Novel Molecular Synapomorphies Demarcate Different Main Groups/Subgroups of Plasmodium and Piroplasmida Species Clarifying Their Evolutionary Relationships
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of Phylogenetic Trees
2.2. Identification of Conserved Signature Indels (CSIs)
2.3. Homology Modelling and Analysis of Protein Structures
3. Results
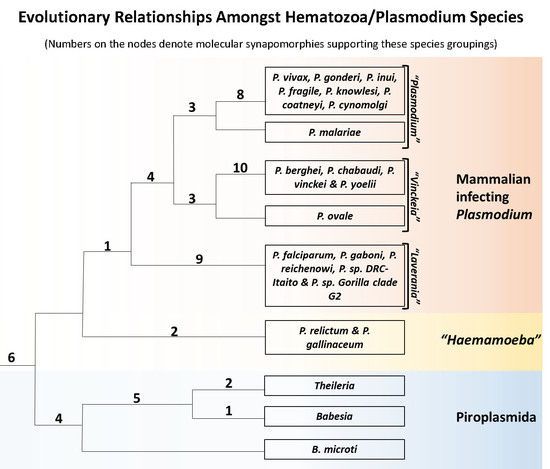
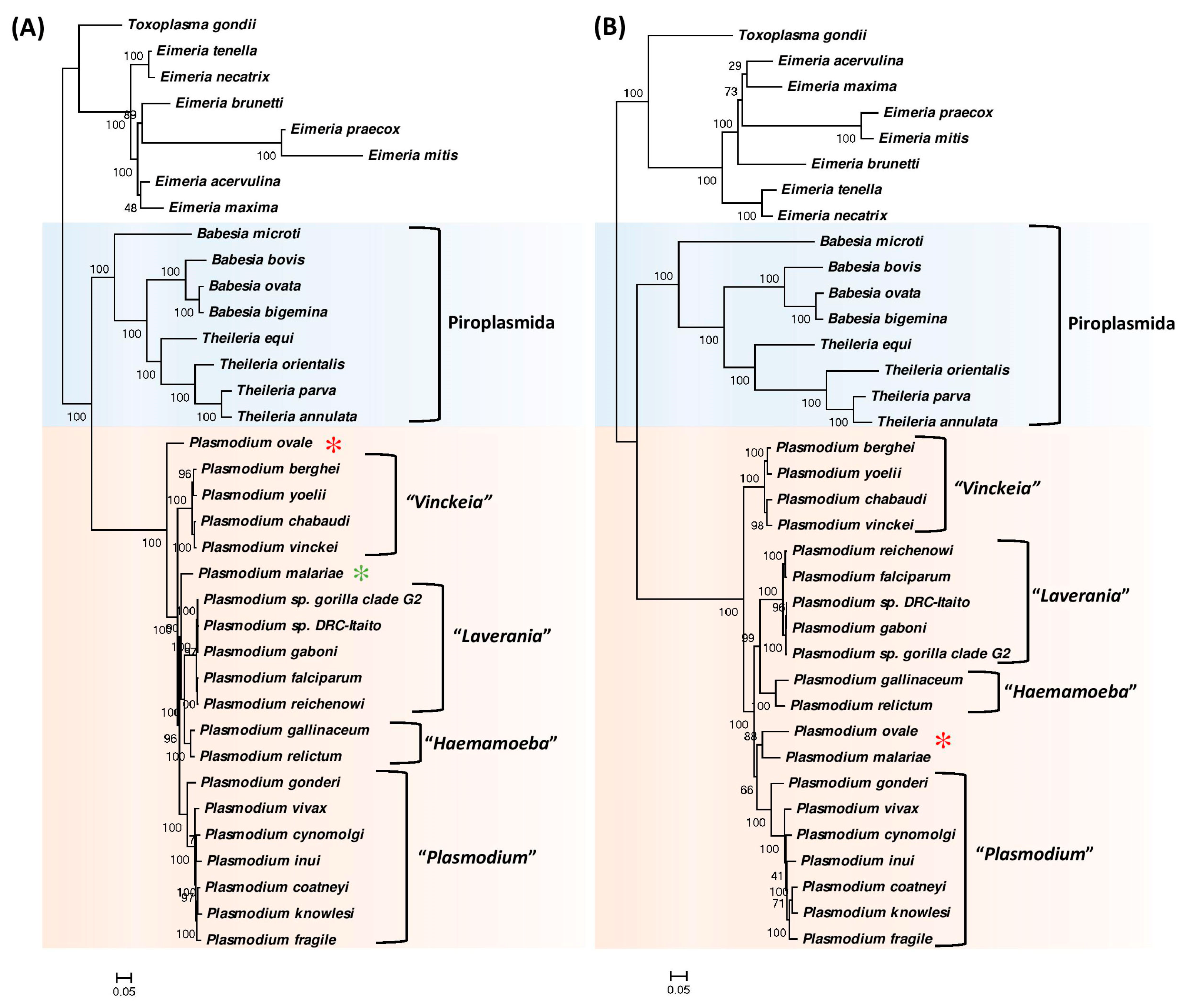
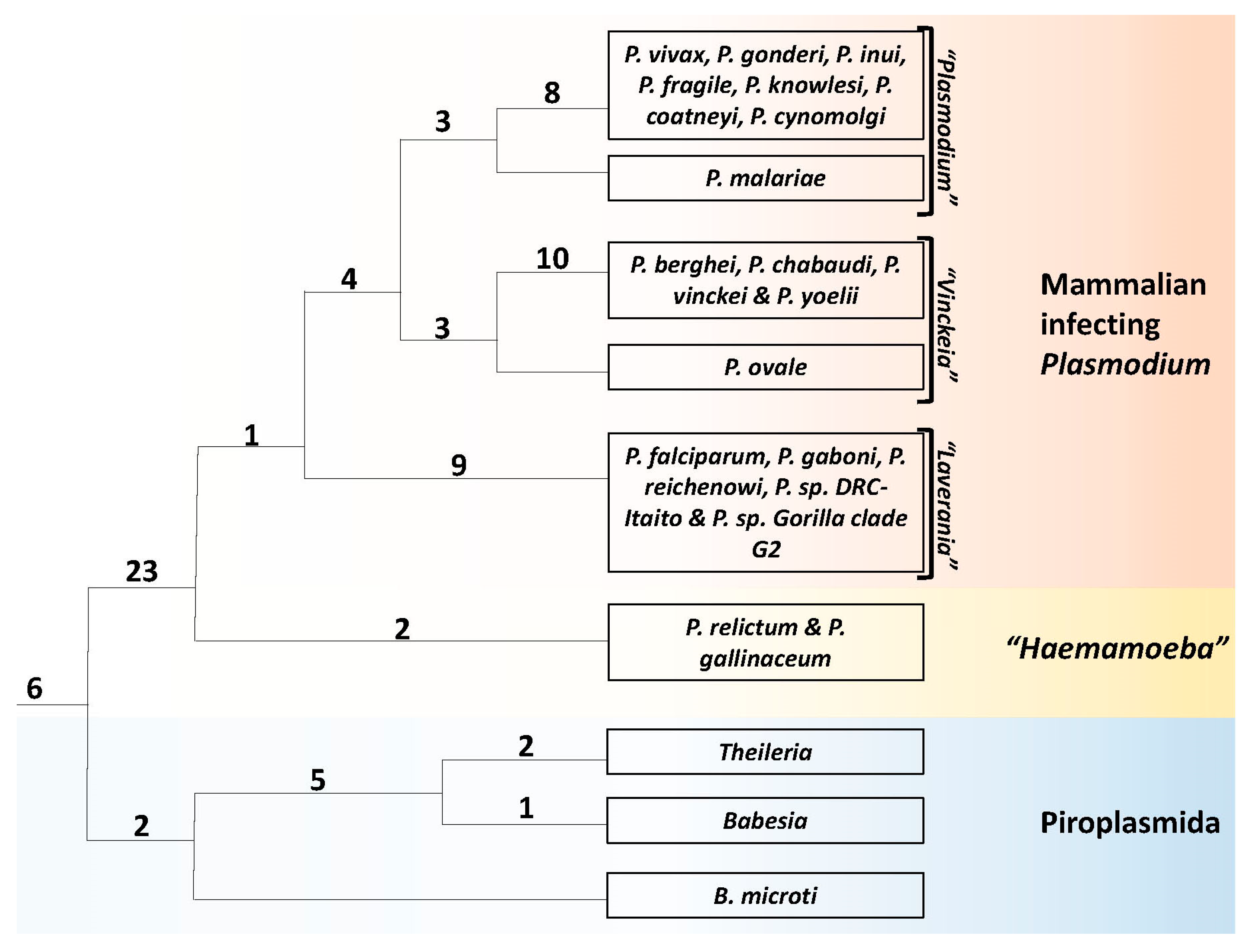
3.1. Phylogenetic Analysis of Hematozoa
3.2. Identification of Molecular Markers Specific for Hematozoa and its Main Groups
3.3. Molecular Markers Specific for the Class Hematozoa
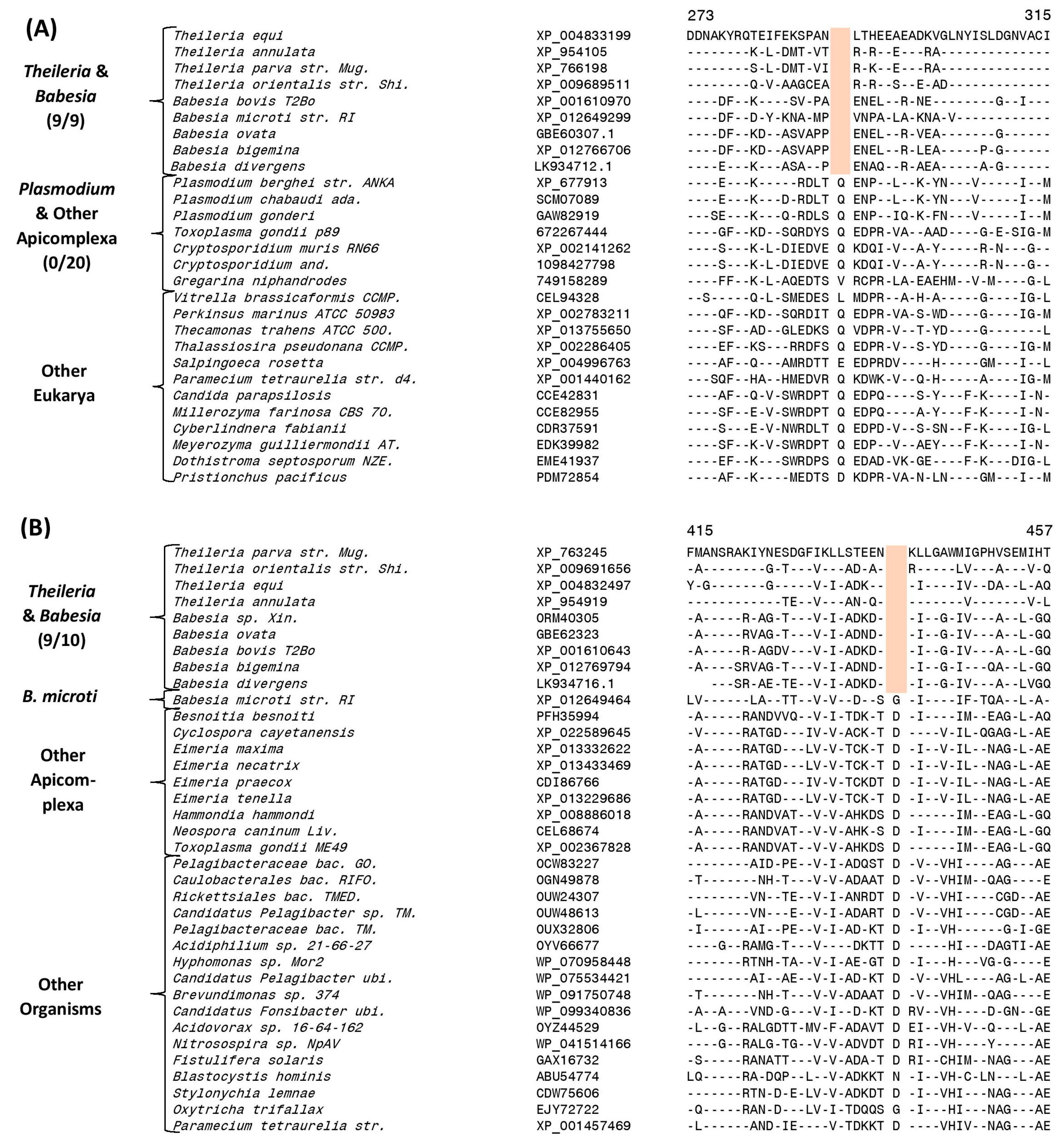
3.4. Molecular Signatures Specific for the Order Piroplasmida
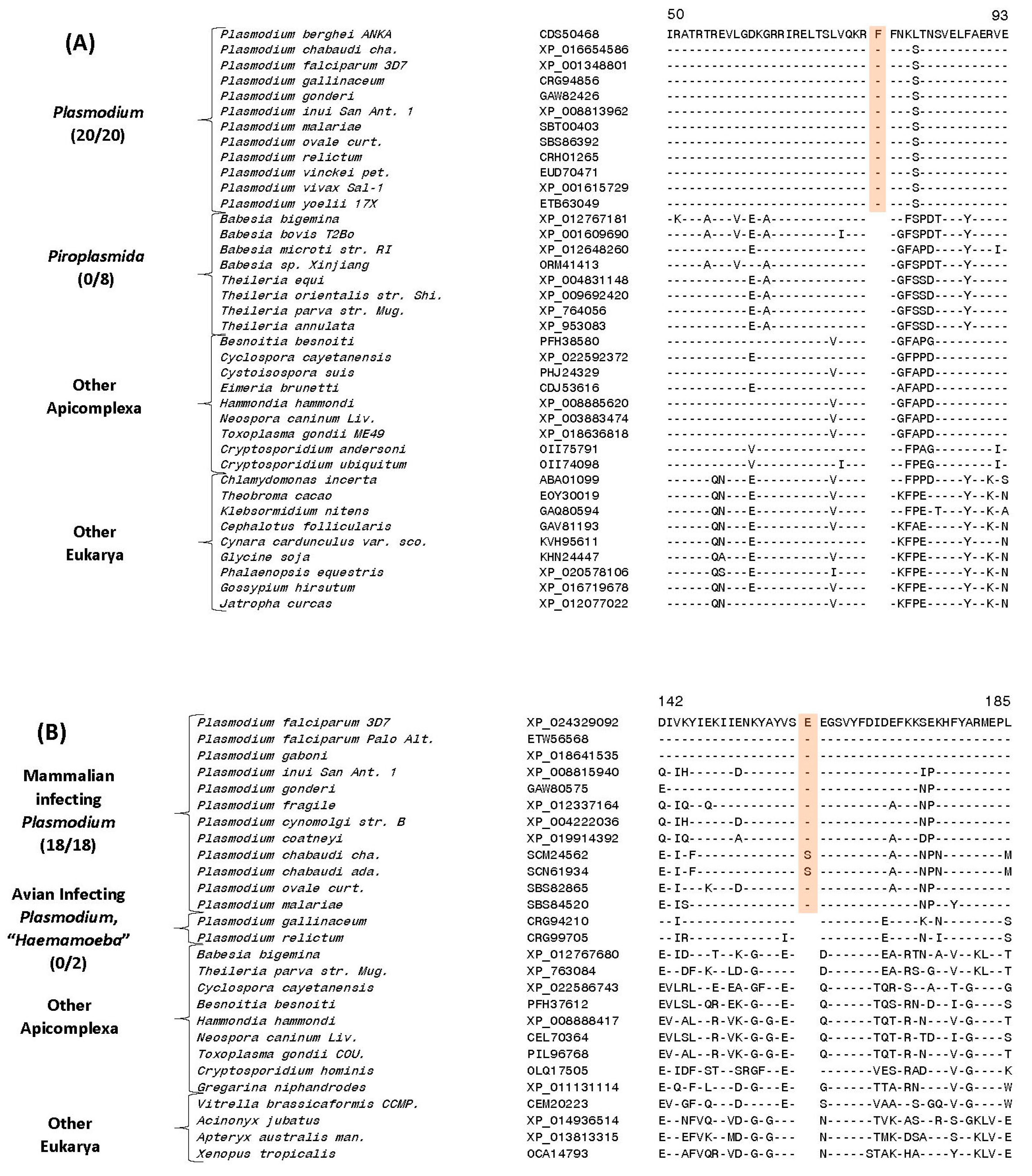
3.5. Molecular Signatures Specific for the Genus Plasmodium and Its Subgenera
3.6. Molecular Signatures for the Mammalian-Infecting Plasmodium Species Clarifying their Evolutionary Relationships
3.7. Localizations of the CSIs in Protein Structures
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Telford, S.R. The Hemoparasites of the Reptilia; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Garnham, P.C. Malaria Parasites and Other Haemosporidia; Blackwell Scientific Publications: Oxford, UK, 1966. [Google Scholar]
- Perkins, S.L. Malaria’s many mates: Past, present, and future of the systematics of the order Haemosporida. J. Parasitol. 2014, 100, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Votypka, J.; Modry, D.; ObornÃ-k, M.; Slapeta, J.; LukeÅ, J. Apicomplexa. In Handbook of the Protists; Archibald, J., Simpson, A., Slamovits, C., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–58. [Google Scholar]
- Allsopp, M.T.; Allsopp, B.A. Molecular sequence evidence for the reclassification of some Babesia species. Ann. NY Acad. Sci. 2006, 1081, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Homer, M.J.; Aguilar-Delfin, I.; Telford, S.R.; Krause, P.J.; Persing, D.H. Babesiosis. Clin. Microbiol. Rev. 2000, 13, 451–469. [Google Scholar] [CrossRef] [PubMed]
- Kamau, J.; de Vos, A.J.; Playford, M.; Salim, B.; Kinyanjui, P.; Sugimoto, C. Emergence of new types of Theileria orientalis in Australian cattle and possible cause of theileriosis outbreaks. Parasit. Vectors 2011, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Lack, J.B.; Reichard, M.V.; Van Den Bussche, R.A. Phylogeny and evolution of the Piroplasmida as inferred from 18S rRNA sequences. Int. J. Parasitol. 2012, 42, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Wenyon, C.M. Protozoology: A Manual for Medical Men, Veterinarians and Zoologists; W. Wood: New York, NY, USA, 1926. [Google Scholar]
- Schreeg, M.E.; Marr, H.S.; Tarigo, J.L.; Cohn, L.A.; Bird, D.M.; Scholl, E.H.; Levy, M.G.; Wiegmann, B.M.; Birkenheuer, A.J. Mitochondrial Genome Sequences and Structures Aid in the Resolution of Piroplasmida phylogeny. PLoS ONE 2016, 11, e0165702. [Google Scholar] [CrossRef]
- Valkiunas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Arisue, N.; Hashimoto, T. Phylogeny and evolution of apicoplasts and apicomplexan parasites. Parasitol. Int. 2015, 64, 254–259. [Google Scholar] [CrossRef]
- Hay, S.I.; Okiro, E.A.; Gething, P.W.; Patil, A.P.; Tatem, A.J.; Guerra, C.A.; Snow, R.W. Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med. 2010, 7. [Google Scholar] [CrossRef]
