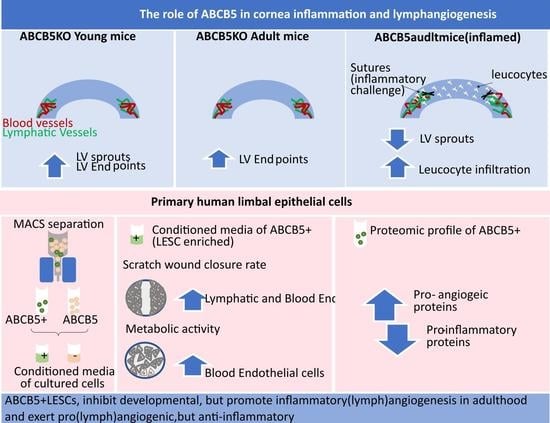
ABCB5+ Limbal Epithelial Stem Cells Inhibit Developmental but Promote Inflammatory (Lymph) Angiogenesis While Preventing Corneal Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Induction and Quantification of Corneal Hemangiogenesis and Lymphangiogenesis
2.3. Immunohistochemistry of Neovascularization
2.4. Primary Human Limbal Epithelial Cell Harvesting and Maintenance
2.5. Maintenance of Human Lymphatic and Blood Endothelial Cells
2.6. ABCB5-Positive and Negative Cell Sorting
2.7. Post-Sorting FACS Analysis
2.8. Collection of Conditioned Media
2.9. Alamar Blue Assay
2.10. Tube Formation Assay
2.11. Proteomics Analysis
Sample Preparation by SP3
2.12. LCMS Data Independent Acquisition
Used System
2.13. Data Independent Acquisition of Samples
2.14. Data Processing and Analysis
2.15. Statistical Analysis
2.16. Study Approval and Ethics Statement for the Use of Human Tissue
3. Results
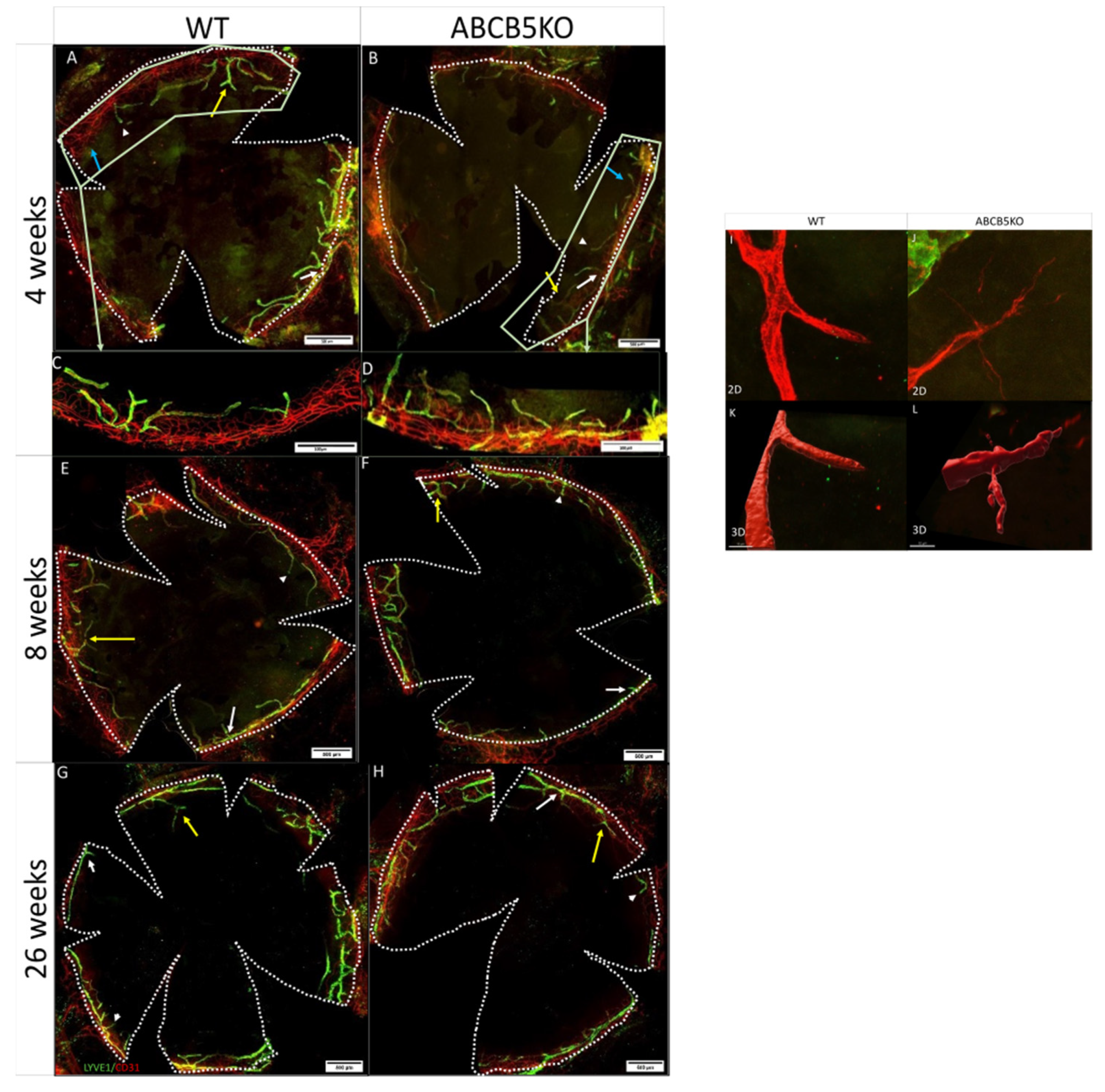
3.1. The Effect of ABCB5 on the Developing and Adult Limbal Vasculature
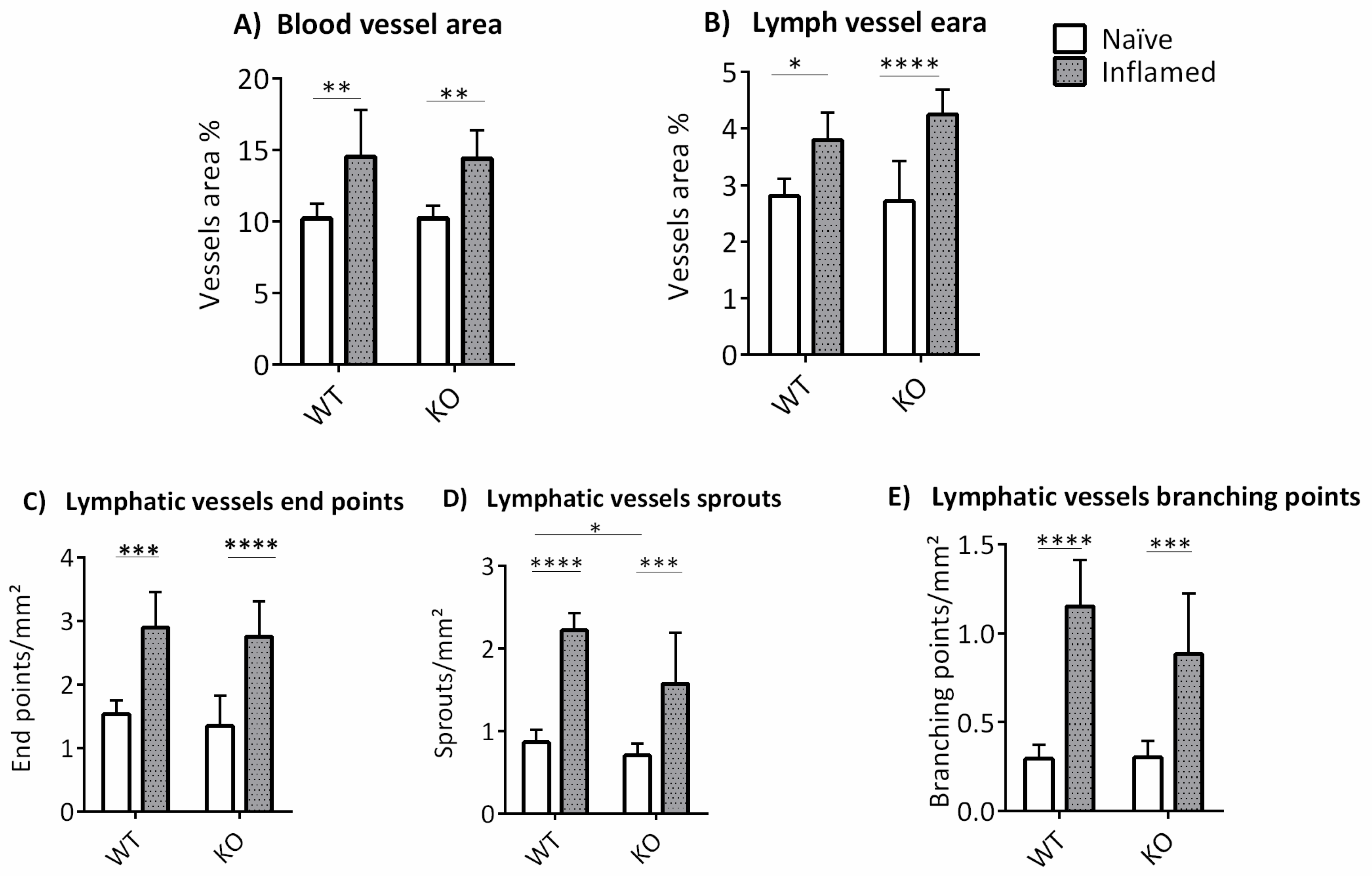
3.2. The Influence of ABCB5 in Suture-Induced Corneal Neovascularization
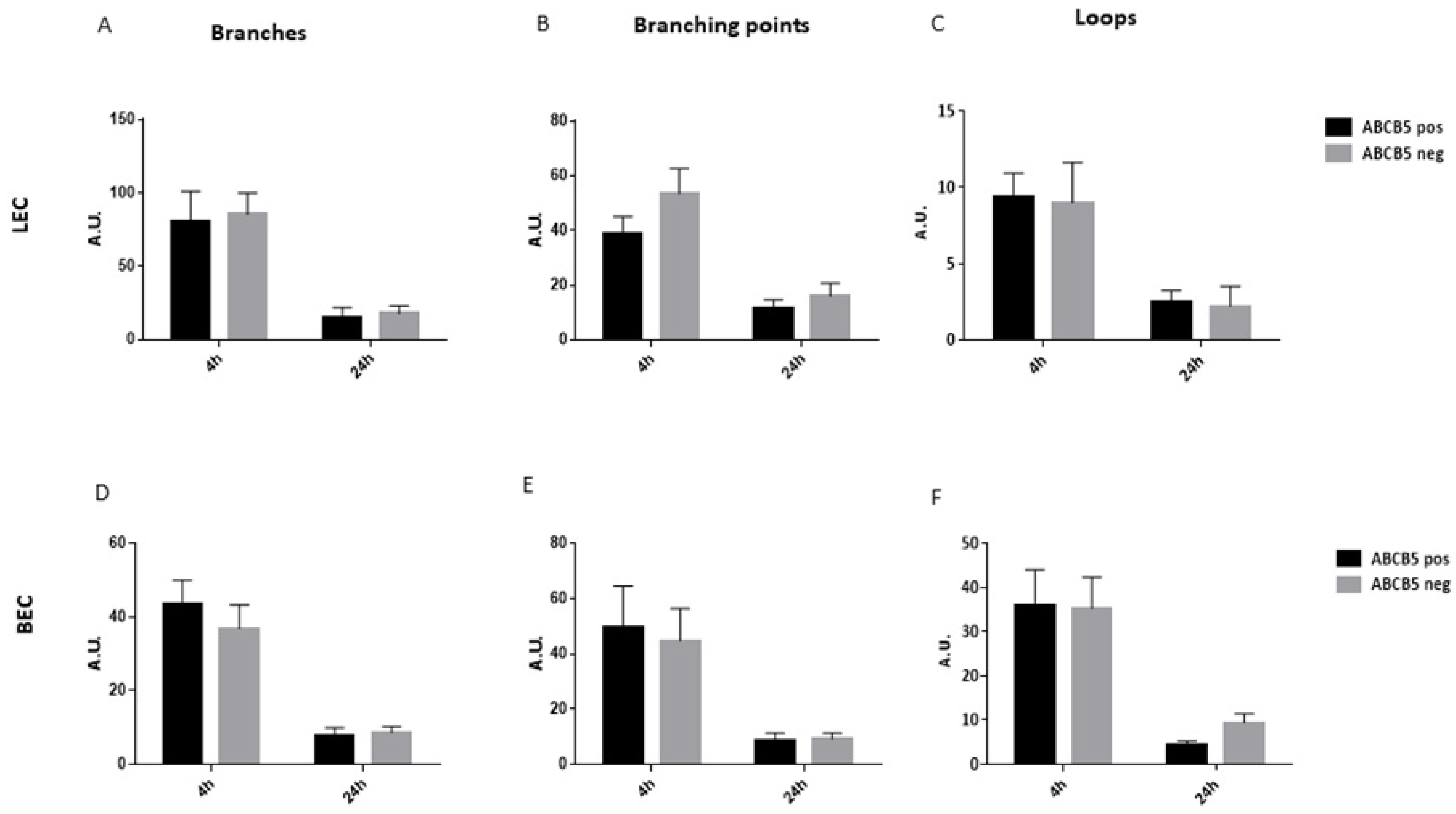
3.3. The Absence of ABCB5 in Human Limbal Epithelial Cells Alters Their Paracrine Activity on Blood and Lymphatic Endothelial Cells
3.4. Proteomics Analysis: Donor Variance and Functional Distribution of Identified Proteins
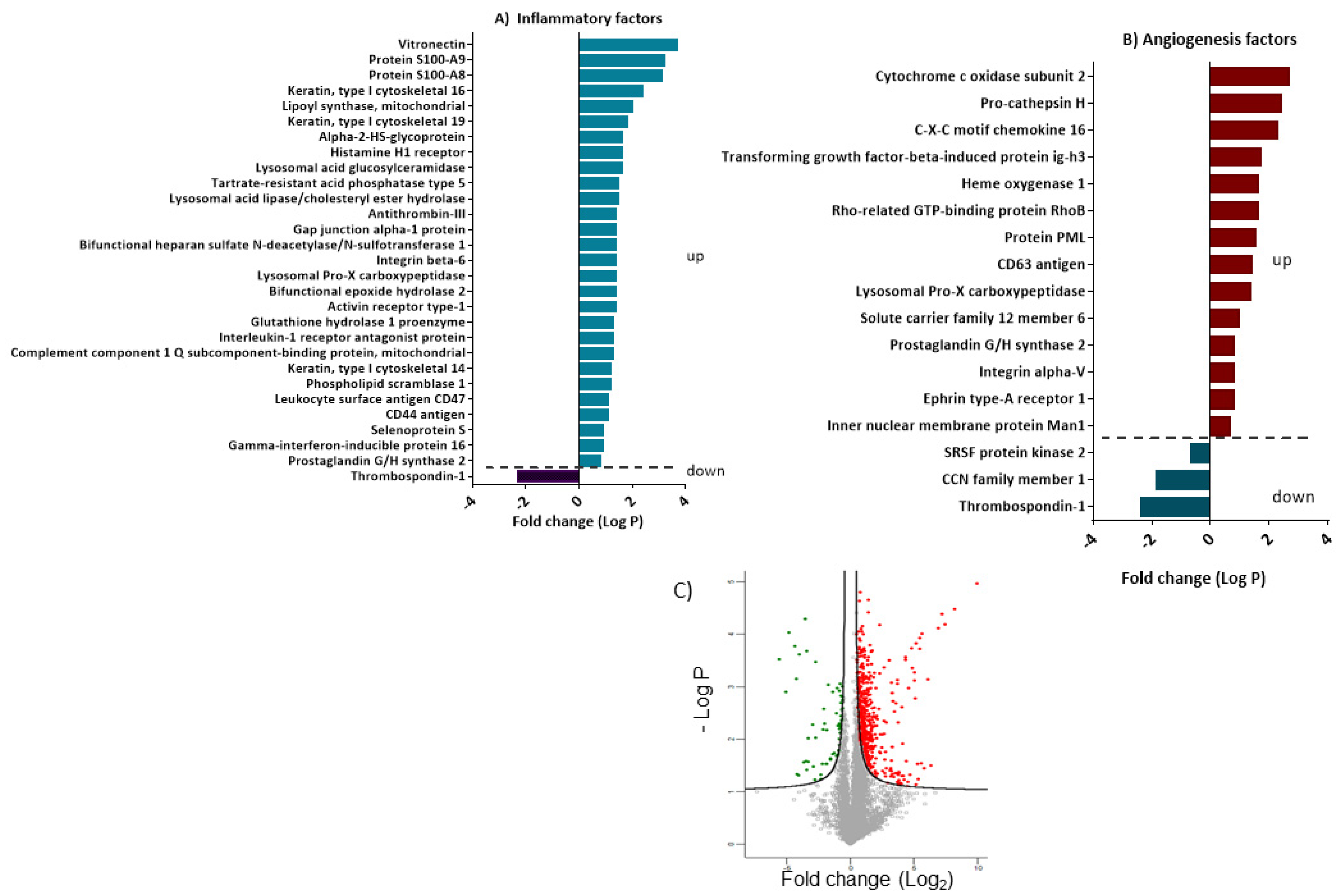
3.5. Differentially Expressed Proteins Involved in Inflammation and Angiogenesis
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frank, N.Y.; Pendse, S.S.; Lapchak, P.H.; Margaryan, A.; Shlain, D.; Doeing, C.; Sayegh, M.H.; Frank, M.H. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J. Biol. Chem. 2003, 278, 47156–47165. [Google Scholar] [CrossRef] [Green Version]
- Schatton, T.; Murphy, G.F.; Frank, N.Y.; Yamaura, K.; Waaga-Gasser, A.M.; Gasser, M.; Zhan, Q.; Jordan, S.; Duncan, L.M.; Weishaupt, C.; et al. Identification of cells initiating human melanomas. Nature 2008, 451, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Ksander, B.R.; Kolovou, P.E.; Wilson, B.J.; Saab, K.R.; Guo, Q.; Ma, J.; McGuire, S.P.; Gregory, M.S.; Vincent, W.J.; Perez, V.L.; et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature 2014, 511, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Vereb, Z.; Albert, R.; Poliska, S.; Olstad, O.K.; Akhtar, S.; Moe, M.C.; Petrovski, G. Comparison of upstream regulators in human ex vivo cultured cornea limbal epithelial stem cells and differentiated corneal epithelial cells. BMC Genom. 2013, 14, 900. [Google Scholar] [CrossRef] [Green Version]
- Notara, M.; Refaian, N.; Braun, G.; Steven, P.; Bock, F.; Cursiefen, C. Short-Term Ultraviolet A Irradiation Leads to Dysfunction of the Limbal Niche Cells and an Antilymphangiogenic and Anti-inflammatory Micromilieu. Investig. Ophthalmol. Vis. Sci. 2016, 57, 928–939. [Google Scholar] [CrossRef] [Green Version]
- Notara, M.; Refaian, N.; Braun, G.; Steven, P.; Bock, F.; Cursiefen, C. Short-term uvb-irradiation leads to putative limbal stem cell damage and niche cell-mediated upregulation of macrophage recruiting cytokines. Stem Cell Res. 2015, 15, 643–654. [Google Scholar] [CrossRef] [Green Version]
