Vimentin Phosphorylation Is Required for Normal Cell Division of Immature Astrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Antibodies
2.3. Astrocyte Cultures
2.4. Immunofluorescence
2.5. Western Blot
2.6. Mitochondrial Membrane Potential Measurement
2.7. Cell Death Measurement
2.8. Data Analysis
3. Results
3.1. Immature VIMSA/SA Astrocytes Contain Vimentin Accumulations
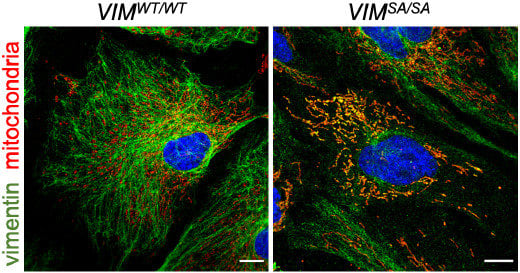
3.2. Vimentin Accumulations in Immature VIMSA/SA Astrocytes Co-Localize with Mitochondria
3.3. Increased Fraction of Bi-Nucleated Cells among Immature VIMSA/SA Astrocytes
3.4. Mitomycin C Treatment or Serum Starvation Reduces the Fraction of Immature VIMSA/SA Astrocytes with Vimentin Accumulations
3.5. The Fraction of Immature VIMSA/SA Astrocytes with Abundant Vimentin Accumulations Is Reduced in the Absence of GFAP
3.6. Immature VIMSA/SA and VIMSA/SAGFAP−/− Astrocytes Have Normal Mitochondrial Membrane Potential
3.7. Immature VIMSA/SA and VIMSA/SAGFAP−/− Astrocytes Show Normal Vulnerability to H2O2, Oxygen/Glucose Deprivation, and Chemical Ischemia
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Pekny, M.; Pekna, M. Astrocyte reactivity and reactive astrogliosis: Costs and benefits. Physiol. Rev. 2014, 94, 1077–1098. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Pekna, M.; Messing, A.; Steinhauser, C.; Lee, J.M.; Parpura, V.; Hol, E.M.; Sofroniew, M.V.; Verkhratsky, A. Astrocytes: A central element in neurological diseases. Acta Neuropathol. 2016, 131, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S.; Sofroniew, M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 2015, 18, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Wilhelmsson, U.; Tatlisumak, T.; Pekna, M. Astrocyte activation and reactive gliosis–A new target in stroke? Neurosci. Lett. 2019, 689, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsson, U.; Li, L.; Pekna, M.; Berthold, C.H.; Blom, S.; Eliasson, C.; Renner, O.; Bushong, E.; Ellisman, M.; Morgan, T.E.; et al. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J. Neurosci. 2004, 24, 5016–5021. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsson, U.; Faiz, M.; de Pablo, Y.; Sjoqvist, M.; Andersson, D.; Widestrand, A.; Potokar, M.; Stenovec, M.; Smith, P.L.; Shinjyo, N.; et al. Astrocytes negatively regulate neurogenesis through the Jagged1-mediated Notch pathway. Stem Cells 2012, 30, 2320–2329. [Google Scholar] [CrossRef] [PubMed]
- de Pablo, Y.; Nilsson, M.; Pekna, M.; Pekny, M. Intermediate filaments are important for astrocyte response to oxidative stress induced by oxygen-glucose deprivation and reperfusion. Histochem. Cell Biol. 2013, 140, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lundkvist, A.; Andersson, D.; Wilhelmsson, U.; Nagai, N.; Pardo, A.C.; Nodin, C.; Stahlberg, A.; Aprico, K.; Larsson, K.; et al. Protective role of reactive astrocytes in brain ischemia. J. Cereb Blood Flow Metab. 2008, 28, 468–481. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Cui, Y.; Roberts, C.; Lu, M.; Wilhelmsson, U.; Pekny, M.; Chopp, M. Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia 2014, 62, 2022–2033. [Google Scholar] [CrossRef]
- Herrmann, J.E.; Imura, T.; Song, B.; Qi, J.; Ao, Y.; Nguyen, T.K.; Korsak, R.A.; Takeda, K.; Akira, S.; Sofroniew, M.V. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci. 2008, 28, 7231–7243. [Google Scholar] [CrossRef]