- Sachs, J.; Malaney, P. The economic and social burden of malaria. Nature 2002, 415, 680–685. [Google Scholar] [CrossRef]
- Martinsen, E.S.; Paperna, I.; Schall, J.J. Morphological versus molecular identification of avian Haemosporidia: An exploration of three species concepts. Parasitology 2006, 133, 279–288. [Google Scholar] [CrossRef]
- Martinsen, E.S.; Perkins, S.L.; Schall, J.J. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): Evolution of life-history traits and host switches. Mol. Phylogenet. Evol. 2008, 47, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Telford, S.R.; Johnson, R.N.; Young, D.G. Additional Plasmodium species from Anolis lizards of Hispaniola and Panama. Int. J. Parasitol. 1989, 19, 275–284. [Google Scholar] [CrossRef]
- Perkins, S.L.; Martinsen, E.S.; Falk, B.G. Do molecules matter more than morphology? Promises and pitfalls in parasites. Parasitology 2011, 138, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Borner, J.; Pick, C.; Thiede, J.; Kolawole, O.M.; Kingsley, M.T.; Schulze, J.; Cottontail, V.M.; Wellinghausen, N.; Schmidt-Chanasit, J.; Bruchhaus, I.; et al. Phylogeny of haemosporidian blood parasites revealed by a multi-gene approach. Mol. Phylogenet. Evol. 2016, 94, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Perkins, S.L.; Sarkar, I.N.; Carter, R. The phylogeny of rodent malaria parasites: Simultaneous analysis across three genomes. Infect. Genet. Evol. 2007, 7, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Honma, H.; Kawai, S.; Motooka, D.; Nakamura, S.; Tougan, T.; Horii, T.; Arisue, N. Draft Genome Sequence of Plasmodium gonderi, a Malaria Parasite of African Old World Monkeys. Genome Announc. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, S.; Sullivan, S.A.; Kawai, S.; Nakamura, S.; Kim, H.R.; Goto, N.; Arisue, N.; Palacpac, N.M.; Honma, H.; Yagi, M.; et al. Plasmodium cynomolgi genome sequences provide insight into Plasmodium vivax and the monkey malaria clade. Nat. Genet. 2012, 44, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Perkins, S.L.; Schall, J.J. A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. J. Parasitol. 2002, 88, 972–978. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; Fallon, S.M.; Bermingham, E. Evolutionary relationships, cospeciation, and host switching in avian malaria parasites. Syst. Biol. 2004, 53, 111–119. [Google Scholar] [CrossRef]
- Dimitrov, D.; Zehtindjiev, P.; Bensch, S. Genetic diversity of avian blood parasites in SE Europe: Cytochrome b lineages of the genera Plasmodium and Haemoproteus (Haemosporida) from Bulgaria. Acta Parasitol. 2010, 55, 201–209. [Google Scholar] [CrossRef]
- Bensch, S.; Hellgren, O.; Perez-Tris, J. MalAvi: A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Arisue, N.; Hashimoto, T.; Mitsui, H.; Palacpac, N.M.; Kaneko, A.; Kawai, S.; Hasegawa, M.; Tanabe, K.; Horii, T. The Plasmodium apicoplast genome: Conserved structure and close relationship of P. ovale to rodent malaria parasites. Mol. Biol. Evol. 2012, 29, 2095–2099. [Google Scholar] [CrossRef] [PubMed]