- Maddula, S.; Davis, D.K.; Burrow, M.K.; Ambati, B.K.; Lim, P.; Fuchsluger, T.A.; Jurkunas, U.V. Horizons in therapy for corneal angiogenesis limbal stem cell deficiency and corneal neovascularization. Ophthalmology 2011, 118, 591–599. [Google Scholar] [CrossRef] [Green Version]
- Lim, P.; Fuchsluger, T.A.; Jurkunas, U.V. Limbal stem cell deficiency and corneal neovascularization. Semin. Ophthalmol. 2009, 24, 139–148. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Saint-Geniez, M.; Hamrah, P.; Jin, Y.; Rashid, S.; Pytowski, B.; Persaud, K.; Wu, Y.; Streilein, J.W.; et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc. Natl. Acad. Sci. USA 2006, 103, 11405–11410. [Google Scholar]
- Ambati, B.K.; Patterson, E.; Jani, P.; Jenkins, C.; Higgins, E.; Singh, N.; Suthar, T.; Vira, N.; Smith, K.; Caldwell, R. Soluble vascular endothelial growth factor receptor-1 contributes to the corneal antiangiogenic barrier. Br. J. Ophthalmol. 2007, 91, 505–508. [Google Scholar] [CrossRef] [Green Version]
- Ambati, B.K.; Joussen, A.M.; Ambati, J.; Moromizato, Y.; Guha, C.; Javaherian, K.; Gillies, S.; O’Reilly, M.S.; Adamis, A.P. Angiostatin inhibits and regresses corneal neovascularization. Arch. Ophthalmol. 2002, 120, 1063–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Amin, S.; Richter, E.; Rashid, S.; Scoglietti, V.; Jani, P.D.; Wang, J.; Kaur, R.; Ambati, J.; Dong, Z.; et al. Flt-1 intraceptors inhibit hypoxia-induced VEGF expression in vitro and corneal neovascularization in vivo. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1647–1652. [Google Scholar] [CrossRef]
- Singh, N.; Higgins, E.; Amin, S.; Jani, P.; Richter, E.; Patel, A.; Kaur, R.; Wang, J.; Ambati, J.; Dong, Z.; et al. Unique homologous siRNA blocks hypoxia-induced VEGF upregulation in human corneal cells and inhibits and regresses murine corneal neovascularization. Cornea 2007, 26, 65–72. [Google Scholar] [CrossRef] [PubMed]
- van Setten, G.B. Vascular endothelial growth factor (VEGF) in normal human corneal epithelium: Detection and physiological importance. Acta Ophthalmol. Scand. 1997, 75, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Edelman, J.L.; Castro, M.R.; Wen, Y. Correlation of VEGF expression by leukocytes with the growth and regression of blood vessels in the rat cornea. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1112–1123. [Google Scholar]
- Joussen, A.M.; Poulaki, V.; Mitsiades, N.; Stechschulte, S.U.; Kirchhof, B.; Dartt, D.A.; Fong, G.H.; Rudge, J.; Wiegand, S.J.; Yancopoulos, G.D.; et al. VEGF-dependent conjunctivalization of the corneal surface. Investig. Ophthalmol. Vis. Sci. 2003, 44, 117–123. [Google Scholar] [CrossRef]
- Ma, D.H.; Chen, J.K.; Zhang, F.; Lin, K.Y.; Yao, J.Y.; Yu, J.S. Regulation of corneal angiogenesis in limbal stem cell deficiency. Prog. Retin. Eye Res. 2006, 25, 563–590. [Google Scholar] [CrossRef]
- Hos, D.; Bachmann, B.; Bock, F.; Onderka, J.; Cursiefen, C. Age-related changes in murine limbal lymphatic vessels and corneal lymphangiogenesis. Exp. Eye Res. 2008, 87, 427–432. [Google Scholar] [CrossRef]
