Breaking Barriers: Modulation of Tumor Microenvironment to Enhance Bacillus Calmette–Guérin Immunotherapy of Bladder Cancer
Abstract
1. Introduction
2. BCG and Tumor Immunity
3. BCG, Immune Checkpoints, and Immune Checkpoint Inhibitors (ICI)
4. BCG and Lymphoid/Myeloid Imbalance
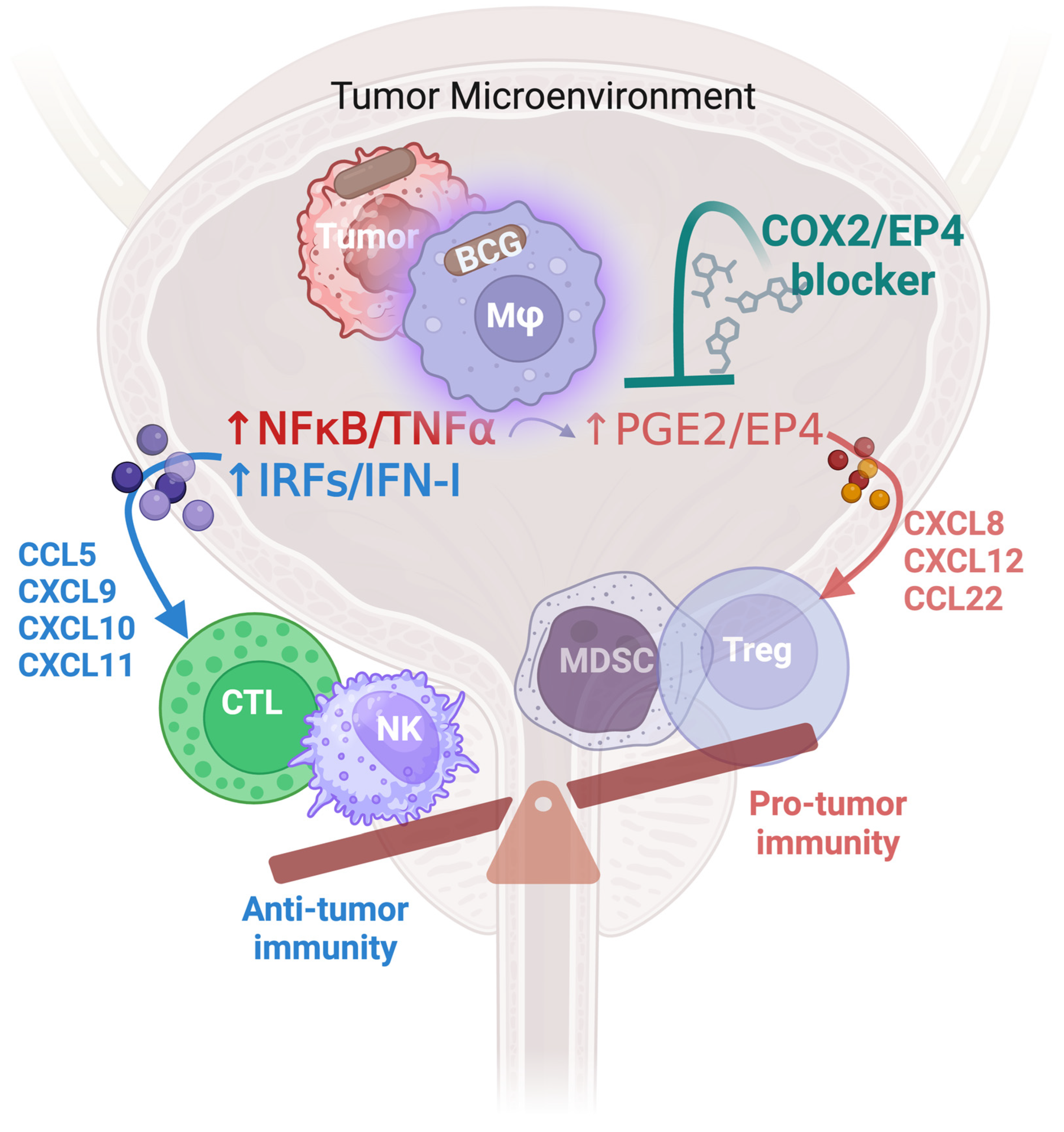
5. BCG and Chemokines Attracting Effector versus Suppressive Populations of Immune Cells: Rationale for Modulating PGE2 Production and Signaling
6. Beyond Cyclooxygenase Inhibitors: Emerging Targets and Biomarkers to Counteract PGE2-Driven Suppression and Enhance Type-1 Inflammatory Pathways
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Joseph, R.W.; Loriot, Y.; Hoffman-Censits, J.; Perez-Gracia, J.L.; Petrylak, D.P.; Derleth, C.L.; Tayama, D.; Zhu, Q.; Ding, B.; et al. Atezolizumab in platinum-treated locally advanced or metastatic urothelial carcinoma: Post-progression outcomes from the phase II IMvigor210 study. Ann. Oncol. 2017, 28, 3044–3050. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, N.N.; Yuno, A.; Trepel, J.B.; Apolo, A.B. Immunotherapy: A new treatment paradigm in bladder cancer. Curr. Opin. Oncol. 2017, 29, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Tabayoyong, W.; Gao, J. The emerging role of immunotherapy in advanced urothelial cancers. Curr. Opin. Oncol. 2018, 30, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Catalano, M.; Santi, R.; Palmieri, V.E.; Vannini, G.; Galli, I.C.; Buttitta, E.; Villari, D.; Rossi, V.; Nesi, G. Immune Checkpoint Inhibitors in Urothelial Bladder Cancer: State of the Art and Future Perspectives. Cancers 2021, 13, 4411. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 1976, 116, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Power, N.E.; Izawa, J. Comparison of guidelines on non-muscle invasive bladder cancer (EAU, CUA, AUA, NCCN, NICE). Bladder Cancer 2016, 2, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.; Rouprêt, M.; Shariat, S.F.; Sylvester, R. European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ)—2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Boorjian, S.A.; Chou, R.; Clark, P.E.; Daneshmand, S.; Konety, B.R.; Pruthi, R.; Quale, D.Z.; Ritch, C.R.; Seigne, J.D.; et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J. Urol. 2016, 196, 1021–1029. [Google Scholar] [CrossRef]
- Shelley, M.; Kynaston, H.; Court, J.; Wilt, T.; Coles, B.; Burgon, K.; Mason, M. A systematic review of intravesical bacillus Calmette-Guérin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int. 2001, 88, 209–216. [Google Scholar] [CrossRef]
- Shelley, M.D.; Wilt, T.; Court, J.; Coles, B.; Kynaston, H.; Mason, M.D. Intravesical bacillus Calmette-Guérin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: A meta-analysis of randomized trials. BJU Int. 2004, 93, 485–490. [Google Scholar] [CrossRef]
- Cambier, S.; Sylvester, R.J.; Collette, L.; Gontero, P.; Brausi, M.A.; Van Andel, G.; Kirkels, W.J.; Da Silva, F.C.; Oosterlinck, W.; Prescott, S. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non–muscle-invasive stage Ta–T1 urothelial bladder cancer patients treated with 1–3 years of maintenance bacillus Calmette-Guérin. Eur. Urol. 2016, 69, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Hemdan, T.; Johansson, R.; Jahnson, S.; Hellström, P.; Tasdemir, I.; Malmström, P.-U.; Members of the Urothelial Cancer Group of the Nordic Association of Urology. 5-Year outcome of a randomized prospective study comparing bacillus Calmette-Guérin with epirubicin and interferon-α2b in patients with T1 bladder cancer. J. Urol. 2014, 191, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Lam, H.-M.; Rosser, C.; Theodorescu, D.; Parks, W.C.; Chan, K.S. The dynamic roles of the bladder tumour microenvironment. Nat. Rev. Urol. 2022, 19, 515–533. [Google Scholar] [CrossRef]
- Pierconti, F.; Raspollini, M.R.; Martini, M.; Larocca, L.M.; Bassi, P.F.; Bientinesi, R.; Baroni, G.; Minervini, A.; Petracco, G.; Pini, G.M.; et al. PD-L1 expression in bladder primary in situ urothelial carcinoma: Evaluation in BCG-unresponsive patients and BCG responders. Virchows Arch. 2020, 477, 269–277. [Google Scholar] [CrossRef]
- Rouanne, M.; Adam, J.; Radulescu, C.; Letourneur, D.; Bredel, D.; Mouraud, S.; Goubet, A.-G.; Leduc, M.; Chen, N.; Tan, T.Z.; et al. BCG therapy downregulates HLA-I on malignant cells to subvert antitumor immune responses in bladder cancer. J. Clin. Investig. 2022, 132, e145666. [Google Scholar] [CrossRef]