- Ansari, H.R.; Templeton, T.J.; Subudhi, A.K.; Ramaprasad, A.; Tang, J.; Lu, F.; Naeem, R.; Hashish, Y.; Oguike, M.C.; Benavente, E.D.; et al. Genome-scale comparison of expanded gene families in Plasmodium ovale wallikeri and Plasmodium ovale curtisi with Plasmodium malariae and with other Plasmodium species. Int. J. Parasitol. 2016, 46, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, G.G.; Bohme, U.; Sanders, M.; Reid, A.J.; Cotton, J.A.; Maiga-Ascofare, O.; Djimde, A.A.; Apinjoh, T.O.; Amenga-Etego, L.; Manske, M.; et al. Plasmodium malariae and P. ovale genomes provide insights into malaria parasite evolution. Nature 2017, 542, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Barta, J.R.; Ogedengbe, J.D.; Martin, D.S.; Smith, T.G. Phylogenetic position of the adeleorinid coccidia (Myzozoa, Apicomplexa, Coccidia, Eucoccidiorida, Adeleorina) inferred using 18S rDNA sequences. J. Eukaryot. Microbiol. 2012, 59, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J.; Kooij, T.W. Phylogenetic profiles of all membrane transport proteins of the malaria parasite highlight new drug targets. Microb. Cell 2016, 3, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.A.; Ellis, J.T. Effects of nucleotide sequence alignment on phylogeny estimation: A case study of 18S rDNAs of apicomplexa. Mol. Biol. Evol. 1997, 14, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, R.; Tsuji, M.; Oda, K.; Zamoto-Niikura, A.; Wei, Q.; Kawabuchi-Kurata, T.; Nishida, A.; Ishihara, C. Babesia microti-group parasites compared phylogenetically by complete sequencing of the CCTeta gene in 36 isolates. J. Vet. Med. Sci. 2009, 71, 55–68. [Google Scholar] [CrossRef]
- Corradetti, A.; Garnham, P.C.C.; Laird, M. New Classification of the Avian Malaria Parasites. Parassitologia 1963, 5, 1–4. [Google Scholar]
- Bray, R.S. Studies on malaria in chimpanzees. VI. Laverania falciparum. Am. J. Trop. Med. Hyg. 1958, 7, 20–24. [Google Scholar] [CrossRef]
- Garnham, P.C. The Subgenera of Plasmodium in mammals. Ann. Soc. Belges. Med. Trop. Parasitol. Mycol. 1964, 44, 267–271. [Google Scholar] [PubMed]
- Galen, S.C.; Borner, J.; Martinsen, E.S.; Schaer, J.; Austin, C.C.; West, C.J.; Perkins, S.L. The polyphyly of Plasmodium: Comprehensive phylogenetic analyses of the malaria parasites (order Haemosporida) reveal widespread taxonomic conflict. R. Soc. Open. Sci. 2018, 5, 171780. [Google Scholar] [CrossRef] [PubMed]
- Pick, C.; Ebersberger, I.; Spielmann, T.; Bruchhaus, I.; Burmester, T. Phylogenomic analyses of malaria parasites and evolution of their exported proteins. BMC Evol. Biol. 2011, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.P.; Higgins, D.G.; McCutchan, T.F. Plasmodium falciparum appears to have arisen as a result of lateral transfer between avian and human hosts. Proc. Natl. Acad. Sci. USA 1991, 88, 3140–3144. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S. Molecular signatures that are distinctive characteristics of the vertebrates and chordates and supporting a grouping of vertebrates with the tunicates. Mol. Phylogenet. Evol. 2016, 94, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Springer, M.S.; Stanhope, M.J.; Madsen, O.; de Jong, W.W. Molecules consolidate the placental mammal tree. Trends Ecol. Evol. 2004, 19, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.C.; Lake, J.A. Evidence that eukaryotes and eocyte prokaryotes are immediate relatives. Science 1992, 257, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Baldauf, S.L.; Palmer, J.D. Animals and fungi are each other’s closest relatives: Congruent evidence from multiple proteins. Proc. Natl. Acad. Sci. USA 1993, 90, 11558–11562. [Google Scholar] [CrossRef]
- Gupta, R.S. Impact of genomics on the understanding of microbial evolution and classification: The importance of Darwin’s views on classification. FEMS Microbiol. Rev. 2016, 40, 520–553. [Google Scholar] [CrossRef]