- Bock, F.; Onderka, J.; Hos, D.; Horn, F.; Martus, P.; Cursiefen, C. Improved semiautomatic method for morphometry of angiogenesis and lymphangiogenesis in corneal flatmounts. Exp. Eye Res. 2008, 87, 462–470. [Google Scholar] [CrossRef]
- Hughes, C.S.; Moggridge, S.; Müller, T.; Sorensen, P.H.; Morin, G.B.; Krijgsveld, J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019, 14, 68–85. [Google Scholar] [CrossRef]
- Searle, B.C.; Pino, L.K.; Egertson, J.D.; Ting, Y.S.; Lawrence, R.T.; MacLean, B.X.; Villén, J.; MacCoss, M.J. Chromatogram libraries improve peptide detection and quantification by data independent acquisition mass spectrometry. Nat. Commun. 2018, 9, 5128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gessulat, S.; Schmidt, T.; Zolg, D.P.; Samaras, P.; Schnatbaum, K.; Zerweck, J.; Knaute, T.; Rechenberger, J.; Delanghe, B.; Huhmer, A.; et al. Prosit: Proteome-wide prediction of peptide tandem mass spectra by deep learning. Nat. Methods 2019, 16, 509–518. [Google Scholar] [CrossRef]
- Demichev, V.; Messner, C.B.; Vernardis, S.I.; Lilley, K.S.; Ralser, M. DIA-NN: Neural networks and interference correction enable deep proteome coverage in high throughput. Nat. Methods 2020, 17, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig. 2004, 113, 1040–1050. [Google Scholar] [CrossRef]
- Daruich, A.; Duncan, M.; Robert, M.P.; Lagali, N.; Semina, E.V.; Aberdam, D.; Ferrari, S.; Romano, V.; des Roziers, C.B.; Benkortebi, R.; et al. Congenital aniridia beyond black eyes: From phenotype and novel genetic mechanisms to innovative therapeutic approaches. Prog. Retin. Eye Res. 2022, 101133. [Google Scholar] [CrossRef]
- Norrick, A.; Esterlechner, J.; Niebergall-Roth, E.; Dehio, U.; Sadeghi, S.; Schröder, H.M.; Ballikaya, S.; Stemler, N.; Ganss, C.; Dieter, K.; et al. Process development and safety evaluation of ABCB5(+) limbal stem cells as advanced-therapy medicinal product to treat limbal stem cell deficiency. Stem Cell Res. Ther. 2021, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, X.; Tse, J.; Tilahun, F.; Qiu, M.; Chen, L. Spontaneous lymphatic vessel formation and regression in the murine cornea. Investig. Ophthalmol. Vis. Sci. 2011, 52, 334–338. [Google Scholar] [CrossRef] [Green Version]
- Erusalimsky, J.D.; Kurz, D.J. Endothelial cell senescence. Handb. Exp. Pharmacol. 2006, 213–248. [Google Scholar] [CrossRef]
- Minamino, T.; Miyauchi, H.; Yoshida, T.; Tateno, K.; Kunieda, T.; Komuro, I. Vascular cell senescence and vascular aging. J. Mol. Cell. Cardiol. 2004, 36, 175–183. [Google Scholar] [CrossRef]
- Cursiefen, C.; Ikeda, S.; Nishina, P.M.; Smith, R.S.; Ikeda, A.; Jackson, D.; Mo, J.S.; Chen, L.; Dana, M.R.; Pytowski, B.; et al. Spontaneous corneal hem- and lymphangiogenesis in mice with destrin-mutation depend on VEGFR3 signaling. Am. J. Pathol. 2005, 166, 1367–1377. [Google Scholar] [CrossRef] [Green Version]
- Francesconi, C.M.; Hutcheon, A.E.; Chung, E.H.; Dalbone, A.C.; Joyce, N.C.; Zieske, J.D. Expression patterns of retinoblastoma and E2F family proteins during corneal development. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1054–1062. [Google Scholar]