- Cambier, C.; Takaki, K.K.; Larson, R.P.; Hernandez, R.E.; Tobin, D.M.; Urdahl, K.B.; Cosma, C.L.; Ramakrishnan, L. Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature 2014, 505, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Arbues, A.; Lugo-Villarino, G.; Neyrolles, O.; Guilhot, C.; Astarie-Dequeker, C. Playing hide-and-seek with host macrophages through the use of mycobacterial cell envelope phthiocerol dimycocerosates and phenolic glycolipids. Front. Cell. Infect. Microbiol. 2014, 4, 173. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Vaseghi-Shanjani, M.; Afkhami, S.; Grondin, J.A.; Kang, A.; D’agostino, M.R.; Yao, Y.; Jain, S.; Zganiacz, A.; Kroezen, Z.; et al. Parenteral BCG vaccine induces lung-resident memory macrophages and trained immunity via the gut–lung axis. Nat. Immunol. 2022, 23, 1687–1702. [Google Scholar] [CrossRef]
- Van Puffelen, J.H.; Novakovic, B.; van Emst, L.; Kooper, D.; Zuiverloon, T.C.M.; Oldenhof, U.T.H.; Witjes, J.A.; Galesloot, T.E.; Vrieling, A.; Aben, K.K.H.; et al. Intravesical BCG in patients with non-muscle invasive bladder cancer induces trained immunity and decreases respiratory infections. J. Immunother. Cancer 2023, 11, e005518. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A. mTOR-and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef]
- Arts, R.J.; Carvalho, A.; La Rocca, C.; Palma, C.; Rodrigues, F.; Silvestre, R.; Kleinnijenhuis, J.; Lachmandas, E.; Gonçalves, L.G.; Belinha, A.; et al. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. 2016, 17, 2562–2571. [Google Scholar] [CrossRef] [PubMed]
- Van Puffelen, J.H.; Keating, S.T.; Oosterwijk, E.; van der Heijden, A.G.; Netea, M.G.; Joosten, L.A.B.; Vermeulen, S.H. Trained immunity as a molecular mechanism for BCG immunotherapy in bladder cancer. Nat. Rev. Urol. 2020, 17, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.J.W.; Blok, B.A.; Aaby, P.; Joosten, L.A.B.; de Jong, D.; van der Meer, J.W.M.; Benn, C.S.; van Crevel, R.; Netea, M.G. Long-term in vitro and in vivo effects of γ-irradiated BCG on innate and adaptive immunity. J. Leukoc. Biol. 2015, 98, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, Y.-G.; McDonald, C.; Kanneganti, T.-D.; Hasegawa, M.; Body-Malapel, M.; Inohara, N.; Núñez, G. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J. Immunol. 2007, 178, 2380–2386. [Google Scholar] [CrossRef]
- Siracusano, S.; Vita, F.; Abbate, R.; Ciciliato, S.; Borelli, V.; Bernabei, M.; Zabucchi, G. The role of granulocytes following intravesical BCG prophylaxis. Eur. Urol. 2007, 51, 1589–1597, discussion 1597–1589. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz, N.M.G.P.; Marinho, F.V.; de Araujo, A.C.V.S.C.; Fahel, J.S.; Oliveira, S.C. MyD88-dependent BCG immunotherapy reduces tumor and regulates tumor microenvironment in bladder cancer murine model. Sci. Rep. 2021, 11, 15648. [Google Scholar] [CrossRef]
- Basu, J.; Shin, D.-M.; Jo, E.-K. Mycobacterial signaling through toll-like receptors. Front. Cell. Infect. Microbiol. 2012, 2, 145. [Google Scholar] [CrossRef]
- Higuchi, T.; Shimizu, M.; Owaki, A.; Takahashi, M.; Shinya, E.; Nishimura, T.; Takahashi, H. A possible mechanism of intravesical BCG therapy for human bladder carcinoma: Involvement of innate effector cells for the inhibition of tumor growth. Cancer Immunol. Immunother. 2009, 58, 1245–1255. [Google Scholar] [CrossRef]
- Arnold, I.C.; Zhang, X.; Artola-Boran, M.; Fallegger, A.; Sander, P.; Johansen, P.; Müller, A. BATF3-dependent dendritic cells drive both effector and regulatory T-cell responses in bacterially infected tissues. PLoS Pathog. 2019, 15, e1007866. [Google Scholar] [CrossRef]
- Lee, K.-H.; Jeong, J.; Yoo, C.-G. Positive feedback regulation of heat shock protein 70 (Hsp70) is mediated through Toll-like receptor 4-PI3K/Akt-glycogen synthase kinase-3β pathway. Exp. Cell Res. 2013, 319, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Wu, Y.; Huang, X.; Wang, W.; Ang, B.; Cao, X.; Wan, T. Toll-like receptor 4 (TLR4) is essential for Hsp70-like protein 1 (HSP70L1) to activate dendritic cells and induce Th1 response. J. Biol. Chem. 2011, 286, 30393–30400. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, J. Alarmins and immunity. Immunol. Rev. 2017, 280, 41–56. [Google Scholar] [CrossRef]
- Antonelli, A.C.; Binyamin, A.; Hohl, T.M.; Glickman, M.S.; Redelman-Sidi, G. Bacterial immunotherapy for cancer induces CD4-dependent tumor-specific immunity through tumor-intrinsic interferon-γ signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 18627–18637. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, T.L.; Ritchey, J.K.; Yuan, J.J.; Andriole, G.L.; Catalona, W.J. T-cell subsets required for intravesical BCG immunotherapy for bladder cancer. J. Urol. 1993, 150, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, T.L.; Gillen, D.; Catalona, W.J. Requirement of a thymus dependent immune response for BCG-mediated antitumor activity. J. Urol. 1987, 137, 155–158. [Google Scholar] [CrossRef] [PubMed]