- Gupta, R.S. Protein phylogenies and signature sequences: A reappraisal of evolutionary relationships among archaebacteria, eubacteria, and eukaryotes. Microbiol. Mol. Biol. Rev. 1998, 62, 1435–1491. [Google Scholar]
- Gupta, R.S.; Lo, B.; Son, J. Phylogenomics and Comparative Genomic Studies Robustly Support Division of the Genus Mycobacterium into an Emended Genus Mycobacterium and Four Novel Genera. Front. Microbiol. 2018, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S. Identification of Conserved Indels that are Useful for Classification and Evolutionary Studies. In Methods in Microbiology, New Approaches to Prokaryotic Systematics; Goodfellow, M., Sutcliffe, I., Chun, J., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 153–182. [Google Scholar]
- Bapteste, E.; Philippe, H. The potential value of indels as phylogenetic markers: Position of trichomonads as a case study. Mol. Biol. Evol. 2002, 19, 972–977. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naushad, H.S.; Lee, B.; Gupta, R.S. Conserved signature indels and signature proteins as novel tools for understanding microbial phylogeny and systematics: Identification of molecular signatures that are specific for the phytopathogenic genera Dickeya, Pectobacterium and Brenneria. Int. J. Syst. Evol. Microbiol. 2014, 64, 366–383. [Google Scholar] [CrossRef] [PubMed]
- Rokas, A.; Holland, P.W. Rare genomic changes as a tool for phylogenetics. Trends Ecol. Evol. 2000, 15, 454–459. [Google Scholar] [CrossRef]
- Bhandari, V.; Naushad, H.S.; Gupta, R.S. Protein based molecular markers provide reliable means to understand prokaryotic phylogeny and support Darwinian mode of evolution. Front. Cell. Infect. Microbiol. 2012, 2, 98. [Google Scholar] [CrossRef]
- Adeolu, M.; Alnajar, S.; Naushad, S.; Gupta, S. Genome-based phylogeny and taxonomy of the ’Enterobacteriales’: Proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5575–5599. [Google Scholar]
- Capella-Gutierrez, S.; Silla-Martinez, J.M.; Gabaldon, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Price, A.L.; Kryukov, G.V.; de Bakker, P.I.; Purcell, S.M.; Staples, J.; Wei, L.J.; Sunyaev, S.R. Pooled association tests for rare variants in exon-resequencing studies. Am. J. Hum. Genet. 2010, 86, 832–838. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Soding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Q.; Zheng, L.; Ji, Y.; Xie, Y.T.; Lai, D.H.; Lun, Z.R.; Suo, X.; Gao, N. Cryo-EM structures of the 80S ribosomes from human parasites Trichomonas vaginalis and Toxoplasma gondii. Cell Res. 2017, 27, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Drinkwater, N.; Vinh, N.B.; Mistry, S.N.; Bamert, R.S.; Ruggeri, C.; Holleran, J.P.; Loganathan, S.; Paiardini, A.; Charman, S.A.; Powell, A.K.; et al. Potent dual inhibitors of Plasmodium falciparum M1 and M17 aminopeptidases through optimization of S1 pocket interactions. Eur. J. Med. Chem. 2016, 110, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Eswar, N.; Webb, B.; Marti-Renom, M.A.; Madhusudhan, M.S.; Eramian, D.; Shen, M.Y.; Pieper, U.; Sali, A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Protein Sci. 2007, 50, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Nanda, A.; Khadka, B. Novel molecular, structural and evolutionary characteristics of the phosphoketolases from bifidobacteria and Coriobacteriales. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Kedzierski, L.; Escalante, A.A.; Isea, R.; Black, C.G.; Barnwell, J.W.; Coppel, R.L. Phylogenetic analysis of the genus Plasmodium based on the gene encoding adenylosuccinate lyase. Infect. Genet. Evol. 2002, 1, 297–301. [Google Scholar] [CrossRef]