- Okada, S.; Nakamura, M.; Katoh, H.; Miyao, T.; Shimazaki, T.; Ishii, K.; Yamane, J.; Yoshimura, A.; Iwamoto, Y.; Toyama, Y.; et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 2006, 12, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Burda, J.E.; Sofroniew, M.V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A.W.; Hu, X.; Yoon, H.; Yan, P.; Xiao, Q.; Wang, Y.; Gil, S.C.; Brown, J.; Wilhelmsson, U.; Restivo, J.L.; et al. Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. FASEB J. 2013, 27, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Macauley, S.L.; Pekny, M.; Sands, M.S. The role of attenuated astrocyte activation in infantile neuronal ceroid lipofuscinosis. J. Neurosci. 2011, 31, 15575–15585. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, W.; Kooijman, L.; Orre, M.; Stassen, O.; Pekny, M.; Hol, E.M. GFAP and vimentin deficiency alters gene expression in astrocytes and microglia in wild-type mice and changes the transcriptional response of reactive glia in mouse model for Alzheimer’s disease. Glia 2015, 63, 1036–1056. [Google Scholar] [CrossRef] [PubMed]
- Kinouchi, R.; Takeda, M.; Yang, L.; Wilhelmsson, U.; Lundkvist, A.; Pekny, M.; Chen, D.F. Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nat. Neurosci. 2003, 6, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Widestrand, A.; Faijerson, J.; Wilhelmsson, U.; Smith, P.L.; Li, L.; Sihlbom, C.; Eriksson, P.S.; Pekny, M. Increased neurogenesis and astrogenesis from neural progenitor cells grafted in the hippocampus of GFAP-/- Vim-/- mice. Stem Cells 2007, 25, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, C.; Sahlgren, C.; Berthold, C.H.; Stakeberg, J.; Celis, J.E.; Betsholtz, C.; Eriksson, J.E.; Pekny, M. Intermediate filament protein partnership in astrocytes. J. Biol. Chem. 1999, 274, 23996–24006. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Johansson, C.B.; Eliasson, C.; Stakeberg, J.; Wallen, A.; Perlmann, T.; Lendahl, U.; Betsholtz, C.; Berthold, C.H.; Frisen, J. Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J. Cell Biol. 1999, 145, 503–514. [Google Scholar] [CrossRef]
- Cho, K.S.; Yang, L.; Lu, B.; Feng Ma, H.; Huang, X.; Pekny, M.; Chen, D.F. Re-establishing the regenerative potential of central nervous system axons in postnatal mice. J. Cell Sci. 2005, 118, 863–872. [Google Scholar] [CrossRef]
- Menet, V.; Prieto, M.; Privat, A.; Gimenez y Ribotta, M. Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc. Natl. Acad. Sci. USA 2003, 100, 8999–9004. [Google Scholar] [CrossRef] [PubMed]
- Verardo, M.R.; Lewis, G.P.; Takeda, M.; Linberg, K.A.; Byun, J.; Luna, G.; Wilhelmsson, U.; Pekny, M.; Chen, D.F.; Fisher, S.K. Abnormal reactivity of muller cells after retinal detachment in mice deficient in GFAP and vimentin. Invest. Ophthalmol Vis. Sci. 2008, 49, 3659–3665. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.; Bar, H.; Kreplak, L.; Strelkov, S.V.; Aebi, U. Intermediate filaments: From cell architecture to nanomechanics. Nat. Rev. Mol. Cell Biol. 2007, 8, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.; Aebi, U. Structure, assembly, and dynamics of intermediate filaments. Subcell Biochem. 1998, 31, 319–362. [Google Scholar] [PubMed]
- Inagaki, M.; Matsuoka, Y.; Tsujimura, K.; Ando, S.; Tokui, T.; Takahashi, T.; Inagaki, N. Dynamic property of intermediate filaments: Regulation by phosphorylation. BioEssays 1996, 18, 481–487. [Google Scholar] [CrossRef]
- Ivaska, J.; Pallari, H.M.; Nevo, J.; Eriksson, J.E. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp. Cell Res. 2007, 313, 2050–2062. [Google Scholar] [CrossRef] [PubMed]