- McAveney, K.M.; Gomella, L.G.; Lattime, E.C. Induction of TH1- and TH2-associated cytokine mRNA in mouse bladder following intravesical growth of the murine bladder tumor MB49 and BCG immunotherapy. Cancer Immunol. Immunother. 1994, 39, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, X.; O’Donnell, M.A. Role of Th1 and Th2 cytokines in BCG-induced IFN-γ production: Cytokine promotion and simulation of BCG effect. Cytokine 2003, 21, 17–26. [Google Scholar] [CrossRef]
- Kamat, A.M.; Briggman, J.; Urbauer, D.L.; Svatek, R.; González, G.M.N.; Anderson, R.; Grossman, H.B.; Prat, F.; Dinney, C.P. Cytokine Panel for Response to Intravesical Therapy (CyPRIT): Nomogram of Changes in Urinary Cytokine Levels Predicts Patient Response to Bacillus Calmette-Guérin. Eur. Urol. 2016, 69, 197–200. [Google Scholar] [CrossRef]
- Biot, C.; Rentsch, C.A.; Gsponer, J.R.; Birkhäuser, F.D.; Jusforgues-Saklani, H.; Lemaître, F.; Auriau, C.; Bachmann, A.; Bousso, P.; Demangel, C.; et al. Preexisting BCG-Specific T Cells Improve Intravesical Immunotherapy for Bladder Cancer. Sci. Transl. Med. 2012, 4, 137ra172. [Google Scholar] [CrossRef] [PubMed]
- Leko, V.; McDuffie, L.A.; Zheng, Z.; Gartner, J.J.; Prickett, T.D.; Apolo, A.B.; Agarwal, P.K.; Rosenberg, S.A.; Lu, Y.-C. Identification of Neoantigen-Reactive Tumor-Infiltrating Lymphocytes in Primary Bladder Cancer. J. Immunol. 2019, 202, 3458–3467. [Google Scholar] [CrossRef]
- Domingo-Domenech, J.; Vidal, S.J.; Rodriguez-Bravo, V.; Castillo-Martin, M.; Quinn, S.A.; Rodriguez-Barrueco, R.; Bonal, D.M.; Charytonowicz, E.; Gladoun, N.; de la Iglesia-Vicente, J.; et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell 2012, 22, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Ayyoub, M.; Routy, B.; Kroemer, G. Microbiome and anticancer immunosurveillance. Cell 2016, 165, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Strong, E.J.; Lee, S. Targeting autophagy as a strategy for developing new vaccines and host-directed therapeutics against mycobacteria. Front. Microbiol. 2021, 11, 614313. [Google Scholar] [CrossRef]
- Yamamoto, K.; Venida, A.; Yano, J.; Biancur, D.E.; Kakiuchi, M.; Gupta, S.; Sohn, A.S.; Mukhopadhyay, S.; Lin, E.Y.; Parker, S.J.; et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature 2020, 581, 100–105. [Google Scholar] [CrossRef]
- Gill, J.; Prasad, V. Pembrolizumab for non–muscle-invasive bladder cancer—A costly therapy in search of evidence. JAMA Oncol. 2021, 7, 501–502. [Google Scholar] [CrossRef] [PubMed]
- Rouanne, M.; Betari, R.; Radulescu, C.; Goubar, A.; Signolle, N.; Neuzillet, Y.; Allory, Y.; Marabelle, A.; Adam, J.; Lebret, T. Stromal lymphocyte infiltration is associated with tumour invasion depth but is not prognostic in high-grade T1 bladder cancer. Eur. J. Cancer 2019, 108, 111–119. [Google Scholar] [CrossRef]
- Bayerl, F.; Meiser, P.; Donakonda, S.; Hirschberger, A.; Lacher, S.B.; Pedde, A.-M.; Hermann, C.D.; Elewaut, A.; Knolle, M.; Ramsauer, L.; et al. Tumor-derived prostaglandin E2 programs cDC1 dysfunction to impair intratumoral orchestration of anti-cancer T cell responses. Immunity 2023, 56, 1341–1358.e11. [Google Scholar] [CrossRef]
- Ibrahim, O.M.; Basse, P.H.; Jiang, W.; Guru, K.; Chatta, G.; Kalinski, P. NFκB-Activated COX2/PGE2/EP4 Axis Controls the Magnitude and Selectivity of BCG-Induced Inflammation in Human Bladder Cancer Tissues. Cancers 2021, 13, 1323. [Google Scholar] [CrossRef] [PubMed]
- Prima, V.; Kaliberova, L.N.; Kaliberov, S.; Curiel, D.T.; Kusmartsev, S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc. Natl. Acad. Sci. USA 2017, 114, 1117–1122. [Google Scholar] [CrossRef]
- Chenard, S.; Jackson, C.; Vidotto, T.; Chen, L.; Hardy, C.; Jamaspishvilli, T.; Berman, D.; Siemens, D.R.; Koti, M. Sexual dimorphism in outcomes of non–muscle-invasive bladder cancer: A role of CD163+ macrophages, B cells, and PD-L1 immune checkpoint. Eur. Urol. Open Sci. 2021, 29, 50–58. [Google Scholar] [CrossRef]
- Karapetyan, L.; Gooding, W.; Li, A.; Yang, X.; Knight, A.; Abushukair, H.M.; De Stefano, D.V.; Sander, C.; Karunamurthy, A.; Panelli, M.; et al. Sentinel Lymph Node Gene Expression Signature Predicts Recurrence-Free Survival in Cutaneous Melanoma. Cancers 2022, 14, 4973. [Google Scholar] [CrossRef]
- Storkus, W.J.; Maurer, D.; Lin, Y.; Ding, F.; Bose, A.; Lowe, D.; Rose, A.; DeMark, M.; Karapetyan, L.; Taylor, J.L.; et al. Dendritic cell vaccines targeting tumor blood vessel antigens in combination with dasatinib induce therapeutic immune responses in patients with checkpoint-refractory advanced melanoma. J. Immunother. Cancer 2021, 9, e003675. [Google Scholar] [CrossRef] [PubMed]
