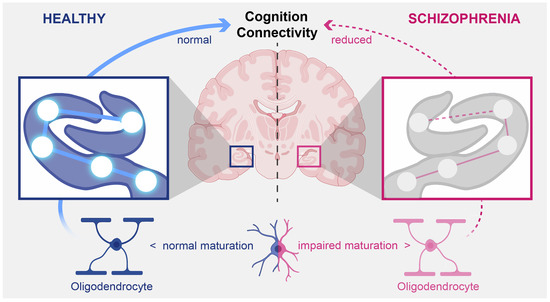
Decreased Oligodendrocyte Number in Hippocampal Subfield CA4 in Schizophrenia: A Replication Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Brains
2.2. Tissue Processing
2.3. Stereological Analyses
2.4. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer, A. Neuere Arbeiten über die Dementia senilis und die auf atheromatöser Gefässerkrankung basierenden Gehirnkrankheiten. Eur. Neurol. 1898, 3, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Plum, F. Prospects for research on schizophrenia. 3. Neurophysiology. Neuropathological findings. Neurosci. Res. Program Bull. 1972, 10, 384–388. [Google Scholar] [PubMed]
- Johnstone, E.C.; Crow, T.J.; Frith, C.D.; Husband, J.; Kreel, L. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet 1976, 2, 924–926. [Google Scholar] [CrossRef]
- Yao, L.; Lui, S.; Liao, Y.; Du, M.Y.; Hu, N.; Thomas, J.A.; Gong, Q.Y. White matter deficits in first episode schizophrenia: An activation likelihood estimation meta-analysis. Prog Neuropsychopharmacol Biol. Psychiatry 2013, 45, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Vargas, T.; Dean, D.J.; Osborne, K.J.; Gupta, T.; Ristanovic, I.; Ozturk, S.; Turner, J.; van Erp, T.G.M.; Mittal, V.A. Hippocampal Subregions Across the Psychosis Spectrum. Schizophr. Bull. 2018, 44, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Wobrock, T.; Falkai, P.; Schneider-Axmann, T.; Guse, B.; Backens, M.; Ecker, U.K.; Heimes, J.; Galea, J.M.; Gruber, O.; et al. Hippocampal integrity and neurocognition in first-episode schizophrenia: A multidimensional study. World J. Biol. Psychiatry 2014, 15, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yao, L.; Zhang, W.; Deng, W.; Xiao, Y.; Li, F.; Sweeney, J.A.; Gong, Q.; Lui, S. Dissociation of fractional anisotropy and resting-state functional connectivity alterations in antipsychotic-naive first-episode schizophrenia. Schizophr. Res. 2019, 204, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, A.; Liu, Y.; Yan, H.; Wang, M.; Sun, Y.; Fan, L.; Song, M.; Xu, K.; Chen, J.; et al. Polygenic effects of schizophrenia on hippocampal grey matter volume and hippocampus-medial prefrontal cortex functional connectivity. Br. J. Psychiatry 2020, 216, 267–274. [Google Scholar] [CrossRef]
- Duan, X.; He, C.; Ou, J.; Wang, R.; Xiao, J.; Li, L.; Wu, R.; Zhang, Y.; Zhao, J.; Chen, H. Reduced Hippocampal Volume and Its Relationship With Verbal Memory and Negative Symptoms in Treatment-Naive First-Episode Adolescent-Onset Schizophrenia. Schizophr. Bull. 2021, 47, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Falkai, P.; Bogerts, B. Cell loss in the hippocampus of schizophrenics. Eur. Arch. Psychiatry Neurol. Sci. 1986, 236, 154–161. [Google Scholar] [CrossRef]
- Bogerts, B. Zur Neuropathologie der Schizophrenien. Fortschr. Neurol. Psychiatr. 1984, 52, 428–437. [Google Scholar] [CrossRef]