- Omary, M.B.; Ku, N.O.; Tao, G.Z.; Toivola, D.M.; Liao, J. “Heads and tails” of intermediate filament phosphorylation: Multiple sites and functional insights. Trends Biochem. Sci. 2006, 31, 383–394. [Google Scholar] [CrossRef]
- Sihag, R.K.; Inagaki, M.; Yamaguchi, T.; Shea, T.B.; Pant, H.C. Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp. Cell Res. 2007, 313, 2098–2109. [Google Scholar] [CrossRef]
- Goto, H.; Yasui, Y.; Kawajiri, A.; Nigg, E.A.; Terada, Y.; Tatsuka, M.; Nagata, K.; Inagaki, M. Aurora-B regulates the cleavage furrow-specific vimentin phosphorylation in the cytokinetic process. J. Biol. Chem. 2003, 278, 8526–8530. [Google Scholar] [CrossRef]
- Kawajiri, A.; Yasui, Y.; Goto, H.; Tatsuka, M.; Takahashi, M.; Nagata, K.; Inagaki, M. Functional significance of the specific sites phosphorylated in desmin at cleavage furrow: Aurora-B may phosphorylate and regulate type III intermediate filaments during cytokinesis coordinatedly with Rho-kinase. Mol. Biol. Cell 2003, 14, 1489–1500. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Goto, H.; Yokoyama, T.; Sillje, H.; Hanisch, A.; Uldschmid, A.; Takai, Y.; Oguri, T.; Nigg, E.A.; Inagaki, M. Phosphorylation by Cdk1 induces Plk1-mediated vimentin phosphorylation during mitosis. J. Cell Biol. 2005, 171, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Yasui, Y.; Goto, H.; Matsui, S.; Manser, E.; Lim, L.; Nagata, K.; Inagaki, M. Protein kinases required for segregation of vimentin filaments in mitotic process. Oncogene 2001, 20, 2868–2876. [Google Scholar] [CrossRef] [PubMed]
- Yasui, Y.; Amano, M.; Nagata, K.; Inagaki, N.; Nakamura, H.; Saya, H.; Kaibuchi, K.; Inagaki, M. Roles of Rho-associated kinase in cytokinesis; mutations in Rho-associated kinase phosphorylation sites impair cytokinetic segregation of glial filaments. J. Cell Biol. 1998, 143, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, K.; Ogawara, M.; Takeuchi, Y.; Imajoh-Ohmi, S.; Ha, M.H.; Inagaki, M. Visualization and function of vimentin phosphorylation by cdc2 kinase during mitosis. J. Biol. Chem. 1994, 269, 31097–31106. [Google Scholar] [PubMed]
- Chou, Y.H.; Bischoff, J.R.; Beach, D.; Goldman, R.D. Intermediate filament reorganization during mitosis is mediated by p34cdc2 phosphorylation of vimentin. Cell 1990, 62, 1063–1071. [Google Scholar] [CrossRef]
- Goto, H.; Kosako, H.; Tanabe, K.; Yanagida, M.; Sakurai, M.; Amano, M.; Kaibuchi, K.; Inagaki, M. Phosphorylation of vimentin by Rho-associated kinase at a unique amino-terminal site that is specifically phosphorylated during cytokinesis. J. Biol. Chem. 1998, 273, 11728–11736. [Google Scholar] [CrossRef] [PubMed]
- Kosako, H.; Amano, M.; Yanagida, M.; Tanabe, K.; Nishi, Y.; Kaibuchi, K.; Inagaki, M. Phosphorylation of glial fibrillary acidic protein at the same sites by cleavage furrow kinase and Rho-associated kinase. J. Biol. Chem. 1997, 272, 10333–10336. [Google Scholar] [CrossRef] [PubMed]
- Inada, H.; Togashi, H.; Nakamura, Y.; Kaibuchi, K.; Nagata, K.; Inagaki, M. Balance between activities of Rho kinase and type 1 protein phosphatase modulates turnover of phosphorylation and dynamics of desmin/vimentin filaments. J. Biol. Chem. 1999, 274, 34932–34939. [Google Scholar] [CrossRef]
- Goto, H.; Inagaki, M. Production of a site- and phosphorylation state-specific antibody. Nat. Protoc. 2007, 2, 2574–2581. [Google Scholar] [CrossRef]