- Huang, S.; Hu, Z.; Wang, P.; Zhang, Y.; Cao, X.; Dong, Y.; Cheng, P.; Xu, H.; Zhu, W.; Tang, B.; et al. Rat epidermal stem cells promote the angiogenesis of full-thickness wounds. Stem Cell Res. Ther. 2020, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Bronckaers, A.; Hilkens, P.; Martens, W.; Gervois, P.; Ratajczak, J.; Struys, T.; Lambrichts, I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol. Ther. 2014, 143, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Komaki, M.; Numata, Y.; Morioka, C.; Honda, I.; Tooi, M.; Yokoyama, N.; Ayame, H.; Iwasaki, K.; Taki, A.; Oshima, N.; et al. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res. Ther. 2017, 8, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, R.; Katagiri, W.; Endo, S.; Kobayashi, T. Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. PLoS ONE 2019, 14, e0225472. [Google Scholar] [CrossRef] [Green Version]
- Duan, C.Y.; Xie, H.T.; Zhao, X.Y.; Xu, W.H.; Zhang, M.C. Limbal niche cells can reduce the angiogenic potential of cultivated oral mucosal epithelial cells. Cell. Mol. Biol. Lett. 2019, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X.; Wu, J.; Lu, W.; Xu, W.; Wu, B. Dental pulp stem cells from human teeth with deep caries displayed an enhanced angiogenesis potential in vitro. J. Dent. Sci. 2021, 16, 318–326. [Google Scholar] [CrossRef]
- Lambrichts, I.; Driesen, R.B.; Dillen, Y.; Gervois, P.; Ratajczak, J.; Vangansewinkel, T.; Wolfs, E.; Bronckaers, A.; Hilkens, P. Dental Pulp Stem Cells: Their Potential in Reinnervation and Angiogenesis by Using Scaffolds. J. Endod. 2017, 43, S12–S16. [Google Scholar] [CrossRef]
- Youssef, A.R.; Emara, R.; Taher, M.M.; Al-Allaf, F.A.; Almalki, M.; Almasri, M.A.; Siddiqui, S.S. Effects of mineral trioxide aggregate, calcium hydroxide, biodentine and Emdogain on osteogenesis, Odontogenesis, angiogenesis and cell viability of dental pulp stem cells. BMC Oral. Health 2019, 19, 133. [Google Scholar] [CrossRef]
- Zaw, S.Y.M.; Kaneko, T.; Zaw, Z.C.T.; Sone, P.P.; Murano, H.; Gu, B.; Okada, Y.; Han, P.; Katsube, K.I.; Okiji, T. Crosstalk between dental pulp stem cells and endothelial cells augments angiogenic factor expression. Oral. Dis. 2020. [Google Scholar] [CrossRef]
- Bizheva, K.; Tan, B.; MacLellan, B.; Hosseinaee, Z.; Mason, E.; Hileeto, D.; Sorbara, L. In-vivo imaging of the palisades of Vogt and the limbal crypts with sub-micrometer axial resolution optical coherence tomography. Biomed. Opt. Express 2017, 8, 4141–4151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Notara, M.; Lentzsch, A.; Coroneo, M.; Cursiefen, C. The Role of Limbal Epithelial Stem Cells in Regulating Corneal (Lymph)angiogenic Privilege and the Micromilieu of the Limbal Niche following UV Exposure. Stem Cells Int. 2018, 2018, 8620172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hos, D.; Matthaei, M.; Bock, F.; Maruyama, K.; Notara, M.; Clahsen, T.; Hou, Y.; Le, V.N.H.; Salabarria, A.C.; Horstmann, J.; et al. Immune reactions after modern lamellar (DALK, DSAEK, DMEK) versus conventional penetrating corneal transplantation. Prog. Retin. Eye Res. 2019, 73, 100768. [Google Scholar] [CrossRef] [PubMed]