- Yolmo, P.; Rahimi, S.; Chenard, S.; Conseil, G.; Jenkins, D.; Sachdeva, K.; Emon, I.; Hamilton, J.; Xu, M.; Rangachari, M.; et al. Atypical B cells mediate poor response to Bacillus Calmette Guérin immunotherapy in non-muscle invasive bladder cancer. bioRxiv 2023. [Google Scholar] [CrossRef]
- De Jong, F.C.; Laajala, T.D.; Hoedemaeker, R.F.; Jordan, K.R.; van der Made, A.C.J.; Boevé, E.R.; van der Schoot, D.K.E.; Nieuwkamer, B.; Janssen, E.A.M.; Mahmoudi, T.; et al. Non–muscle-invasive bladder cancer molecular subtypes predict differential response to intravesical Bacillus Calmette-Guérin. Sci. Transl. Med. 2023, 15, eabn4118. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Yang, X.; Liu, Y.; Liu, Y.; Li, Y.; Sun, L.; Yang, X.; Niu, H. Bacillus Calmette–Guérin and anti-PD-L1 combination therapy boosts immune response against bladder cancer. OncoTargets Ther. 2018, 11, 2891–2899. [Google Scholar] [CrossRef]
- Hashizume, A.; Umemoto, S.; Yokose, T.; Nakamura, Y.; Yoshihara, M.; Shoji, K.; Wada, S.; Miyagi, Y.; Kishida, T.; Sasada, T. Enhanced expression of PD-L1 in non-muscle-invasive bladder cancer after treatment with Bacillus Calmette-Guerin. Oncotarget 2018, 9, 34066–34078. [Google Scholar] [CrossRef]
- Lim, C.J.; Nguyen, P.H.D.; Wasser, M.; Kumar, P.; Lee, Y.H.; Nasir, N.J.M.; Chua, C.; Lai, L.; Hazirah, S.N.; Loh, J.J.H.; et al. Immunological hallmarks for clinical response to BCG in bladder cancer. Front. Immunol. 2021, 11, 3634. [Google Scholar] [CrossRef]
- Inman, B.A.; Sebo, T.J.; Frigola, X.; Dong, H.; Bergstralh, E.J.; Frank, I.; Fradet, Y.; Lacombe, L.; Kwon, E.D. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: Associations with localized stage progression. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2007, 109, 1499–1505. [Google Scholar] [CrossRef]
- Woldu, S.L.; Gerald, T.; Margulis, V.; Halstuch, D.; Ber, Y.; Lifshitz, K.; Margel, D.; Lotan, Y.; Jia, L. PD-L1 expression and BCG response in nonmuscle invasive bladder cancer. J. Clin. Oncol. 2022, 40, 6. [Google Scholar] [CrossRef]
- Rodríguez-Izquierdo, M.; Del Cañizo, C.G.; Rubio, C.; Reina, I.A.; Hernández Arroyo, M.; Rodríguez Antolín, A.; Dueñas Porto, M.; Guerrero-Ramos, F. Immune Predictors of Response after Bacillus Calmette–Guérin Treatment in Non-Muscle-Invasive Bladder Cancer. Cancers 2023, 15, 5554. [Google Scholar] [CrossRef]
- Copland, A.; Sparrow, A.; Hart, P.; Diogo, G.R.; Paul, M.; Azuma, M.; Reljic, R. Bacillus Calmette-Guérin induces PD-L1 expression on antigen-presenting cells via autocrine and paracrine interleukin-STAT3 circuits. Sci. Rep. 2019, 9, 3655. [Google Scholar] [CrossRef]
- Kates, M.; Matoso, A.; Choi, W.; Baras, A.S.; Daniels, M.J.; Lombardo, K.; Brant, A.; Mikkilineni, N.; McConkey, D.J.; Kamat, A.M.; et al. Adaptive immune resistance to intravesical BCG in non–muscle invasive bladder cancer: Implications for prospective BCG-unresponsive trials. Clin. Cancer Res. 2020, 26, 882–891. [Google Scholar] [CrossRef]
- Squibb, B.-M. A Phase 3, Randomized, Double-Blind Trial of Nivolumab in Combination with Intravesical BCG Versus Standard of Care BCG Alone in Participants with High-Risk Non-Muscle Invasive Bladder Cancer That Is Persistent or Recurrent after Treatment with BCG. Available online: https://clinicaltrials.gov/study/NCT04149574 (accessed on 13 April 2024).
- Pfizer. CREST: Combination of Sasanlimab and Alternative BCG Regimens to Evaluate outcomes with Subcutaneous Anti-PD-1 Treatment. Available online: https://clinicaltrials.gov/study/NCT041653172023 (accessed on 13 April 2024).
- Hahn, N. PhAse 1/2 StuDy of Modern ImmunotherApy in BCG-Unresponsive, BCG-RelaPsing, and High-Risk BCG-Naive Non-muscle Invasive UroThelial Carcinoma of the BLADDER. Available online: https://clinicaltrials.gov/study/NCT033171582023 (accessed on 13 April 2024).
- AstraZeneca. Assessment of Efficacy and Safety of Durvalumab Plus BCG Compared to the Standard Therapy with BCG in Non-muscle Invasive Bladder Cancer (POTOMAC). Available online: https://clinicaltrials.gov/study/NCT035286942023 (accessed on 13 April 2024).
- Fragkoulis, C.; Fragkiadis, E.; Sakellakis, M.; Pinitas, A.; Tzannis, K.; Gavalas, N.; Stamatakos, P.; Leventi, A.; Papadopoulos, G.; Stathouros, G.; et al. Intravesical administration of durvalumab to patients with high risk non muscle invasive bladder cancer after BCG failure. A phase II trial by the Hellenic GU Cancer Group. Eur. Urol. 2023, 83, S604–S605. [Google Scholar] [CrossRef]
- Columbia, U.O.B. Phase I/II Trial of Local Cystoscopic Injection of Tremelimumab Plus Systemic Durvalumab (MEDI4736) for High Risk Non-Muscle Invasive Bladder Cancer. Available online: https://clinicaltrials.gov/study/NCT051206222023 (accessed on 13 April 2024).