- Nishizawa, K.; Yano, T.; Shibata, M.; Ando, S.; Saga, S.; Takahashi, T.; Inagaki, M. Specific localization of phosphointermediate filament protein in the constricted area of dividing cells. J. Biol. Chem. 1991, 266, 3074–3079. [Google Scholar]
- Ogawara, M.; Inagaki, N.; Tsujimura, K.; Takai, Y.; Sekimata, M.; Ha, M.H.; Imajoh-Ohmi, S.; Hirai, S.; Ohno, S.; Sugiura, H.; et al. Differential targeting of protein kinase C and CaM kinase II signalings to vimentin. J. Cell. Biol. 1995, 131, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, N.; Goto, H.; Ogawara, M.; Nishi, Y.; Ando, S.; Inagaki, M. Spatial patterns of Ca2+ signals define intracellular distribution of a signaling by Ca2+/Calmodulin-dependent protein kinase II. J. Biol. Chem. 1997, 272, 25195–25199. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.F.; Spinelli, A.M.; Wang, R.; Anfinogenova, Y.; Singer, H.A.; Tang, D.D. Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J. Biol. Chem. 2006, 281, 34716–34724. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, R.; Gannon, O.J.; Rezey, A.C.; Jiang, S.; Gerlach, B.D.; Liao, G.; Tang, D.D. Polo-like Kinase 1 Regulates Vimentin Phosphorylation at Ser-56 and Contraction in Smooth Muscle. J. Biol. Chem. 2016, 291, 23693–23703. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, M.; Tanaka, H.; Inoko, A.; Goto, H.; Yonemura, S.; Kobori, K.; Hayashi, Y.; Kondo, E.; Itohara, S.; Izawa, I.; et al. Defect of mitotic vimentin phosphorylation causes microophthalmia and cataract via aneuploidy and senescence in lens epithelial cells. J. Biol. Chem. 2013, 288, 35626–35635. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Goto, H.; Inoko, A.; Makihara, H.; Enomoto, A.; Horimoto, K.; Matsuyama, M.; Kurita, K.; Izawa, I.; Inagaki, M. Cytokinetic Failure-induced Tetraploidy Develops into Aneuploidy, Triggering Skin Aging in Phosphovimentin-deficient Mice. J. Biol. Chem. 2015, 290, 12984–12998. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Puschmann, T.B.; Marasek, P.; Inagaki, M.; Pekna, M.; Wilhelmsson, U.; Pekny, M. Increased Neuronal Differentiation of Neural Progenitor Cells Derived from Phosphovimentin-Deficient Mice. Mol. Neurobiol. 2018, 55, 5478–5489. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Leveen, P.; Pekna, M.; Eliasson, C.; Berthold, C.H.; Westermark, B.; Betsholtz, C. Mice lacking glial fibrillary acidic protein display astrocytes devoid of intermediate filaments but develop and reproduce normally. EMBO J. 1995, 14, 1590–1598. [Google Scholar] [CrossRef]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 2017, 18, 529. [Google Scholar] [CrossRef]
- Bondarenko, A.; Chesler, M. Rapid astrocyte death induced by transient hypoxia, acidosis, and extracellular ion shifts. Glia 2001, 34, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Gentil, B.J.; Minotti, S.; Beange, M.; Baloh, R.H.; Julien, J.P.; Durham, H.D. Normal role of the low-molecular-weight neurofilament protein in mitochondrial dynamics and disruption in Charcot-Marie-Tooth disease. FASEB J. 2012, 26, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.L.; Lung, H.L.; Wu, K.C.; Le, A.H.; Tang, H.M.; Fung, M.C. Vimentin supports mitochondrial morphology and organization. Biochem. J. 2008, 410, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Nekrasova, O.E.; Mendez, M.G.; Chernoivanenko, I.S.; Tyurin-Kuzmin, P.A.; Kuczmarski, E.R.; Gelfand, V.I.; Goldman, R.D.; Minin, A.A. Vimentin intermediate filaments modulate the motility of mitochondria. Mol. Biol. Cell 2011, 22, 2282–2289. [Google Scholar] [CrossRef] [PubMed]