- Gocheva, V.; Chen, X.; Peters, C.; Reinheckel, T.; Joyce, J.A. Deletion of cathepsin H perturbs angiogenic switching, vascularization and growth of tumors in a mouse model of pancreatic islet cell cancer. Biol. Chem. 2010, 391, 937–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, C.; Patel, R.; Guillen, C.; Wardlaw, A.J. The chemokine CXCL16 is highly and constitutively expressed by human bronchial epithelial cells. Exp. Lung Res. 2009, 35, 272–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiavarina, B.; Costanza, B.; Ronca, R.; Blomme, A.; Rezzola, S.; Chiodelli, P.; Giguelay, A.; Belthier, G.; Doumont, G.; Van Simaeys, G.; et al. Metastatic colorectal cancer cells maintain the TGFβ program and use TGFBI to fuel angiogenesis. Theranostics 2021, 11, 1626–1640. [Google Scholar] [CrossRef]
- Braunstein, S.G.; Deramaudt, T.G.; Rosenblum, D.G.; Dunn, M.G.; Abraham, N.G. Heme oxygenase-1 gene expression as a stress index to ocular irritation. Curr. Eye Res. 1999, 19, 115–122. [Google Scholar] [CrossRef]
- Dulak, J.; Łoboda, A.; Zagórska, A.; Józkowicz, A. Complex role of heme oxygenase-1 in angiogenesis. Antioxid. Redox Signal. 2004, 6, 858–866. [Google Scholar] [CrossRef]
- Bonazzi, A.; Mastyugin, V.; Mieyal, P.A.; Dunn, M.W.; Laniado-Schwartzman, M. Regulation of cyclooxygenase-2 by hypoxia and peroxisome proliferators in the corneal epithelium. J. Biol. Chem. 2000, 275, 2837–2844. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, T.; Saika, S.; Okada, Y.; Kawashima, Y.; Sumioka, T.; Fujita, N.; Suzuki, Y.; Yamanaka, A.; Ohnishi, Y. Expression of cyclooxygenase-2 in corneal cells after photorefractive keratectomy and laser in situ keratomileusis in rabbits. J. Cataract. Refract. Surg. 2004, 30, 2612–2617. [Google Scholar] [CrossRef]
- Okada, Y. Effects of topical antiglaucoma medications on corneal epithelium as evaluated by gene expression patterns. Cornea 2007, 26, S46–S54. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Stevens, J.; Hilton, M.B.; Seaman, S.; Conrads, T.P.; Veenstra, T.D.; Logsdon, D.; Morris, H.; Swing, D.A.; Patel, N.L.; et al. COX-2 inhibition potentiates antiangiogenic cancer therapy and prevents metastasis in preclinical models. Sci. Transl. Med. 2014, 6, 242ra284. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kawai, M.; Kawai, Y.; Mashima, Y. The effect of selective cyclooxygenase-2 inhibitor on corneal angiogenesis in the rat. Curr. Eye Res. 1999, 19, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Ellenberg, D.; Azar, D.T.; Hallak, J.A.; Tobaigy, F.; Han, K.Y.; Jain, S.; Zhou, Z.; Chang, J.H. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog. Retin. Eye Res. 2010, 29, 208–248. [Google Scholar] [CrossRef] [Green Version]
- Foulsham, W.; Dohlman, T.H.; Mittal, S.K.; Taketani, Y.; Singh, R.B.; Masli, S.; Dana, R. Thrombospondin-1 in ocular surface health and disease. Ocul. Surf. 2019, 17, 374–383. [Google Scholar] [CrossRef]
- Uno, K.; Hayashi, H.; Kuroki, M.; Uchida, H.; Yamauchi, Y.; Kuroki, M.; Oshima, K. Thrombospondin-1 accelerates wound healing of corneal epithelia. Biochem. Biophys. Res. Commun. 2004, 315, 928–934. [Google Scholar] [CrossRef]
- Guo, N.; Krutzsch, H.C.; Inman, J.K.; Roberts, D.D. Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res. 1997, 57, 1735–1742. [Google Scholar]