- Russo, A.E.; Memon, A.; Ahmed, S. Bladder Cancer and the Urinary Microbiome—New Insights and Future Directions: A Review. Clin. Genitourin. Cancer 2024, 22, 434–444. [Google Scholar] [CrossRef]
- Inman, B.A.; Hahn, N.M.; Stratton, K.; Kopp, R.; Sankin, A.; Skinner, E.; Pohar, K.; Gartrell, B.A.; Pham, S.; Rishipathak, D.; et al. A Phase 1b/2 Study of Atezolizumab with or Without Bacille Calmette-Guérin in Patients with High-risk Non–muscle-invasive Bladder Cancer. Eur. Urol. Oncol. 2023, 6, 313–320. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Approves Pembrolizumab for BCG-Unresponsive, High-Risk Non-Muscle Invasive Bladder Cancer; The Food and Drug Administration: Silver Spring, MD, USA, 2020.
- Balar, A.V.; Kamat, A.M.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.L.; Roumiguié, M.; Krieger, L.E.M.; Singer, E.A.; Bajorin, D.F.; Grivas, P.; et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Roumiguié, M.; Esen, A.A.; Lebret, T.; De Wit, R.; Shore, N.D.; Bajorin, D.F.; Krieger, L.E.M.; Kandori, S.; Uchio, E.M.; et al. Pembrolizumab (pembro) monotherapy for patients (pts) with high-risk non–muscle-invasive bladder cancer (HR NMIBC) unresponsive to bacillus Calmette–Guérin (BCG): Results from cohort B of the phase 2 KEYNOTE-057 trial. J. Clin. Oncol. 2023, 41, LBA442. [Google Scholar] [CrossRef]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.-O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef] [PubMed]
- DE, R.C.F. FDA Approves Nivolumab for Adjuvant Treatment of Urothelial Carcinoma. FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-adjuvant-treatment-urothelial-carcinoma (accessed on 13 April 2024).
- Hahn, N.M.; O’Donnell, M.A.; Efstathiou, J.A.; Zahurak, M.; Rosner, G.L.; Smith, J.; Kates, M.R.; Bivalacqua, T.J.; Tran, P.T.; Song, D.Y.; et al. A phase 1 trial of durvalumab in combination with bacillus Calmette-Guerin (BCG) or external beam radiation therapy in patients with BCG-unresponsive non-muscle-invasive bladder cancer: The Hoosier Cancer Research Network GU16-243 ADAPT-BLADDER study. Eur. Urol. 2023, 83, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Syn, N.L.; Teng, M.W.L.; Mok, T.S.K.; Soo, R.A. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017, 18, e731–e741. [Google Scholar] [CrossRef] [PubMed]
- Deininger, S.; Törzsök, P.; Oswald, D.; Lusuardi, L. Current systemic treatment options in metastatic urothelial carcinoma after progression on checkpoint inhibition therapy—A systemic review combined with single-group meta-analysis of three studies testing enfortumab vedotin. Cancers 2021, 13, 3206. [Google Scholar] [CrossRef]
- Roupret, M.; Neuzillet, Y.; Bertaut, A.; Pignot, G.; Houede, N.; Champiat, S.; Ficher, S.N.-L.; Chausson, M.; Loriot, Y. ALBAN: An open label, randomized, phase III trial, evaluating efficacy of atezolizumab in addition to one year BCG (bacillus Calmette-Guerin) bladder instillation in BCG-naive patients with high-risk nonmuscle invasive bladder cancer (AFU-GETUG 37). J. Clin. Oncol. 2019, 37, TPS4589. [Google Scholar] [CrossRef]
- Chevalier, M.F.; Trabanelli, S.; Racle, J.; Salomé, B.; Cesson, V.; Gharbi, D.; Bohner, P.; Domingos-Pereira, S.; Dartiguenave, F.; Fritschi, A.-S.; et al. ILC2-modulated T cell–to-MDSC balance is associated with bladder cancer recurrence. J. Clin. Investig. 2017, 127, 2916–2929. [Google Scholar] [CrossRef]
- Gao, Q.; Qiu, S.-J.; Fan, J.; Zhou, J.; Wang, X.-Y.; Xiao, Y.-S.; Xu, Y.; Li, Y.-W.; Tang, Z.-Y. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J. Clin. Oncol. 2007, 25, 2586–2593. [Google Scholar] [CrossRef]
- Sinicrope, F.A.; Rego, R.L.; Ansell, S.M.; Knutson, K.L.; Foster, N.R.; Sargent, D.J. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology 2009, 137, 1270–1279. [Google Scholar] [CrossRef]
- Marvel, D.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the tumor microenvironment: Expect the unexpected. J. Clin. Investig. 2015, 125, 3356–3364. [Google Scholar] [CrossRef] [PubMed]
- Solito, S.; Marigo, I.; Pinton, L.; Damuzzo, V.; Mandruzzato, S.; Bronte, V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann. N. Y. Acad. Sci. 2014, 1319, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhao, X.; Xia, Y.; Zhu, X.; Xiao, P. Increased circulating immunosuppressive CD14+ HLA-DR−/low cells correlate with clinical cancer stage and pathological grade in patients with bladder carcinoma. J. Int. Med. Res. 2011, 39, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Takayama, H.; Nishimura, K.; Tsujimura, A.; Nakai, Y.; Nakayama, M.; Aozasa, K.; Okuyama, A.; Nonomura, N. Increased infiltration of tumor associated macrophages is associated with poor prognosis of bladder carcinoma in situ after intravesical bacillus Calmette-Guerin instillation. J. Urol. 2009, 181, 1894–1900. [Google Scholar] [CrossRef] [PubMed]
- Dadi, S.; Chhangawala, S.; Whitlock, B.M.; Franklin, R.A.; Luo, C.T.; Oh, S.A.; Toure, A.; Pritykin, Y.; Huse, M.; Leslie, C.S.; et al. Cancer Immunosurveillance by Tissue-Resident Innate Lymphoid Cells and Innate-like T Cells. Cell 2016, 164, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Carrega, P.; Loiacono, F.; Di Carlo, E.; Scaramuccia, A.; Mora, M.; Conte, R.; Benelli, R.; Spaggiari, G.M.; Cantoni, C.; Campana, S.; et al. NCR(+)ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures. Nat. Commun. 2015, 6, 8280. [Google Scholar] [CrossRef]