- Escalante, A.A.; Barrio, E.; Ayala, F.J. Evolutionary origin of human and primate malarias: Evidence from the circumsporozoite protein gene. Mol. Biol. Evol. 1995, 12, 616–626. [Google Scholar] [PubMed]
- Imamura, S.; Yabu, T.; Yamashita, M. Protective role of cell division cycle 48 (CDC48) protein against neurodegeneration via ubiquitin-proteasome system dysfunction during zebrafish development. J. Biol. Chem. 2012, 287, 23047–23056. [Google Scholar] [CrossRef] [PubMed]
- McCutchan, T.F.; Kissinger, J.C.; Touray, M.G.; Rogers, M.L.; Li, J.; Sullivan, M.; Braga, E.M.; Krettli, A.U.; Miller, L.H. Comparison of circumsporozoite proteins from avian and mammalian malarias: Biological and phylogenetic implications. Proc. Natl. Acad. Sci. USA 1996, 93, 11889–11894. [Google Scholar] [CrossRef]
- Khadka, B.; Gupta, R.S. Identification of a conserved 8 aa insert in the PIP5K protein in the Saccharomycetaceae family of fungi and the molecular dynamics simulations and structural analysis to investigate its potential functional role. Proteins 2017, 85, 1454–1467. [Google Scholar] [CrossRef]
- Akiva, E.; Itzhaki, Z.; Margalit, H. Built-in loops allow versatility in domain-domain interactions: Lessons from self-interacting domains. Proc. Natl. Acad. Sci. USA 2008, 105, 13292–13297. [Google Scholar] [CrossRef]
- Hashimoto, K.; Panchenko, A.R. Mechanisms of protein oligomerization, the critical role of insertions and deletions in maintaining different oligomeric states. Proc. Natl. Acad. Sci. USA 2010, 107, 20352–20357. [Google Scholar] [CrossRef] [PubMed]
- Alonso, P.; Noor, A.M. The global fight against malaria is at crossroads. Lancet 2017, 390, 2532–2534. [Google Scholar] [CrossRef]
- Cox, F.E. History of the discovery of the malaria parasites and their vectors. Parasit. Vectors 2010, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Phylogenies from molecular sequences: Inference and reliability. Annu. Rev. Genet. 1988, 22, 521–565. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.C.; Creevey, C.J.; O’Connell, M.J. Mitochondrial data are not suitable for resolving placental mammal phylogeny. Mamm. Genome 2014, 25, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Qari, S.H.; Shi, Y.P.; Pieniazek, N.J.; Collins, W.E.; Lal, A.A. Phylogenetic relationship among the malaria parasites based on small subunit rRNA gene sequences: Monophyletic nature of the human malaria parasite, Plasmodium falciparum. Mol. Phylogenet. Evol. 1996, 6, 157–165. [Google Scholar] [CrossRef]
- Escalante, A.A.; Freeland, D.E.; Collins, W.E.; Lal, A.A. The evolution of primate malaria parasites based on the gene encoding cytochrome b from the linear mitochondrial genome. Proc. Natl. Acad. Sci. USA 1998, 95, 8124–8129. [Google Scholar] [CrossRef]
- Krief, S.; Escalante, A.A.; Pacheco, M.A.; Mugisha, L.; Andre, C.; Halbwax, M.; Fischer, A.; Krief, J.M.; Kasenene, J.M.; Crandfield, M.; et al. On the diversity of malaria parasites in African apes and the origin of Plasmodium falciparum from Bonobos. PLoS Pathog. 2010, 6. [Google Scholar] [CrossRef]
- Kokwaro, G. Ongoing challenges in the management of malaria. Malar. J. 2009, 8, S2. [Google Scholar] [CrossRef]