- Milner, D.J.; Mavroidis, M.; Weisleder, N.; Capetanaki, Y. Desmin cytoskeleton linked to muscle mitochondrial distribution and respiratory function. J. Cell. Biol. 2000, 150, 1283–1298. [Google Scholar] [CrossRef]
- Stone, M.R.; O’Neill, A.; Lovering, R.M.; Strong, J.; Resneck, W.G.; Reed, P.W.; Toivola, D.M.; Ursitti, J.A.; Omary, M.B.; Bloch, R.J. Absence of keratin 19 in mice causes skeletal myopathy with mitochondrial and sarcolemmal reorganization. J. Cell. Sci. 2007, 120, 3999–4008. [Google Scholar] [CrossRef] [PubMed]
- Shinjyo, N.; de Pablo, Y.; Pekny, M.; Pekna, M. Complement Peptide C3a Promotes Astrocyte Survival in Response to Ischemic Stress. Mol. Neurobiol. 2016, 53, 3076–3087. [Google Scholar] [CrossRef]
- Bornheim, R.; Muller, M.; Reuter, U.; Herrmann, H.; Bussow, H.; Magin, T.M. A dominant vimentin mutant upregulates Hsp70 and the activity of the ubiquitin-proteasome system, and causes posterior cataracts in transgenic mice. J. Cell. Sci. 2008, 121, 3737–3746. [Google Scholar] [CrossRef]
- Bousquet, O.; Basseville, M.; Vila-Porcile, E.; Billette de Villemeur, T.; Hauw, J.J.; Landrieu, P.; Portier, M.M. Aggregation of a subpopulation of vimentin filaments in cultured human skin fibroblasts derived from patients with giant axonal neuropathy. Cell. Motil. Cytoskeleton 1996, 33, 115–129. [Google Scholar] [CrossRef]
- Kueper, T.; Grune, T.; Prahl, S.; Lenz, H.; Welge, V.; Biernoth, T.; Vogt, Y.; Muhr, G.M.; Gaemlich, A.; Jung, T.; et al. Vimentin is the specific target in skin glycation. Structural prerequisites, functional consequences, and role in skin aging. J. Biol. Chem. 2007, 282, 23427–23436. [Google Scholar] [CrossRef]
- Perez-Sala, D.; Oeste, C.L.; Martinez, A.E.; Carrasco, M.J.; Garzon, B.; Canada, F.J. Vimentin filament organization and stress sensing depend on its single cysteine residue and zinc binding. Nat. Commun. 2015, 6, 7287. [Google Scholar] [CrossRef] [PubMed]
- Viedma-Poyatos, A.; de Pablo, Y.; Pekny, M.; Perez-Sala, D. The cysteine residue of glial fibrillary acidic protein is a critical target for lipoxidation and required for efficient network organization. Free Radic. Biol. Med. 2018, 120, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Chernoivanenko, I.S.; Matveeva, E.A.; Gelfand, V.I.; Goldman, R.D.; Minin, A.A. Mitochondrial membrane potential is regulated by vimentin intermediate filaments. FASEB J. 2015, 29, 820–827. [Google Scholar] [CrossRef] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pablo, Y.d.; Marasek, P.; Pozo-Rodrigálvarez, A.; Wilhelmsson, U.; Inagaki, M.; Pekna, M.; Pekny, M. Vimentin Phosphorylation Is Required for Normal Cell Division of Immature Astrocytes. Cells 2019, 8, 1016. https://doi.org/10.3390/cells8091016
Pablo Yd, Marasek P, Pozo-Rodrigálvarez A, Wilhelmsson U, Inagaki M, Pekna M, Pekny M. Vimentin Phosphorylation Is Required for Normal Cell Division of Immature Astrocytes. Cells. 2019; 8(9):1016. https://doi.org/10.3390/cells8091016
Chicago/Turabian StylePablo, Yolanda de, Pavel Marasek, Andrea Pozo-Rodrigálvarez, Ulrika Wilhelmsson, Masaki Inagaki, Marcela Pekna, and Milos Pekny. 2019. "Vimentin Phosphorylation Is Required for Normal Cell Division of Immature Astrocytes" Cells 8, no. 9: 1016. https://doi.org/10.3390/cells8091016
APA StylePablo, Y. d., Marasek, P., Pozo-Rodrigálvarez, A., Wilhelmsson, U., Inagaki, M., Pekna, M., & Pekny, M. (2019). Vimentin Phosphorylation Is Required for Normal Cell Division of Immature Astrocytes. Cells, 8(9), 1016. https://doi.org/10.3390/cells8091016