- Jovanovic, I.P.; Pejnovic, N.N.; Radosavljevic, G.D.; Pantic, J.M.; Milovanovic, M.Z.; Arsenijevic, N.N.; Lukic, M.L. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int. J. Cancer 2014, 134, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Martino, A.; Badell, E.; Abadie, V.; Balloy, V.; Chignard, M.; Mistou, M.-Y.; Combadière, B.; Combadière, C.; Winter, N. Mycobacterium bovis bacillus calmette-guérin vaccination mobilizes innate myeloid-derived suppressor cells restraining in vivo T cell priming via IL-1R–dependent nitric oxide production. J. Immunol. 2010, 184, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Papotto, P.H.; Maeda, S.; Tomimori, J.; Xavier, M.B.; Rizzo, L.V.; Kallas, E.G.; Carvalho, K.I. New players in the same old game: Disturbance of group 2 innate lymphoid cells in HIV-1 and mycobacterium leprae co-infected patients. PLoS Neglected Trop. Dis. 2015, 9, e0004030. [Google Scholar] [CrossRef]
- Bernink, J.H.; Krabbendam, L.; Germar, K.; de Jong, E.; Gronke, K.; Kofoed-Nielsen, M.; Munneke, J.M.; Hazenberg, M.D.; Villaudy, J.; Buskens, C.J.; et al. Interleukin-12 and -23 control plasticity of CD127+ group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 2015, 43, 146–160. [Google Scholar] [CrossRef]
- Silver, J.S.; Kearley, J.; Copenhaver, A.M.; Sanden, C.; Mori, M.; Yu, L.; Pritchard, G.H.; Berlin, A.A.; Hunter, C.A.; Bowler, R.; et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat. Immunol. 2016, 17, 626–635. [Google Scholar] [CrossRef]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Obermajer, N.; Muthuswamy, R.; Lesnock, J.; Edwards, R.P.; Kalinski, P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood 2011, 118, 5498–5505. [Google Scholar] [CrossRef]
- Obermajer, N.; Muthuswamy, R.; Odunsi, K.; Edwards, R.P.; Kalinski, P. PGE2-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer EnvironmentPGE2 controls CXCR4-driven accumulation of MDSCs. Cancer Res. 2011, 71, 7463–7470. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.L.; Obermajer, N.; Odunsi, K.; Edwards, R.P.; Kalinski, P. Synergistic COX2 Induction by IFNγ and TNFα Self-Limits Type-1 Immunity in the Human Tumor Microenvironment. Cancer Immunol. Res. 2016, 4, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Obermajer, N.; Kalinski, P. Key role of the positive feedback between PGE(2) and COX2 in the biology of myeloid-derived suppressor cells. Oncoimmunology 2012, 1, 762–764. [Google Scholar] [CrossRef]
- Theodoraki, M.-N.; Yerneni, S.; Sarkar, S.N.; Orr, B.; Muthuswamy, R.; Voyten, J.; Modugno, F.; Jiang, W.; Grimm, M.; Basse, P.H.; et al. Helicase-Driven Activation of NFκB-COX2 Pathway Mediates the Immunosuppressive Component of dsRNA-Driven Inflammation in the Human Tumor Microenvironment. Cancer Res. 2018, 78, 4292–4302. [Google Scholar] [CrossRef]
- Rodríguez-Ubreva, J.; Català-Moll, F.; Obermajer, N.; Álvarez-Errico, D.; Ramirez, R.N.; Company, C.; Vento-Tormo, R.; Moreno-Bueno, G.; Edwards, R.P.; Mortazavi, A.; et al. Prostaglandin E2 Leads to the Acquisition of DNMT3A-Dependent Tolerogenic Functions in Human Myeloid-Derived Suppressor Cells. Cell Rep. 2017, 21, 154–167. [Google Scholar] [CrossRef]
- Muthuswamy, R.; Berk, E.; Junecko, B.F.; Zeh, H.J.; Zureikat, A.H.; Normolle, D.; Luong, T.M.; Reinhart, T.A.; Bartlett, D.L.; Kalinski, P. NF-κB hyperactivation in tumor tissues allows tumor-selective reprogramming of the chemokine microenvironment to enhance the recruitment of cytolytic T effector cells. Cancer Res. 2012, 72, 3735–3743. [Google Scholar] [CrossRef] [PubMed]
- Muthuswamy, R.; Corman, J.M.; Dahl, K.; Chatta, G.S.; Kalinski, P. Functional reprogramming of human prostate cancer to promote local attraction of effector CD8(+) T cells. Prostate 2016, 76, 1095–1105. [Google Scholar] [CrossRef]
- Muthuswamy, R.; Urban, J.; Lee, J.-J.; Reinhart, T.A.; Bartlett, D.; Kalinski, P. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. 2008, 68, 5972–5978. [Google Scholar] [CrossRef] [PubMed]
- Muthuswamy, R.; Wang, L.; Pitteroff, J.; Gingrich, J.R.; Kalinski, P. Combination of IFNα and poly-I:C reprograms bladder cancer microenvironment for enhanced CTL attraction. J. Immunother. Cancer 2015, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, O.M.; Pandey, R.K.; Chatta, G.; Kalinski, P. Role of tumor microenvironment in the efficacy of BCG therapy. Trends Res. 2020, 3. [Google Scholar] [CrossRef] [PubMed]
- Pallotta, M.T.; Rossini, S.; Suvieri, C.; Coletti, A.; Orabona, C.; Macchiarulo, A.; Volpi, C.; Grohmann, U. Indoleamine 2,3-dioxygenase 1 (IDO1): An up-to-date overview of an eclectic immunoregulatory enzyme. FEBS J. 2021, 289, 6099–6118. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liang, H.; Fang, X.; Zhang, S.; Xing, Z.; Shi, L.; Kuang, C.; Seliger, B.; Yang, Q. What is the prospect of indoleamine 2,3-dioxygenase 1 inhibition in cancer? Extrapolation from the past. J. Exp. Clin. Cancer Res. 2021, 40, 60. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Wu, Y.-H.; Song, Y.; Yu, B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, J.; Zhang, Z.; Guo, Y.; Wu, Y.; Wang, R.; Wang, L.; Mao, S.; Yao, X. Overexpression of Indoleamine 2,3-Dioxygenase 1 Promotes Epithelial-Mesenchymal Transition by Activation of the IL-6/STAT3/PD-L1 Pathway in Bladder Cancer. Transl. Oncol. 2019, 12, 485–492. [Google Scholar] [CrossRef]
- Santos, H.; Matheus, L.H.G.; Silva, A.; Dalmazzo, S.V.; Santos, A.A.; Santos, L.; Souza, D.M.; Reis, S.T.; Nascimento, I.P.; Dellê, H. Indoleamine 2,3-Dioxygenase-1 Expression is Changed During Bladder Cancer Cell Invasion. Int. J. Tryptophan Res. 2022, 15, 11786469211065612. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Zhou, Q. Prognostic role of indoleamine 2,3-dioxygenase 1 expression in solid tumors: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 954495. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.T.; Nguyen, T.T.; Nguyen, T.Q.; Luu, D.T.; Pham, T.N.D.; Bui, T.T.T.; Duong, T.T.; Phan, D.A.T.; Ngo, Q.D. Abstract 5884: Comprehensive analyzing the expression of IDO1 and TDO2 in bladder cancer. Cancer Res. 2022, 82, 5884. [Google Scholar] [CrossRef]