- Cursiefen, C.; Maruyama, K.; Bock, F.; Saban, D.; Sadrai, Z.; Lawler, J.; Dana, R.; Masli, S. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J. Exp. Med. 2011, 208, 1083–1092. [Google Scholar] [CrossRef]
- Sekiyama, E.; Nakamura, T.; Cooper, L.J.; Kawasaki, S.; Hamuro, J.; Fullwood, N.J.; Kinoshita, S. Unique distribution of thrombospondin-1 in human ocular surface epithelium. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1352–1358. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Dietrich, T.; Saito, K.; Sorokin, L.; Sasaki, T.; Paulsson, M.; Kruse, F.E. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp. Eye Res. 2007, 85, 845–860. [Google Scholar] [CrossRef]
- Wei, W.; Clarke, C.J.P.; Somers, G.R.; Cresswell, K.S.; Loveland, K.A.; Trapani, J.A.; Johnstone, R.W. Expression of IFI 16 in epithelial cells and lymphoid tissues. Histochem. Cell. Biol. 2003, 119, 45–54. [Google Scholar] [CrossRef]
- Cao, T.; Shao, S.; Li, B.; Jin, L.; Lei, J.; Qiao, H.; Wang, G. Up-regulation of Interferon-inducible protein 16 contributes to psoriasis by modulating chemokine production in keratinocytes. Sci. Rep. 2016, 6, 25381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Liang, Y.; Qiao, Y.; Zhao, X.; Yang, Y.; Yang, S.; Li, B.; Zhao, Q.; Dong, L.; Quan, S.; et al. Expression of soluble epoxide hydrolase in renal tubular epithelial cells regulates macrophage infiltration and polarization in IgA nephropathy. Am. J. Physiol. Ren. Physiol. 2018, 315, F915–F926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reisdorf, W.C.; Xie, Q.; Zeng, X.; Xie, W.; Rajpal, N.; Hoang, B.; Burgert, M.E.; Kumar, V.; Hurle, M.R.; Rajpal, D.K.; et al. Preclinical evaluation of EPHX2 inhibition as a novel treatment for inflammatory bowel disease. PLoS ONE 2019, 14, e0215033. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, W.M.; Chaurasia, S.S.; Medeiros, F.W.; Mohan, R.R.; Sinha, S.; Wilson, S.E. Topical interleukin-1 receptor antagonist inhibits inflammatory cell infiltration into the cornea. Exp. Eye Res. 2008, 86, 753–757. [Google Scholar] [CrossRef] [Green Version]
- Böcker, U.; Damião, A.; Holt, L.; Han, D.S.; Jobin, C.; Panja, A.; Mayer, L.; Sartor, R.B. Differential expression of interleukin 1 receptor antagonist isoforms in human intestinal epithelial cells. Gastroenterology 1998, 115, 1426–1438. [Google Scholar] [CrossRef]
- Daig, R.; Rogler, G.; Aschenbrenner, E.; Vogl, D.; Falk, W.; Gross, V.; Schölmerich, J.; Andus, T. Human intestinal epithelial cells secrete interleukin-1 receptor antagonist and interleukin-8 but not interleukin-1 or interleukin-6. Gut 2000, 46, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, M.; Azuma, C.; Kanai, T.; Koyama, M.; Kimura, T.; Saji, F. Interleukin-1 receptor antagonist expression in epithelial cells of human endometrium. Int. J. Gynaecol. Obstet. 1995, 49, 305–310. [Google Scholar] [CrossRef]
- Gabay, C.; Smith, M.F., Jr.; Arend, W.P. The human intracellular interleukin 1 receptor antagonist promoter appropriately regulates gene expression in keratinocytes and gastrointestinal epithelial cells in vivo. Cytokine 1999, 11, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, G.J.; Van Le, L.; Valea, F.; Haskill, S.; Roberts, W.; Arend, W.P.; Stuart, A.; Peters, W.P. Expression of interleukin-1α, interleukin-1β, and an interleukin-1 receptor antagonist in human retinal pigment epithelial cells. Exp. Eye Res. 1992, 55, 325–335. [Google Scholar] [CrossRef]