- Roeske, M.J.; Konradi, C.; Heckers, S.; Lewis, A.S. Hippocampal volume and hippocampal neuron density, number and size in schizophrenia: A systematic review and meta-analysis of postmortem studies. Mol. Psychiatry 2021, 26, 3524–3535. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.W.; Rakic, P. Elimination of neurons from the rhesus monkey’s lateral geniculate nucleus during development. J. Comp. Neurol. 1988, 272, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Steyskal, C.; Bernstein, H.G.; Schneider-Axmann, T.; Parlapani, E.; Schaeffer, E.L.; Gattaz, W.F.; Bogerts, B.; Schmitz, C.; Falkai, P. Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathol. 2009, 117, 395–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaral, D.; Lavenex, P. Hippocampal Neuroanatomy. In The Hippocampus Book, 1st ed.; Andrersen, P., Morris, R., Amaral, D., Eds.; Oxford University Press: Oxford, UK, 2006; Volume 1, pp. 37–115. [Google Scholar]
- Falkai, P.; Malchow, B.; Wetzestein, K.; Nowastowski, V.; Bernstein, H.G.; Steiner, J.; Schneider-Axmann, T.; Kraus, T.; Hasan, A.; Bogerts, B.; et al. Decreased Oligodendrocyte and Neuron Number in Anterior Hippocampal Areas and the Entire Hippocampus in Schizophrenia: A Stereological Postmortem Study. Schizophr. Bull. 2016, 42, S4–S12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreczmanski, P.; Schmidt-Kastner, R.; Heinsen, H.; Steinbusch, H.W.; Hof, P.R.; Schmitz, C. Stereological studies of capillary length density in the frontal cortex of schizophrenics. Acta Neuropathol. 2005, 109, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Kreczmanski, P.; Heinsen, H.; Mantua, V.; Woltersdorf, F.; Masson, T.; Ulfig, N.; Schmidt-Kastner, R.; Korr, H.; Steinbusch, H.W.; Hof, P.R.; et al. Volume, neuron density and total neuron number in five subcortical regions in schizophrenia. Brain 2007, 130, 678–692. [Google Scholar] [CrossRef] [Green Version]
- Casanova, M.F.; de Zeeuw, L.; Switala, A.; Kreczmanski, P.; Korr, H.; Ulfig, N.; Heinsen, H.; Steinbusch, H.W.; Schmitz, C. Mean cell spacing abnormalities in the neocortex of patients with schizophrenia. Psychiatry Res. 2005, 133, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Höistad, M.; Heinsen, H.; Wicinski, B.; Schmitz, C.; Hof, P.R. Stereological assessment of the dorsal anterior cingulate cortex in schizophrenia: Absence of changes in neuronal and glial densities. Neuropathol. Appl. Neurobiol. 2013, 39, 348–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinsen, H. Serial thick, frozen, gallocyanin stained sections of human central nervous system. J. Histotechnol. 1991, 14, 167–173. [Google Scholar] [CrossRef]
- Heinsen, H.; Arzberger, T.; Schmitz, C. Celloidin mounting (embedding without infiltration)—A new, simple and reliable method for producing serial sections of high thickness through complete human brains and its application to stereological and immunohistochemical investigations. J. Chem. Neuroanat. 2000, 20, 49–59. [Google Scholar] [CrossRef]
- van Kooten, I.A.J.; Palmen, S.J.M.C.; von Cappeln, P.; Steinbusch, H.W.M.; Korr, H.; Heinsen, H.; Hof, P.R.; van Engeland, H.; Schmitz, C. Neurons in the fusiform gyrus are fewer and smaller in autism. Brain 2008, 131, 987–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hof, P.R.; Haroutunian, V.; Friedrich, V.L., Jr.; Byne, W.; Buitron, C.; Perl, D.P.; Davis, K.L. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol. Psychiatry 2003, 53, 1075–1085. [Google Scholar] [CrossRef]