- Matheus, L.H.G.; Dalmazzo, S.V.; Brito, R.B.O.; Pereira, L.A.; de Almeida, R.J.; Camacho, C.P.; Dellê, H. 1-Methyl-D-tryptophan activates aryl hydrocarbon receptor, a pathway associated with bladder cancer progression. BMC Cancer 2020, 20, 869. [Google Scholar] [CrossRef] [PubMed]
- Meireson, A.; Devos, M.; Brochez, L. IDO Expression in Cancer: Different Compartment, Different Functionality? Front. Immunol. 2020, 11, 531491. [Google Scholar] [CrossRef] [PubMed]
- Vidotto, T.; Nersesian, S.; Graham, C.; Siemens, D.R.; Koti, M. DNA damage repair gene mutations and their association with tumor immune regulatory gene expression in muscle invasive bladder cancer subtypes. J. Immunother. Cancer 2019, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, A.; Lashinger, L.; Millikan, R.; Grossman, H.B.; Benedict, W.; Dinney, C.P.N.; McConkey, D.J. Role of tumor necrosis factor-related apoptosis-inducing ligand in interferon-induced apoptosis in human bladder cancer cells. Cancer Res. 2004, 64, 8973–8979. [Google Scholar] [CrossRef] [PubMed]
- Joudi, F.N.; Smith, B.J.; O’donnell, M.A. Final results from a national multicenter phase II trial of combination bacillus Calmette-Guérin plus interferon α-2B for reducing recurrence of superficial bladder cancer☆. Urol. Oncol. Semin. Orig. Investig. 2006, 24, 344–348. [Google Scholar] [CrossRef]
- Nepple, K.G.; Lightfoot, A.J.; Rosevear, H.M.; O’Donnell, M.A.; Lamm, D.L.; Bladder Cancer Genitourinary Oncology Study Group. Bacillus Calmette-Guérin with or without interferon α-2b and megadose versus recommended daily allowance vitamins during induction and maintenance intravesical treatment of nonmuscle invasive bladder cancer. J. Urol. 2010, 184, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Lamm, D.; Brausi, M.; O’Donnell, M.A.; Witjes, J.A. Interferon alfa in the treatment paradigm for non–muscle-invasive bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 35.e21–35.e30. [Google Scholar] [CrossRef]
- Fuge, O.; Vasdev, N.; Allchorne, P.; Green, J.S. Immunotherapy for bladder cancer. Res. Rep. Urol. 2015, 7, 65–79. [Google Scholar]
- Sharma, P.; Shen, Y.; Wen, S.; Yamada, S.; Jungbluth, A.A.; Gnjatic, S.; Bajorin, D.F.; Reuter, V.E.; Herr, H.; Old, L.J.; et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 3967–3972. [Google Scholar] [CrossRef] [PubMed]
- Pichler, R.; Fritz, J.; Zavadil, C.; Schäfer, G.; Culig, Z.; Brunner, A. Tumor-infiltrating immune cell subpopulations influence the oncologic outcome after intravesical Bacillus Calmette-Guérin therapy in bladder cancer. Oncotarget 2016, 7, 39916–39930. [Google Scholar] [CrossRef] [PubMed]
- Fenner, A. BCG enriches Treg cells. Nat. Rev. Urol. 2018, 15, 591. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.; Kirby, J.; Atkins, H.; Davies, B.; Kelly, J. Cyclooxygenase-2 inhibition: A potential mechanism for increasing the efficacy of bacillus calmette-guerin immunotherapy for bladder cancer. J. Urol. 2005, 174, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Kirby, J.A.; Davies, B.R.; Leung, H.; Kelly, J.D. Celecoxib has potent antitumour effects as a single agent and in combination with BCG immunotherapy in a model of urothelial cell carcinoma. Eur. Urol. 2008, 54, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Kurtova, A.V.; Xiao, J.; Mo, Q.; Pazhanisamy, S.; Krasnow, R.; Lerner, S.P.; Chen, F.; Roh, T.T.; Lay, E.; Ho, P.L.; et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature 2015, 517, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.R.; Pelly, V.S.; Moeini, A.; Chiang, S.-C.; Flanagan, E.; Bromley, C.P.; Clark, C.; Earnshaw, C.H.; Koufaki, M.A.; Bonavita, E.; et al. Chemotherapy-induced COX-2 upregulation by cancer cells defines their inflammatory properties and limits the efficacy of chemoimmunotherapy combinations. Nat. Commun. 2022, 13, 2063. [Google Scholar] [CrossRef]
- Bell, C.R.; Zelenay, S. COX-2 upregulation by tumour cells post-chemotherapy fuels the immune evasive dark side of cancer inflammation. Cell Stress 2022, 6, 76–78. [Google Scholar] [CrossRef]
- Pelly, V.S.; Moeini, A.; Roelofsen, L.M.; Bonavita, E.; Bell, C.R.; Hutton, C.; Blanco-Gomez, A.; Banyard, A.; Bromley, C.P.; Flanagan, E.; et al. Anti-Inflammatory Drugs Remodel the Tumor Immune Environment to Enhance Immune Checkpoint Blockade Efficacy. Cancer Discov. 2021, 11, 2602–2619. [Google Scholar] [CrossRef]
- Zelenay, S.; Reis e Sousa, C. Reducing prostaglandin E2production to raise cancer immunogenicity. Oncoimmunology 2016, 5, e1123370. [Google Scholar] [CrossRef]
- Zelenay, S.; van der Veen, A.G.; Böttcher, J.P.; Snelgrove, K.J.; Rogers, N.; Acton, S.E.; Chakravarty, P.; Girotti, M.R.; Marais, R.; Quezada, S.A.; et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell 2015, 162, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Koblish, H.K.; Horton, B.; Scherle, P.A.; Newton, R.; Gajewski, T.F. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8 T cells directly within the tumor microenvironment. J. Immunother. Cancer 2014, 2, 3. [Google Scholar] [CrossRef]
- Squibb, B.-M. A Phase 2, Randomized, Open-label Study of Nivolumab or Nivolumab/BMS-986205 Alone or Combined with Intravesical BCG in Participants with BCG-Unresponsive, High-Risk, Non-Muscle Invasive Bladder Cancer. 2023. Available online: https://clinicaltrials.gov/study/NCT03519256 (accessed on 13 April 2024).
