J.; Melville, J.; Haines, M.L.; Boysen, K.; Hodkinson, L.; Kilian, A.; Date, K.L.S.; Potvin, D.A.; Parris, K.M. Identifying hybridization and admixture using SNPs: Application of the DArTseq platform in phylogeographic research on vertebrates. R. Soc. Open Sci. 2017, 4, 161061. [Google Scholar] [CrossRef] [PubMed]
- Guillot, G.; Santos, F.; Estoup, A. Population Genetics Analysis Using R and Geneland; Technical University of Denmark: Kongens Lyngby, Denmark, 2009. [Google Scholar]
- Perez, M.F.; Franco, F.F.; Bombonato, J.R.; Bonatelli, I.A.S.; Khan, G.; Romeiro-Brito, M.; Fegies, A.C.; Ribeiro, P.M.; Silva, G.A.R.; Moraes, E.M. Assessing population structure in the face of isolation by distance: Are we neglecting the problem? Divers. Distrib. 2018, 24, 1883–1889. [Google Scholar] [CrossRef] [Green Version]
- Earl, D.A. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Jakobsson, M.; Rosenberg, N.A. CLUMPP: A cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 2007, 23, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, N.M.; Mayzel, J.; Jakobsson, M.; Rosenberg, N.A.; Mayrose, I. Clumpak: A program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 2015, 15, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Hudson, R.R. Generating samples under a Wright-Fisher neutral model of genetic variation. Bioinformatics 2002, 18, 337–338. [Google Scholar] [CrossRef]
- Oliveira, E.A. Evolução Cromossômica na Família Arapaimidae (Teleostei: Osteoglossiformes): Uma Abordagem Populacional e Intercontinental. Ph.D. Thesis, Universidade Federal de São Carlos, São Carlos, Brazil, 2019. [Google Scholar]
- Perez, M.F.; Bonatelli, I.A.S.; Moraes, E.M.; Carstens, B.C. Model-based analysis supports interglacial refugia over long-dispersal events in the diversification of two South American cactus species. Heredity 2016, 116, 550–557. [Google Scholar] [CrossRef]
- Flagel, L.; Brandvain, Y.; Schrider, D.R. The Unreasonable Effectiveness of Convolutional Neural Networks in Population Genetic Inference. Mol. Biol. Evol. 2019, 36, 220–238. [Google Scholar] [CrossRef]
- Verba, J.T.; Rabello Neto, J.G.; Zuanon, J.; Farias, I. Evidence of multiple paternity and cooperative parental care in the so called monogamous silver arowana Osteoglossum bicirrhosum (Osteoglossiformes: Osteoglossidae). Neotrop. Ichthyol. 2014, 12, 145–151. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Ripley, B.D. Pattern recognition and neural networks. In Pattern Recognition and Neural Networks; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Buja, A. Flexible discriminant analysis by optimal scoring. J. Am. Stat. Assoc. 1994, 89, 1255–1270. [Google Scholar] [CrossRef]
- Friedman, J. Multivariate adaptive regression splines (with discussion). Ann. Stat. 1991, 19, 1–67. [Google Scholar] [CrossRef]
- Busby, J.R. BIOCLIM—A bioclimate analysis and prediction system. Plant Prot. Q. 1991, 6, 8–9. [Google Scholar]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.I. Classification and Regression Trees; Chapman and Hall: Wadsworth, NY, USA, 1984. [Google Scholar]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; Series: Chapman & Hall/CRC Monographs on Statistics and Applied Probability; Chapman and Hall/CRC Press: London, UK, 1989. [Google Scholar]
- Ridgeway, G. The State of Boosting. Comput. Sci. Stat. 1999, 31, 172–181. [Google Scholar]
- Breiman, L. Randomforest. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G. WorldClim interpolated global terrestrial climate surfaces. Int. J. Climatol. 2004. [Google Scholar] [CrossRef]
- Suzuki, A.; Taki, Y.; Urushido, T. Karyotypes of two species of arowana, Osteoglossum bicirrhosum and O. ferreirai. Jpn. J. Ichthyol. 1982, 29, 220–222. [Google Scholar]
- Gatti, R.; Atum, Y.; Schiaffino, L.; Jochumsen, M.; Manresa, J.B. Convolutional Neural Networks Improve the Prediction of Hand Movement Speed and Force from Single-trial EEG. bioRxiv 2019, 492660. [Google Scholar] [CrossRef]
- da Silva, T.; Hrbek, T.; Farias, I.P. Microsatellite markers for the silver arowana (Osteoglossum bicirrhosum, Osteoglossidae, Osteoglossiformes). Mol. Ecol. Resour. 2009, 9, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Cooke, G.M.; Chao, N.L.; Beheregaray, L.B. Natural selection in the water: Freshwater invasion and adaptation by water colour in the Amazonian pufferfish. J. Evol. Biol. 2012, 25, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
