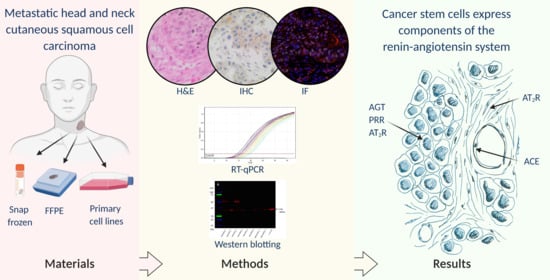
Cancer Stem Cells in Metastatic Head and Neck Cutaneous Squamous Cell Carcinoma Express Components of the Renin-Angiotensin System
Abstract
1. Introduction
2. Materials and Methods
2.1. mHNcSCC Tissue Samples
2.2. mHNcSCC-Derived Primary Cell Lines
2.3. Histochemical and Immunohistochemical Staining
2.4. Image Capture and Analysis
2.5. RT-qPCR
2.6. Western Blotting
3. Results
3.1. Angiotensinogen, PRR, ACE and AT2R but Not Renin or ACE2 Were Present on mHNcSCC Tissue Samples
3.2. Angiotensinogen, PRR, and AT2R Were Expressed by the CSCs in the TNs and PTS, and ACE Was Expressed by the Endothelium of the Tumor Microvessels within mHNcSCC Tissue Samples
3.3. RT-qPCR Demonstrated Transcript Expression of Angiotensinogen, PRR, ACE, ACE2, and AT1R, but Not Renin or AT2R
3.4. Western Blotting Demonstrated Protein Expression of Angiotensinogen, PRR, and ACE
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alam, M.; Ratner, D. Cutaneous squamous-cell carcinoma. N. Engl. J. Med. 2001, 344, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Brougham, N.D.L.S.; Dennett, E.R.; Cameron, R.; Tan, S.T. The incidence of metastasis from cutaneous squamous cell carcinoma and the impact of its risk factors. J. Surg. Oncol. 2012, 106, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.S.; Stern, R.S. Nonmelanoma cancers of the skin. N. Engl. J. Med. 1992, 327, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, R.; Zelac, D.E. Squamous cell carcinoma of the skin. Plast. Reconstr. Surg. 2004, 114, 82e–94e. [Google Scholar] [CrossRef] [PubMed]
- Baccelli, I.; Trumpp, A. The evolving concept of cancer and metastasis stem cells. J. Cell Biol. 2012, 198, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.T.; Guzman, M.L.; Noble, M. Cancer stem cells. N. Engl. J. Med. 2006, 355, 1253–1261. [Google Scholar] [CrossRef]
- Bradshaw, A.; Wickremsekera, A.; Tan, S.T.; Peng, L.; Davis, P.F.; Itinteang, T. Cancer stem cell hierarchy in glioblastoma multiforme. Front. Surg. 2016, 3, 21. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- van Schaijik, B.; Davis, P.F.; Wickremesekera, A.C.; Tan, S.T.; Itinteang, T. Subcellular localisation of the stem cell markers OCT4, SOX2, NANOG, KLF4 and c-MYC in cancer: A review. J. Clin. Pathol. 2018, 71, 88–91. [Google Scholar] [CrossRef]
- Yu, H.H.; Featherston, T.; Tan, S.T.; Chibnall, A.M.; Brasch, H.D.; Davis, P.F.; Itinteang, T. Characterization of cancer stem cells in moderately differentiated buccal mucosal squamous cell carcinoma. Front. Surg. 2016, 3, 46. [Google Scholar] [CrossRef]
- Baillie, R.; Itinteang, T.; Yu, H.H.; Brasch, H.D.; Davis, P.F.; Tan, S.T. Cancer stem cells in moderately differentiated oral tongue squamous cell carcinoma. J. Clin. Pathol. 2016, 69, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Ram, R.; Brasch, H.D.; Dunne, J.C.; Davis, P.F.; Tan, S.T.; Itinteang, T. The identification of three cancer stem cell subpopulations within moderately differentiated lip squamous cell carcinoma. Front. Surg. 2017, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Cane, R.; Kennedy-Smith, A.; Brasch, H.D.; Savage, S.; Marsh, R.W.; Itinteang, T.; Tan, S.T.; Itinteang, T. Characterization of cancer stem cells in renal clear cell carcinoma. J. Stem Cell Regen. Biol. 2019, 5, 6–17. [Google Scholar] [CrossRef]