- Schwarz, Y.A.; Amin, R.S.; Stark, J.M.; Trapnell, B.C.; Wilmott, R.W. Interleukin-1 receptor antagonist inhibits interleukin-8 expression in A549 respiratory epithelial cells infected in vitro with a replication-deficient recombinant adenovirus vector. Am. J. Respir. Cell Mol. Biol. 1999, 21, 388–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.Z.; Wu, J.F.; Cass, D.; Erle, D.J.; Corry, D.; Young, S.G.; Farese, R.V., Jr.; Sheppard, D. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J. Cell Biol. 1996, 133, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Kellner, P.; Nestler, F.; Leimert, A.; Bucher, M.; Czeslick, E.; Sablotzki, A.; Raspè, C. Antithrombin III, but not C1 esterase inhibitor reduces inflammatory response in lipopolysaccharide-stimulated human monocytes in an ex-vivo whole blood setting. Cytokine 2014, 70, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Minnema, M.C.; Chang, A.C.; Jansen, P.M.; Lubbers, Y.T.; Pratt, B.M.; Whittaker, B.G.; Taylor, F.B.; Hack, C.E.; Friedman, B. Recombinant human antithrombin III improves survival and attenuates inflammatory responses in baboons lethally challenged with Escherichia coli. Blood 2000, 95, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Ito, T.; Yoneda, M.; Takamoto, S.; Nakade, Y.; Okamoto, S.; Okada, M.; Yokohama, S.; Aso, K.; Makino, I. Antithrombin III prevents concanavalin A-induced liver injury through inhibition of macrophage inflammatory protein-2 release and production of prostacyclin in mice. J. Hepatol. 2002, 36, 766–773. [Google Scholar] [CrossRef] [Green Version]
- Yamashiro, K.; Kiryu, J.; Tsujikawa, A.; Honjo, M.; Nonaka, A.; Miyamoto, K.; Honda, Y.; Tanihara, H.; Ogura, Y. Inhibitory effects of antithrombin III against leukocyte rolling and infiltration during endotoxin-induced uveitis in rats. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1553–1560. [Google Scholar]
- Zhao, T.; Ding, X.; Du, H.; Yan, C. Lung Epithelial Cell-Specific Expression of Human Lysosomal Acid Lipase Ameliorates Lung Inflammation and Tumor Metastasis in Lipa(−/−) Mice. Am. J. Pathol. 2016, 186, 2183–2192. [Google Scholar] [CrossRef] [Green Version]
- Hamano, N.; Terada, N.; Maesako, K.; Ikeda, T.; Fukuda, S.; Wakita, J.; Yamashita, T.; Konno, A. Expression of histamine receptors in nasal epithelial cells and endothelial cells--the effects of sex hormones. Int. Arch. Allergy Immunol. 1998, 115, 220–227. [Google Scholar] [CrossRef]
- Guo, Y.; Ramachandran, C.; Satpathy, M.; Srinivas, S.P. Histamine-induced myosin light chain phosphorylation breaks down the barrier integrity of cultured corneal epithelial cells. Pharm. Res. 2007, 24, 1824–1833. [Google Scholar] [CrossRef]
- Sharif, N.A.; Wiernas, T.K.; Howe, W.E.; Griffin, B.W.; Offord, E.A.; Pfeifer, A.M. Human corneal epithelial cell functional responses to inflammatory agents and their antagonists. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2562–2571. [Google Scholar]