- Barby, F.; Rab, P.; Lavoue, S.; Ezaz, T.; Bertollo, L.A.C.; Kilian, A.; Maruyama, S.R.; Oliveira, E.A.; Artoni, R.F.; Santos, M.H.; et al. From chromosomes to genome: Insights into the evolutionary relationships and biogeography of Old World knifefishes (Notopteridae; Osteoglossiformes). Genes 2018, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.H.; Ong, D.; Wong, C.C.; Lim, L.C.; Orban, L. A strain-specific and a sex-associated STS marker for Asian arowana (Scleropages formosus, Osteoglossidae). Aquac. Res. 2003, 34, 951–957. [Google Scholar] [CrossRef]
- Landguth, E.L.; Fedy, B.C.; Oyler-McCance, S.J.; Garey, A.L.; Emel, S.L.; Mumma, M.; Wagner, H.H.; Fortin, M.; Cushman, S.A. Effects of sample size, number of markers, and allelic richness on the detection of spatial genetic pattern. Mol. Ecol. Res. 2012, 12, 276–284. [Google Scholar] [CrossRef]
- Bagley, J.C.; Sandel, M.; Travis, J.; de Lourdes Lozano-Vilano, M.; Johnson, J.B. Paleoclimatic modeling and phylogeography of least killifish, Heterandria formosa: Insights into Pleistocene expansion-contraction dynamics and evolutionary history of North American Coastal Plain freshwater biota. BMC Evol. Biol. 2013, 13, 223. [Google Scholar] [CrossRef] [PubMed]
- McMahan, C.D.; Ginger, L.; Cage, M.; David, K.T.; Chakrabarty, P.; Johnston, M.; Matamoros, W.A. Pleistocene to holocene expansion of the black-belt cichlid in Central America, Vieja maculicauda (Teleostei: Cichlidae). PLoS ONE 2017, 12, e0178439. [Google Scholar] [CrossRef] [PubMed]
- Oberdorff, T.; Jézéquel, C.; Campero, M.; Carvajal-Vallejos, F.; Cornu, J.F.; Dias, M.S.; Tedesco, P.A. Opinion Paper: How vulnerable are Amazonian freshwater fishes to ongoing climate change? J. App. Icht. 2015, 31, 4–9. [Google Scholar] [CrossRef]
- Rossetti, D.F.; Valeriano, M.M. Evolution of the lowest amazon basin modeled from the integration of geological and SRTM topographic data. Catena 2007, 70, 253–265. [Google Scholar] [CrossRef] [Green Version]
- Vitorino, C.A.; Nogueira, F.; Souza, I.L.; Araripe, J.; Venere, P.C. Low genetic diversity and structuring of the arapaima (Osteoglossiformes, Arapaimidae) population of the Araguaia-Tocantins basin. Front. Genet. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Torati, L.S.; Taggart, J.B.; Varela, E.S.; Araripe, J.; Wehner, S.; Migaud, H. Genetic diversity and structure in Arapaima gigas populations from Amazon and Araguaia-Tocantins river basins. BMC Genet. 2019, 20, 13. [Google Scholar] [CrossRef] [PubMed]





| Species | Sampling Site | River Basin | DArT_N | Cito_N |
|---|---|---|---|---|
| Osteoglossum bicirrhosum | Javaé River (Loroti, TO) | Tocantins-Araguaia | 5 | (06♀ 08 ♂) |
| Osteoglossum bicirrhosum | Xavantinho River (Luciara, MT) | Tocantins-Araguaia | 6 | (06♀ 05 ♂) |
| Osteoglossum bicirrhosum | Solimões River (Catalão, AM) | Amazon | 2 | (10♀ 09♂) |
| Osteoglossum ferreirai | Negro River (Tarumã, AM) | Amazon | 20 | (11♀ 10 ♂) |
| Locality | Species | Basin | A | HO | HE | GIS |
|---|---|---|---|---|---|---|
| Luciara | O. bicirrhosum | TAb | 1.202 | 0.121 | 0.137 | 0.117 |
| Loroti | O. bicirrhosum | TAb | 1.211 | 0.128 | 0.145 | 0.123 |
| Catalão | O. bicirrhosum | AMb | 1.433 | 0.275 | 0.358 | 0.232 |
| Tarumã | O. ferreirai | AMb | 1.235 | 0.112 | 0.176 | 0.364 |
| Parameter | RMSE | Spearman’s ρ | Median | Interval |
|---|---|---|---|---|
| Ne | 0.4971 | 0.6409 | 468,195.3679 | 452,302.7–481,932.6 |
| DT | 0.4816 | 0.6571 | 1,049,955.4491 | 1,013,20–1,074,667.3 |
| θrF-A | 0.6539 | 0.5670 | 0.0551 | 0.0538–0.0561 |
| θrC-A | 0.6454 | 0.5702 | 0.5470 | 0.5337–0.5596 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, F.H.S.d.; Perez, M.F.; Bertollo, L.A.C.; Oliveira, E.A.d.; Lavoué, S.; Gestich, C.C.; Ráb, P.; Ezaz, T.; Liehr, T.; Viana, P.F.; et al. Interspecific Genetic Differences and Historical Demography in South American Arowanas (Osteoglossiformes, Osteoglossidae, Osteoglossum). Genes 2019, 10, 693. https://doi.org/10.3390/genes10090693
Souza FHSd, Perez MF, Bertollo LAC, Oliveira EAd, Lavoué S, Gestich CC, Ráb P, Ezaz T, Liehr T, Viana PF, et al. Interspecific Genetic Differences and Historical Demography in South American Arowanas (Osteoglossiformes, Osteoglossidae, Osteoglossum). Genes. 2019; 10(9):693. https://doi.org/10.3390/genes10090693
Chicago/Turabian StyleSouza, Fernando Henrique Santos de, Manolo Fernandez Perez, Luiz Antônio Carlos Bertollo, Ezequiel Aguiar de Oliveira, Sebastien Lavoué, Carla Cristina Gestich, Petr Ráb, Tariq Ezaz, Thomas Liehr, Patrik Ferreira Viana, and et al. 2019. "Interspecific Genetic Differences and Historical Demography in South American Arowanas (Osteoglossiformes, Osteoglossidae, Osteoglossum)" Genes 10, no. 9: 693. https://doi.org/10.3390/genes10090693