- Mathison, B.A.; Pritt, B.S. Update on Malaria Diagnostics and Test Utilization. J. Clin. Microbiol. 2017, 55, 2009–2017. [Google Scholar] [CrossRef]
- Ahmod, N.Z.; Gupta, R.S.; Shah, H.N. Identification of a Bacillus anthracis specific indel in the yeaC gene and development of a rapid pyrosequencing assay for distinguishing B. anthracis from the B. cereus group. J. Microbiol. Methods 2011, 87, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Y.; Paschos, A.; Gupta, R.S.; Schellhorn, H.E. Insertion/deletion-based approach for the detection of Escherichia coli O157:H7 in freshwater environments. Environ. Sci. Technol. 2014, 48, 11462–11470. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.M.N.; Gupta, R.S. Novel Sequence Features of DNA Repair Genes/Proteins from Deinococcus Species Implicated in Protection from Oxidatively Generated Damage. Genes 2018, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Gupta, R.S. Conserved inserts in the Hsp60 (GroEL) and Hsp70 (DnaK) proteins are essential for cellular growth. Mol. Genet. Genom. 2009, 281, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Miotto, O.; Almagro-Garcia, J.; Manske, M.; Macinnis, B.; Campino, S.; Rockett, K.A.; Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; et al. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat. Genet. 2013, 45, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Hemming-Schroeder, E.; Umukoro, E.; Lo, E.; Fung, B.; Tomas-Domingo, P.; Zhou, G.; Zhong, D.; Dixit, A.; Atieli, H.; Githeko, A.; et al. Impacts of Antimalarial Drugs on Plasmodium falciparum Drug Resistance Markers, Western Kenya, 2003–2015. Am. J. Trop. Med. Hyg. 2018, 98, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S. Impact of Genomics on Clarifying the Evolutionary Relationships amongst Mycobacteria: Identification of Molecular Signatures Specific for the Tuberculosis-Complex of Bacteria with Potential Applications for Novel Diagnostics and Therapeutics. High Throughput 2018, 7, 31. [Google Scholar] [CrossRef]
- Cherkasov, A.; Nandan, D.; Reiner, N.E. Selective targeting of indel-inferred differences in spatial structures of highly homologous proteins. Proteins 2005, 58, 950–954. [Google Scholar] [CrossRef]



| Protein Name | Accession Number | Figure Number | Indel Size | Indel Position | Specificity |
|---|---|---|---|---|---|
| Cell division cycle protein 48 homologue | CDS48458 | 2, S1 | 1 aa ins | 106–156 | Class Hematozoa |
| 30S ribosomal protein S9, putative | CDU21364 | S2 | 1 aa ins | 449–503 | |
| 40S ribosomal protein S12, putative | XP_677491 | S3 | 1 aa ins | 62–109 | |
| 20S proteasome β 4 subunit | CDS50559 | S4 | 1 aa ins | 04–59 | |
| Golgi reassembly-stacking protein 1 | XP_012769739 | S5 | 1 aa del | 90–136 | |
| Pyruvate kinase 2 | XP_680419 | S6 | 1 aa ins | 461–515 | |
| Succinyl-CoA synthetase β chain | XP_004833199 | 3 (A), S7 | 1 aa del | 273–315 | Order Piroplasmida |
| Hypothetical protein, conserved | XP_954617 | S8 | 1 aa ins | 1856–1907 | |
| Dihydrolipoamide dehydrogenase | XP_763245 | 3 (B), S9 | 1 aa del | 415–465 | Order Piroplasmida, except B. microti |
| Conserved hypothetical protein | XP_004832720 | S10 | 1 aa del | 19–74 | |
| Conserved hypothetical protein | XP_004832649 | S11 | 2 aa del | 334–388 | |
| Intron-binding aquarius β like | XP_012767741 | S12 | 2 aa del | 1280–1322 | |
| Intron-binding aquarius β like | XP_012767742 | S13 | 5/8 aa del | 1303–1346 | |