- Selemon, L.D.; Mrzljak, J.; Kleinman, J.E.; Herman, M.M.; Goldman-Rakic, P.S. Regional Specificity in the Neuropathologic Substrates of Schizophrenia: A Morphometric Analysis of Broca’s Area 44 and Area 9. Arch. Gen. Psychiatry 2003, 60, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stark, A.; Uylings, H.; Sanz-Arigita, E.; Pakkenberg, B. Glial Cell Loss in the Anterior Cingulate Cortex, a Subregion of the Prefrontal Cortex, in Subjects With Schizophrenia. Am. J. Psychiatry 2004, 161, 882–888. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, C.; Hof, P.R. Design-based stereology in neuroscience. Neuroscience 2005, 130, 813–831. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C. Variation of fractionator estimates and its prediction. Anat. Embryol. 1998, 198, 371–397. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Hof, P.R. Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J. Chem. Neuroanat. 2000, 20, 93–114. [Google Scholar] [CrossRef]
- Chen, F.; Bertelsen, A.B.; Holm, I.E.; Nyengaard, J.R.; Rosenberg, R.; Dorph-Petersen, K.A. Hippocampal volume and cell number in depression, schizophrenia; suicide subjects. Brain Res. 2020, 1727, 146546. [Google Scholar] [CrossRef] [PubMed]
- Falkai, P.; Steiner, J.; Malchow, B.; Shariati, J.; Knaus, A.; Bernstein, H.G.; Schneider-Axmann, T.; Kraus, T.; Hasan, A.; Bogerts, B.; et al. Oligodendrocyte and Interneuron Density in Hippocampal Subfields in Schizophrenia and Association of Oligodendrocyte Number with Cognitive Deficits. Front. Cell. Neurosci. 2016, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Katsel, P.; Davis, K.L.; Haroutunian, V. Variations in myelin and oligodendrocyte-related gene expression across multiple brain regions in schizophrenia: A gene ontology study. Schizophr. Res. 2005, 79, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Dracheva, S.; Davis, K.L.; Chin, B.; Woo, D.A.; Schmeidler, J.; Haroutunian, V. Myelin-associated mRNA and protein expression deficits in the anterior cingulate cortex and hippocampus in elderly schizophrenia patients. Neurobiol. Dis. 2006, 21, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Segal, D.; Schmitz, C.; Hof, P.R. Spatial distribution and density of oligodendrocytes in the cingulum bundle are unaltered in schizophrenia. Acta Neuropathol. 2009, 117, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, A.; Simons, M.; Cantuti-Castelvetri, L.; Falkai, P. A new role for oligodendrocytes and myelination in schizophrenia and affective disorders? Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 371–372. [Google Scholar] [CrossRef] [Green Version]
- Nave, K.A. Oligodendrocytes and the “micro brake” of progenitor cell proliferation. Neuron 2010, 65, 577–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassoli, J.S.; Guest, P.C.; Malchow, B.; Schmitt, A.; Falkai, P.; Martins-de-Souza, D. Disturbed macro-connectivity in schizophrenia linked to oligodendrocyte dysfunction: From structural findings to molecules. npj Schizophr. 2015, 1, 15034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stedehouder, J.; Couey, J.J.; Brizee, D.; Hosseini, B.; Slotman, J.A.; Dirven, C.M.F.; Shpak, G.; Houtsmuller, A.B.; Kushner, S.A. Fast-spiking Parvalbumin Interneurons are Frequently Myelinated in the Cerebral Cortex of Mice and Humans. Cereb. Cortex 2017, 27, 5001–5013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stedehouder, J.; Kushner, S.A. Myelination of parvalbumin interneurons: A parsimonious locus of pathophysiological convergence in schizophrenia. Mol. Psychiatry 2017, 22, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.O.; Ardekani, B.A.; Nierenberg, J.; Butler, P.D.; Javitt, D.C.; Hoptman, M.J. Voxelwise Correlational Analyses of White Matter Integrity in Multiple Cognitive Domains in Schizophrenia. Am. J. Psychiatry 2006, 163, 2008–2010. [Google Scholar] [CrossRef] [PubMed]