- Cui, X.; Shen, D.; Kong, C.; Zhang, Z.; Zeng, Y.; Lin, X.; Liu, X. NF-κB suppresses apoptosis and promotes bladder cancer cell proliferation by upregulating survivin expression in vitro and in vivo. Sci. Rep. 2017, 7, 40723. [Google Scholar] [CrossRef] [PubMed]
- Pikarsky, E.; Porat, R.M.; Stein, I.; Abramovitch, R.; Amit, S.; Kasem, S.; Gutkovich-Pyest, E.; Urieli-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 2004, 431, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. Nuclear factor-κB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Maeda, S.; Hikiba, Y.; Nakagawa, H.; Hayakawa, Y.; Shibata, W.; Yanai, A.; Ogura, K.; Omata, M. Constitutive NF-κB activation in colorectal carcinoma plays a key role in angiogenesis, promoting tumor growth. Clin. Cancer Res. 2009, 15, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, O.M.; El-Deeb, N.M.; Abbas, H.; Elmasry, S.M.; El-Aassar, M. Alginate based tamoxifen/metal dual core-folate decorated shell: Nanocomposite targeted therapy for breast cancer via ROS-driven NF-κB pathway modulation. Int. J. Biol. Macromol. 2020, 146, 119–131. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, N.M.; Ibrahim, O.M.; Mohamed, M.A.; Farag, M.M.S.; Farrag, A.A.; El-Aassar, M.R. Alginate/κ-carrageenan oral microcapsules loaded with Agaricus bisporus polysaccharides MH751906 for natural killer cells mediated colon cancer immunotherapy. Int. J. Biol. Macromol. 2022, 205, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Kanda, S.; Nomata, K.; Eguchi, J.; Kanetake, H. Expression of Cyclooxygenase-2 and Ep4 receptor in transitional cell carcinoma of the upper urinary tract. J. Urol. 2005, 173, 56–60. [Google Scholar] [CrossRef]
- Gandhi, S.; Opyrchal, M.; Grimm, M.J.; Slomba, R.T.; Kokolus, K.M.; Witkiewicz, A.; Attwood, K.; Groman, A.; Williams, L.; Tarquini, M.L.; et al. Systemic infusion of TLR3-ligand and IFN-α in patients with breast cancer reprograms local tumor microenvironments for selective CTL influx. J. Immunother. Cancer 2023, 11, e007381. [Google Scholar] [CrossRef]
- Orr, B.; Mahdi, H.; Fang, Y.; Strange, M.; Uygun, I.; Rana, M.; Zhang, L.; Mora, A.S.; Pusateri, A.; Elishaev, E.; et al. Phase I Trial Combining Chemokine-Targeting with Loco-Regional Chemoimmunotherapy for Recurrent, Platinum-Sensitive Ovarian Cancer Shows Induction of CXCR3 Ligands and Markers of Type 1 Immunity. Clin. Cancer Res. 2022, 28, 2038–2049. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.S.; Strange, M.; Fang, Y.; Uygun, I.; Zhang, L.; Tseng, G.C.; Kalinski, P.; Edwards, R.P.; Vlad, A.M. Longitudinal Modulation of Loco-Regional Immunity in Ovarian Cancer Patients Receiving Intraperitoneal Chemotherapy. Cancers 2022, 14, 5647. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zeng, H.; Jin, K.; Shao, F.; Liu, Z.; Chang, Y.; Wang, Y.; Zhu, Y.; Wang, Z.; Xu, L.; et al. NKG2A and PD-L1 expression panel predicts clinical benefits from adjuvant chemotherapy and PD-L1 blockade in muscle-invasive bladder cancer. J. Immunother. Cancer 2022, 10, e004569. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.S.; Funt, S.A.; Borch, A.; Munk, K.K.; Bjerregaard, A.-M.; Reading, J.L.; Maher, C.; Regazzi, A.; Wong, P.; Al-Ahmadie, H.; et al. Neoantigen-specific CD8 T cell responses in the peripheral blood following PD-L1 blockade might predict therapy outcome in metastatic urothelial carcinoma. Nat. Commun. 2022, 13, 1935. [Google Scholar] [CrossRef]
- Wang, L.; Saci, A.; Szabo, P.M.; Chasalow, S.D.; Castillo-Martin, M.; Domingo-Domenech, J.; Siefker-Radtke, A.; Sharma, P.; Sfakianos, J.P.; Gong, Y.; et al. EMT- and stroma-related gene expression and resistance to PD-1 blockade in urothelial cancer. Nat. Commun. 2018, 9, 3503. [Google Scholar] [CrossRef]
| Treatment | Study Phase | Trial | Reference |
|---|---|---|---|
| Nivolumab and BCG | Phase III | NCT04149574 | [68] |
| Sasanlimab and BCG | Phase III | NCT04165317 | [69] |
| Durvalumab and BCG or EBRT | Phase I | NCT03317158 | [70] |
| Durvalumab and BCG | Phase III | NCT03528694 | [71] |
| Durvalumab | Phase II | NCT03759496 | [72] |
| Durvalumab and Tremelimumab | Phase I/II | NCT05120622 | [73] |
| Durvalumab and S-488210/S-488211 | Phase I/II | NCT04106115 | [74] |
| Atezolizumab | Phase Ib/II | NCT02792192 | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, O.M.; Kalinski, P. Breaking Barriers: Modulation of Tumor Microenvironment to Enhance Bacillus Calmette–Guérin Immunotherapy of Bladder Cancer. Cells 2024, 13, 699. https://doi.org/10.3390/cells13080699
Ibrahim OM, Kalinski P. Breaking Barriers: Modulation of Tumor Microenvironment to Enhance Bacillus Calmette–Guérin Immunotherapy of Bladder Cancer. Cells. 2024; 13(8):699. https://doi.org/10.3390/cells13080699
Chicago/Turabian StyleIbrahim, Omar M., and Pawel Kalinski. 2024. "Breaking Barriers: Modulation of Tumor Microenvironment to Enhance Bacillus Calmette–Guérin Immunotherapy of Bladder Cancer" Cells 13, no. 8: 699. https://doi.org/10.3390/cells13080699
APA StyleIbrahim, O. M., & Kalinski, P. (2024). Breaking Barriers: Modulation of Tumor Microenvironment to Enhance Bacillus Calmette–Guérin Immunotherapy of Bladder Cancer. Cells, 13(8), 699. https://doi.org/10.3390/cells13080699