| Cysteinyl-tRNA synthetase | XP_001608890 | S14 | 2 aa ins | 261–316 | Genus Babesia (except B. microti) |
| Cysteinyl-tRNA synthetase | XP_001608890 | S14 | 1 aa ins | 261–316 | Genus Theileria |
| Eukaryotic translation initiation factor 4a | XP_764692 | S15 | 1 aa del | 225–279 |
| Protein Name | Accession number | Figure Number | Indel Size | Indel Position | Specificity |
|---|---|---|---|---|---|
| 40S ribosomal protein S3 | CDS50468 | 4 (A), S16 | 1 aa ins | 50–103 | Genus Plasmodium |
| 26S proteasome regulatory subunit RPN2 | CDS50476 | S17 | 2 aa ins | 777–826 | |
| 26S proteasome regulatory subunit 4 | XP_673015 | S18 | 1 aa del | 64–116 | |
| 40S ribosomal protein S25, putative | XP_001348379 | S19 | 1 aa del | 35–79 | |
| 50S ribosomal protein L1, mitochondrial, putative * | XP_674529 | S20 | 1 aa del | 126–167 | |
| 60S ribosomal protein L35, putative | XP_001347931 | S21 | 1 aa del | 25–64 | |
| Asparagine-tRNA ligase, putative* | XP_680201 | S22 | 1 aa del | 436–487 | |
| ATP-dependent RNA helicase DBP10 | CDS45652 | S23 | 1 aa ins | 385–438 | |
| Alternative splicing regulator, putative | XP_001349840 | S24 | 1 aa del | 296–338 | |
| Adenosinetriphosphatase protein | XP_677822 | S25 | 1 aa del | 387–437 | |
| DNA2/NAM7 helicase | CDS49097 | S26 | 3/4 aa ins | 950–1013 | |
| Elongation factor Tu family | CDS46726 | S27 | 1 aa ins | 335–380 | |
| Multidrug resistance protein 2 | CDS50130 | S28 | 1 aa del | 438–488 | |
| Pre-mRNA-processing-splicing factor 8 | XP_022714222 | S29 | 2 aa ins | 1174–1230 | |
| Pyruvate dehydrogenase E1 component, α subunit | CDS46715 | S30 | 1 aa ins | 218–272 | |
| Pyruvate dehydrogenase E1 component, α subunit | CDS46715 | S31 | 1 aa ins | 406–451 | |
| Ras-related protein Rab-5A | XP_677676 | S32 | 2 aa del | 103–162 | |
| Ribosomal protein L27a, putative | XP_677367 | S33 | 1 aa ins | 97–142 | |
| RuvB-like helicase isoform 1 | XP_673190 | S34 | 1 aa ins | 121–178 | |
| RuvB-like helicase isoform 1 | XP_673190 | S35 | 1 aa del | 96–144 | |
| RuvB-like helicase isoform 2 | CDS46888 | S36 | 1 aa ins | 136–194 | |
| Splicing factor 3A subunit 2 | XP_676664 | S37 | 1 aa del | 111–157 | |
| Translation initiation factor SUI1 | SCM24886 | S38 | 1 aa del | 181–221 | |
| Cysteine-tRNA ligase protein | XP_024329092 | 4 (B), S39 | 1 aa ins | 135–185 | Mammalian infecting clades |
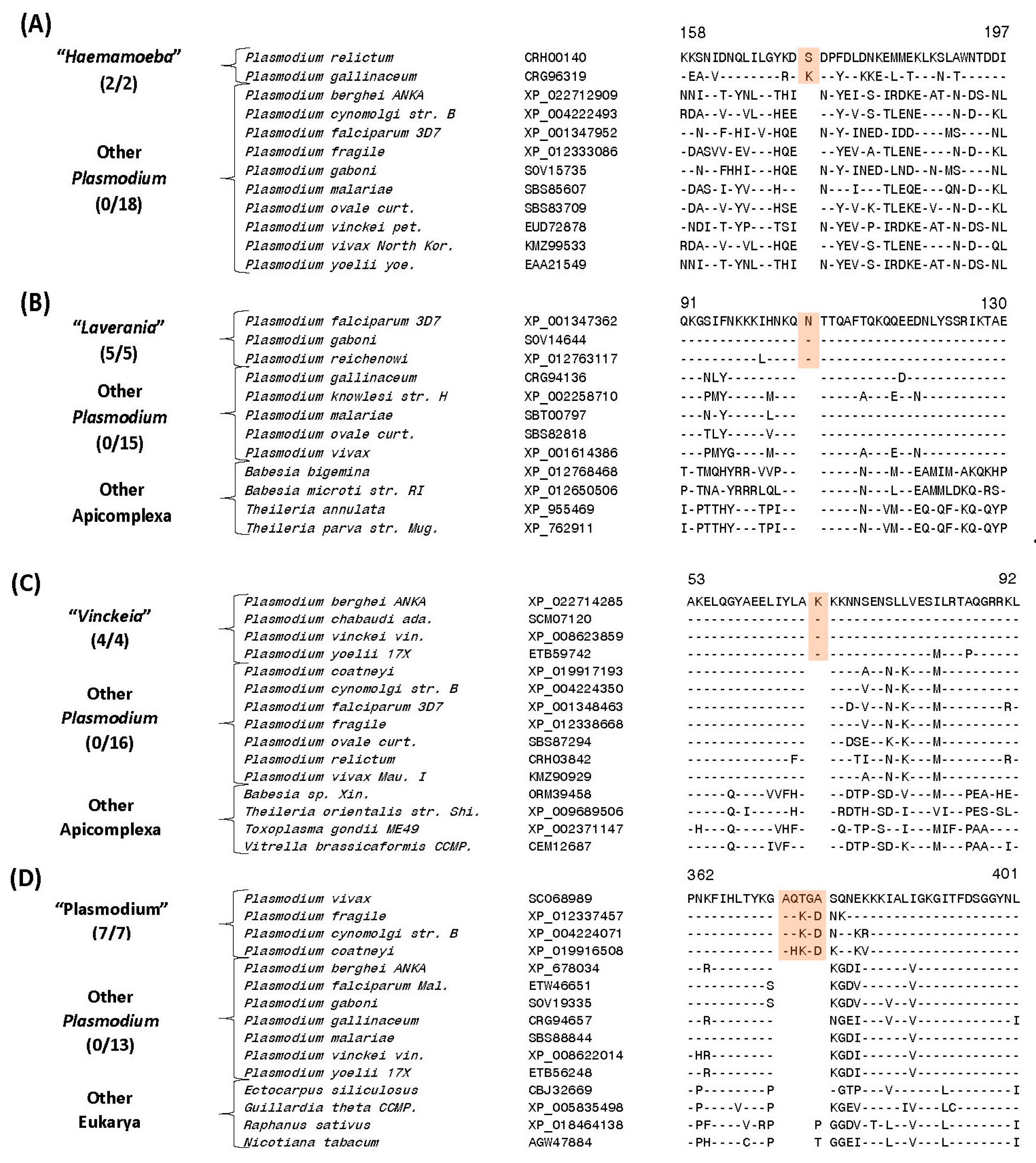
| Protein phosphatase | CRH00140 | 5 (A), S40 | 1 aa ins | 146–197 | Avian clade, “Haemamoeba” |
| Conserved Plasmodium protein | CRG96454 | S41 | 1 aa ins | 5–58 |
| Protein Name | Accession number | Figure Number | Indel Size | Indel Position | Specificity |
|---|---|---|---|---|---|
| Eukaryotic translation initiation factor 3 subunit D | XP_001347362 | 5 (B), S42 | 1 aa ins | 82–130 | Subgenus “Laverania” a |
| Conserved hypothetical protein | XP_001349841 | S43 | 1 aa ins | 322–365 | |
| Aconitate hydratase protein | XP_001350142 | S44 | 1 aa ins | 450–494 | |
| Conserved Plasmodium protein | XP_024329193 | S45 | 1 aa ins | 528–575 | |
| Cation-transporting ATPase | XP_001349175.1 | S46 | 1 aa ins | 576–633 | |
| Serine/threonine protein kinase | XP_001349887 | S47 | 2 aa ins | 175–222 | |
| Tetratricopeptide repeat family protein | KOB86259 | S48 | 1 aa ins | 548–594 | |
| Thioredoxin-like protein | XP_001348359 | S49 | 1 aa ins | 125–171 | |