- Soltani, S.; Zakeri-Milani, P.; Barzegar-Jalali, M.; Jelvehgari, M. Design of eudragit RL nanoparticles by nanoemulsion method as carriers for ophthalmic drug delivery of ketotifen fumarate. Iran. J. Basic Med. Sci. 2016, 19, 550–560. [Google Scholar] [PubMed]
- Skronska-Wasek, W.; Durlanik, S.; Le, H.Q.; Schroeder, V.; Kitt, K.; Garnett, J.P.; Pflanz, S. The antimicrobial peptide S100A8/A9 produced by airway epithelium functions as a potent and direct regulator of macrophage phenotype and function. Eur. Respir. J. 2022, 59, 2002732. [Google Scholar] [CrossRef] [PubMed]
- Notara, M.; Behboudifard, S.; Kluth, M.A.; Maßlo, C.; Ganss, C.; Frank, M.H.; Schumacher, B.; Cursiefen, C. UV light-blocking contact lenses protect against short-term UVB-induced limbal stem cell niche damage and inflammation. Sci. Rep. 2018, 8, 12564. [Google Scholar] [CrossRef] [Green Version]
- Khiatah, B.; Qi, M.; Du, W.; T-Chen, K.; van Megen, K.M.; Perez, R.G.; Isenberg, J.S.; Kandeel, F.; Roep, B.O.; Ku, H.T.; et al. Intra-pancreatic tissue-derived mesenchymal stromal cells: A promising therapeutic potential with anti-inflammatory and pro-angiogenic profiles. Stem Cell Res. Ther. 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.S.; Kim, S. Human adipose mesenchymal stem cells modulate inflammation and angiogenesis through exosomes. Sci. Rep. 2022, 12, 2776. [Google Scholar] [CrossRef] [PubMed]
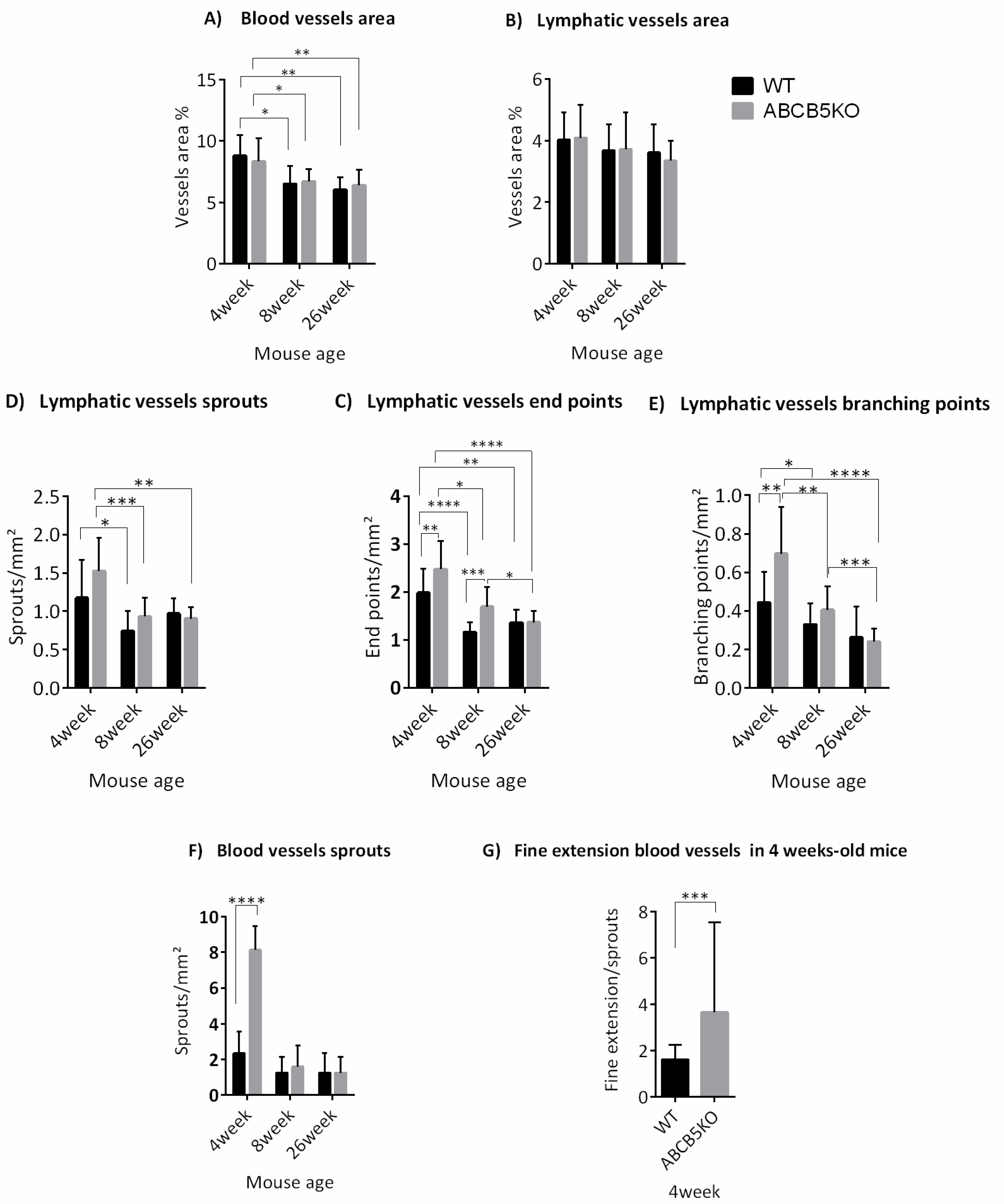




| Mouse Age | Changes in Vascular Network |
|---|---|
| 4 weeks | ↑Blood vessel sprouts ↑lymphatic vessel endpoints ↑lymphatic vessel branching points |
| 8 weeks | ↑Lymphatic vessel endpoints |
| 26 weeks | No difference |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meshko, B.; Volatier, T.L.A.; Hadrian, K.; Deng, S.; Hou, Y.; Kluth, M.A.; Ganss, C.; Frank, M.H.; Frank, N.Y.; Ksander, B.; et al. ABCB5+ Limbal Epithelial Stem Cells Inhibit Developmental but Promote Inflammatory (Lymph) Angiogenesis While Preventing Corneal Inflammation. Cells 2023, 12, 1731. https://doi.org/10.3390/cells12131731
Meshko B, Volatier TLA, Hadrian K, Deng S, Hou Y, Kluth MA, Ganss C, Frank MH, Frank NY, Ksander B, et al. ABCB5+ Limbal Epithelial Stem Cells Inhibit Developmental but Promote Inflammatory (Lymph) Angiogenesis While Preventing Corneal Inflammation. Cells. 2023; 12(13):1731. https://doi.org/10.3390/cells12131731
Chicago/Turabian StyleMeshko, Berbang, Thomas L. A. Volatier, Karina Hadrian, Shuya Deng, Yanhong Hou, Mark Andreas Kluth, Christoph Ganss, Markus H. Frank, Natasha Y. Frank, Bruce Ksander, and et al. 2023. "ABCB5+ Limbal Epithelial Stem Cells Inhibit Developmental but Promote Inflammatory (Lymph) Angiogenesis While Preventing Corneal Inflammation" Cells 12, no. 13: 1731. https://doi.org/10.3390/cells12131731
APA StyleMeshko, B., Volatier, T. L. A., Hadrian, K., Deng, S., Hou, Y., Kluth, M. A., Ganss, C., Frank, M. H., Frank, N. Y., Ksander, B., Cursiefen, C., & Notara, M. (2023). ABCB5+ Limbal Epithelial Stem Cells Inhibit Developmental but Promote Inflammatory (Lymph) Angiogenesis While Preventing Corneal Inflammation. Cells, 12(13), 1731. https://doi.org/10.3390/cells12131731