- Nestor, P.G.; Kubicki, M.; Kuroki, N.; Gurrera, R.J.; Niznikiewicz, M.; Shenton, M.E.; McCarley, R.W. Episodic memory and neuroimaging of hippocampus and fornix in chronic schizophrenia. Psychiatry Res. 2007, 155, 21–28. [Google Scholar] [CrossRef]
- Mitterauer, B.J. Possible role of glia in cognitive impairment in schizophrenia. CNS Neurosci. Ther. 2011, 17, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Takahashi, S.; Malchow, B.; Papazova, I.; Stöcklein, S.; Ertl-Wagner, B.; Papazov, B.; Kumpf, U.; Wobrock, T.; Keller-Varady, K.; et al. Cognitive and functional deficits are associated with white matter abnormalities in two independent cohorts of patients with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Voineskos, A.N.; Felsky, D.; Kovacevic, N.; Tiwari, A.K.; Zai, C.; Chakravarty, M.M.; Lobaugh, N.J.; Shenton, M.E.; Rajji, T.K.; Miranda, D.; et al. Oligodendrocyte genes, white matter tract integrity; cognition in schizophrenia. Cereb. Cortex 2013, 23, 2044–2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konradi, C.; Yang, C.K.; Zimmerman, E.I.; Lohmann, K.M.; Gresch, P.; Pantazopoulos, H.; Berretta, S.; Heckers, S. Hippocampal interneurons are abnormal in schizophrenia. Schizophr. Res. 2011, 131, 165–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, T.; Bazmi, H.H.; Mirnics, K.; Wu, Q.; Sampson, A.R.; Lewis, D.A. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am. J. Psychiatry 2008, 165, 479–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, S.J.; Webster, M.J.; Sivagnanasundaram, S.; Duncan, C.; Elashoff, M.; Weickert, C.S. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am. J. Psychiatry 2010, 167, 1479–1488. [Google Scholar] [CrossRef]
- Mellios, N.; Huang, H.S.; Baker, S.P.; Galdzicka, M.; Ginns, E.; Akbarian, S. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol. Psychiatry 2009, 65, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Fachim, H.A.; Srisawat, U.; Dalton, C.F.; Reynolds, G.P. Parvalbumin promoter hypermethylation in postmortem brain in schizophrenia. Epigenomics 2018, 10, 519–524. [Google Scholar] [CrossRef]
- Glaser, E.R.; Green, G.; Hendricks, S. Sterology for Biological Research, 2nd ed.; MBF Press: Williston, VT, USA, 2007. [Google Scholar]
- Schmitz, C.; Born, M.; Dolezel, P.; Rutten, B.P.; de Saint-Georges, L.; Hof, P.R.; Korr, H. Prenatal protracted irradiation at very low dose rate induces severe neuronal loss in rat hippocampus and cerebellum. Neuroscience 2005, 130, 935–948. [Google Scholar] [CrossRef]
- Konopaske, G.T.; Dorph-Petersen, K.-A.; Sweet, R.A.; Pierri, J.N.; Zhang, W.; Sampson, A.R.; Lewis, D.A. Effect of Chronic Antipsychotic Exposure on Astrocyte and Oligodendrocyte Numbers in Macaque Monkeys. Biol. Psychiatry 2008, 63, 759–765. [Google Scholar] [CrossRef]