| Pre-mRNA-processing-splicing factor 8 * | XP_001351366 | S50 | 1 aa ins | 2635–2685 | |
| Mitochondrial ribosomal protein L17-2 precursor | XP_022714285 | 5 (C), S51 | 1 aa ins | 44–92 | Subgenus “Vinckeia” P. |
| Gdp-mannose 4,6 dehydratase | XP_678388 | S52 | 1 aa ins | 119–156 | |
| 14-3-3 protein | XP_022714317 | S53 | 1 aa ins | 385–431 | |
| Conserved Plasmodium protein | XP_679884 | S54 | 1 aa del | 380–429 | |
| Conserved Plasmodium protein | XP_022714261 | S55 | 4 aa ins | 113–167 | |
| Conserved Plasmodium protein | XP_678492 | S56 | 1 aa ins | 893–932 | |
| M17 leucyl aminopeptidase protein | XP_678034 | S57 | 1 aa ins | 199–249 | |
| PelOta protein homologue, putative | CAH99124.1 | S58 | 1 aa del | 181–232 | |
| LCCL domain-containing protein | XP_679041 | S59 | 1 aa ins | 166–212 | |
| DEAD-box family helicase 4 protein | XP_019913407 | S60 | 1 aa ins | 442–491 | |
| DEAD-box family helicase 4 protein | XP_019913407 | S60 | 2 aa ins | 442–491 | Subgenus “Plasmodium” |
| Conserved Plasmodium protein | CRG96454 | S41 | 1 aa del | 5–58 | |
| Leucine aminopeptidase protein | SCO68989 | 5 (D), S61 | 4/5 aa ins | 346–401 | |
| Conserved hypothetical protein | XP_001616802 | S62 | 1 aa ins | 384–433 | |
| Hypothetical protein PVBG_03892 | KMZ97769 | S63 | 1 aa ins | 879–922 | |
| Hypothetical protein PVMG_00581 | KMZ97919 | S64 | 1 aa ins | 140–179 | |
| Hypothetical protein PVNG_02680 | KMZ97919 | S65 | 1 aa ins | 278–323 | |
| Serine/threonine protein kinase + | SCO74371 | S66 | 1 aa ins | 546–598 | |
| Biotin-acetyl-CoA-carboxylase ligase protein | 68069783 | 6 (A), S67 | 1 aa ins | 28–68 | “Vinckeia-Plasmodium” clade |
| Hypothetical protein PVIIG_05030 | KMZ81663 | S68 | 1 aa ins | 347–394 | |
| Conserved Plasmodium protein | XP_679973 | S69 | 1 aa del | 227–276 | |
| Conserved Plasmodium protein | KMZ97769 | S70 | 2 aa ins | 688–737 | |
| Conserved Plasmodium protein | KMZ97769 | S70 | 1 aa ins | 688–737 | “Plasmodium-Malariae” clade |
| Ubiquitin-activating enzyme E1 protein | 901876239 | 6 (B), S71 | 2 aa ins | 153–204 | |
| Hypothetical protein PVNG_01558 | KNA00692 | S72 | 1 aa ins | 277–323 | |
| Phosphoinositide-specific phospholipase C protein | 1269288476 | 6 (C), S73 | 1/2 aa ins | 190–238 | “Vinckeia-Ovale” clade |
| DEAD/DEAH helicase protein | 1269289107 | S74 | 1 aa ins | 144–192 | |
| ATPase protein | XP_022712410 | S75 | 2 aa ins | 764–811 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, R.; Gupta, R.S. Novel Molecular Synapomorphies Demarcate Different Main Groups/Subgroups of Plasmodium and Piroplasmida Species Clarifying Their Evolutionary Relationships. Genes 2019, 10, 490. https://doi.org/10.3390/genes10070490
Sharma R, Gupta RS. Novel Molecular Synapomorphies Demarcate Different Main Groups/Subgroups of Plasmodium and Piroplasmida Species Clarifying Their Evolutionary Relationships. Genes. 2019; 10(7):490. https://doi.org/10.3390/genes10070490
Chicago/Turabian StyleSharma, Rahul, and Radhey S. Gupta. 2019. "Novel Molecular Synapomorphies Demarcate Different Main Groups/Subgroups of Plasmodium and Piroplasmida Species Clarifying Their Evolutionary Relationships" Genes 10, no. 7: 490. https://doi.org/10.3390/genes10070490
APA StyleSharma, R., & Gupta, R. S. (2019). Novel Molecular Synapomorphies Demarcate Different Main Groups/Subgroups of Plasmodium and Piroplasmida Species Clarifying Their Evolutionary Relationships. Genes, 10(7), 490. https://doi.org/10.3390/genes10070490