- Wang, H.; Xu, H.; Niu, J.; Mei, F.; Li, X.; Kong, J.; Cai, W.; Xiao, L. Haloperidol activates quiescent oligodendroglia precursor cells in the adult mouse brain. Schizophr. Res. 2010, 119, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Fard, M.K.; van der Meer, F.; Sánchez, P.; Cantuti-Castelvetri, L.; Mandad, S.; Jäkel, S.; Fornasiero, E.F.; Schmitt, S.; Ehrlich, M.; Starost, L.; et al. BCAS1 expression defines a population of early myelinating oligodendrocytes in multiple sclerosis lesions. Sci. Transl. Med. 2017, 9, 7816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miron, V.E.; Kuhlmann, T.; Antel, J.P. Cells of the oligodendroglial lineage, myelination, and remyelination. Biochim. Biophys. Acta 2011, 1812, 184–193. [Google Scholar] [CrossRef] [PubMed]






| No. | A | O | Cause of Death | PMI | Fix | Diagnosis | |
|---|---|---|---|---|---|---|---|
| [y] | [y] | [h] | [d] | DSM-IV | ICD-10 | ||
| S1 | 22 | 19 | Suicide by jumping from high building | 88 | 130 | 295.30 | F20.00 |
| S2 | 36 | 28 | Suicide by strangulation | <72 | 115 | 295.30 | F20.00 |
| S4 | 50 | 17 | Peritonitis | <24 | 203 | 295.30 | F20.00 |
| S5 | 50 | 22 | Suicide by strangulation | 18 | 170 | 295.30 | F20.00 |
| S6 | 51 | 17 | Septicemia | 33 | 127 | 295.60 | F20.50 |
| S7 | 54 | 20 | Septicemia | 27 | 250 | 295.60 | F20.50 |
| S8 | 55 | 22 | Right-sided heart failure | 25 | 84 | 295.30 | F20.00 |
| S9 | 57 | 37 | Septicemia | 76 | 163 | 295.30 | F20.00 |
| S10 | 60 | 24 | Pulmonary embolism | <48 | 311 | 295.30 | F20.01 |
| S11 | 62 | 19 | Aspiration | 7 | 171 | 295.30 | F20.00 |
| S12 | 63 | 22 | Acute myocardial infarct | 15 | 338 | 295.60 | F20.50 |
| C2 | 36 | - | Gunshot | 24 | 143 | - | - |
| C3 | 47 | - | Acute myocardial infarct | <24 | 133 | - | - |
| C5 | 50 | - | Avalanche accident | 23 | 498 | - | - |
| C6 | 51 | - | Septicemia | 7 | 285 | - | - |
| C7 | 54 | - | Acute myocardial infarct | 18 | 168 | - | - |
| C8 | 56 | - | Acute myocardial infarct | 60 | 3570 | - | - |
| C9 | 58 | - | Acute myocardial infarct | 28 | 126 | - | - |
| C10 | 60 | - | Gastrointestinal hemorrhage | 18 | 101 | - | - |
| C11 | 60 | - | Gastrointestinal hemorrhage | 27 | 302 | - | - |
| C12 | 62 | - | Acute myocardial infarct | <24 | 3696 | - | - |
| C13 | 65 | - | Bronchopneumonia | 6 | 2289 | - | - |
| DG Neurons | CA4 Neurons | CA4 Astrocytes | CA4 Oligodendrocytes | |
|---|---|---|---|---|
| Objective used | 40 | 40 | 40 | 40 |
| B [µm2] | 625 | 1225 | 1225 | 1225 |
| H [µm] | 15 | 20 | 20 | 20 |
| D [µm] | 360 | 200 | 200 | 200 |
| ∑ CS | 449 | 1131 | 377 | 1131 |
| ∑ Q- | 321 | 358 | 506 | 472 |
| CEpred. [n] | 0.056 | 0.053 | 0.044 | 0.046 |
| ∑ P (Cavalieri) | 114 | 1070 | 1070 | 1070 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitt, A.; Tatsch, L.; Vollhardt, A.; Schneider-Axmann, T.; Raabe, F.J.; Roell, L.; Heinsen, H.; Hof, P.R.; Falkai, P.; Schmitz, C. Decreased Oligodendrocyte Number in Hippocampal Subfield CA4 in Schizophrenia: A Replication Study. Cells 2022, 11, 3242. https://doi.org/10.3390/cells11203242
Schmitt A, Tatsch L, Vollhardt A, Schneider-Axmann T, Raabe FJ, Roell L, Heinsen H, Hof PR, Falkai P, Schmitz C. Decreased Oligodendrocyte Number in Hippocampal Subfield CA4 in Schizophrenia: A Replication Study. Cells. 2022; 11(20):3242. https://doi.org/10.3390/cells11203242
Chicago/Turabian StyleSchmitt, Andrea, Laura Tatsch, Alisa Vollhardt, Thomas Schneider-Axmann, Florian J. Raabe, Lukas Roell, Helmut Heinsen, Patrick R. Hof, Peter Falkai, and Christoph Schmitz. 2022. "Decreased Oligodendrocyte Number in Hippocampal Subfield CA4 in Schizophrenia: A Replication Study" Cells 11, no. 20: 3242. https://doi.org/10.3390/cells11203242