- Munro, M.J.; Wickremesekera, S.K.; Peng, L.; Marsh, R.W.; Itinteang, T.; Tan, S.T. Cancer stem cell subpopulations in primary colon adenocarcinoma. PLoS ONE 2019, 14, e0221963. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, S.; Wickremesekera, S.K.; Brasch, H.D.; Van Schaijik, B.; Marsh, R.W.; Tan, S.T.; Itinteang, T. Expression and localization of cathepsins B, D and G in cancer stem cells in liver metastasis from colon adenocarcinoma. Front. Surg. 2018, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Yoganandarajah, V.; Patel, J.; van Schaijik, B.; Bockett, N.; Brasch, H.D.; Paterson, E.; Sim, D.; Davis, P.F.; Roth, I.M.; Itinteang, T.; et al. Identification of cancer stem cell subpopulations in head and neck metastatic malignant Melanoma. Cells 2020, 9, 324. [Google Scholar] [CrossRef]
- Wickremesekera, A.C.; Brasch, H.D.; Lee, V.M.; Davis, P.F.; Parker, A.; Koeck, H.; Itinteang, T.; Tan, S.T.; Wickremesekera, A.R.; Brasch, H.D.; et al. Cancer stem cell subpopulations in metastatic melanoma to the brain express components of the renin-angiotensin system. J. Cancer Metastasis Treat. 2019, 5, 62. [Google Scholar] [CrossRef]
- Koh, S.P.; Brasch, H.D.; de Jongh, J.; Itinteang, T.; Tan, S.T. Cancer stem cell subpopulations in moderately differentiated head and neck cutaneous squamous cell carcinoma. Heliyon 2019, 5, e02257. [Google Scholar] [CrossRef]
- Kilmister, E.J.; Patel, J.; van Schaijik, B.; Bockett, N.; Brasch, H.D.; Paterson, E.; Sim, D.; Davis, P.F.; Roth, I.M.; Itinteang, T.; et al. Cancer stem cell subpopulations are present within metastatic head and neck cutaneous squamous cell carcinoma. Front. Oncol. 2020, 10, 1091. [Google Scholar] [CrossRef]
- Peach, M.J. Renin-angiotensin system: Biochemistry and mechanisms of action. Physiol. Rev. 1977, 57, 313–370. [Google Scholar] [CrossRef] [PubMed]
- Tikellis, C.; Thomas, M.C. Angiotensin-Converting Enzyme 2 (ACE2) Is a key modulator of the renin angiotensin system in health and disease. Int. J. Pept. 2012, 2012, 256294. [Google Scholar] [CrossRef] [PubMed]
- Ager, E.I.; Neo, J.; Christophi, C. The renin-angiotensin system and malignancy. Carcinogenesis 2008, 29, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.J.; Wickremesekera, A.C.; Davis, P.F.; Marsh, R.; Tan, S.T.; Itinteang, T. Renin-angiotensin system and cancer: A review. Integr. Cancer Sci. Ther. 2017, 4, 1–6. [Google Scholar] [CrossRef]
- Lever, A.F.; Hole, D.J.; Gillis, C.R.; McCallum, I.R.; McInnes, G.T.; MacKinnon, P.L.; Meredith, P.A.; Murray, L.S.; Reid, J.L.; Robertson, J.W. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet 1998, 352, 179–184. [Google Scholar] [CrossRef]
- Sun, H.; Li, T.; Zhuang, R.; Cai, W.; Zheng, Y. Do renin-angiotensin system inhibitors influence the recurrence, metastasis, and survival in cancer patients? Medicine 2017, 96, e6394. [Google Scholar] [CrossRef]
- Christian, J.B.; Lapane, K.L.; Hume, A.L.; Eaton, C.B.; Weinstock, M.A. Association of ACE inhibitors and angiotensin receptor blockers with keratinocyte cancer prevention in the randomized VATTC trial. J. Natl. Cancer Inst. 2008, 100, 1223–1232. [Google Scholar] [CrossRef]
- Zambidis, E.T.; Soon Park, T.; Yu, W.; Tam, A.; Levine, M.; Yuan, X.; Pryzhkova, M.; Péault, B. Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood 2008, 112, 3601–3614. [Google Scholar] [CrossRef]
- Sugimoto, M.; Furuta, T.; Shirai, N.; Kodaira, C.; Nishino, M.; Ikuma, M.; Sugimura, H.; Hishida, A. Role of angiotensinogen gene polymorphism on Helicobacter pylori infection-related gastric cancer risk in Japanese. Carcinogenesis 2007, 28, 2036–2040. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, K.; Qin, H.; Yang, L.; Zhang, L.; Cao, Y. Genetic association between angiotensinogen polymorphisms and lung cancer risk. Medicine 2015, 94, e1250. [Google Scholar] [CrossRef]
- Vairaktaris, E.; Yapijakis, C.; Vylliotis, A.; Derka, S.; Vassiliou, S.; Nkenke, E.; Serefoglou, Z.; Ragos, V.; Critselis, E.; Avgoustidis, D.; et al. Angiotensinogen polymorphism is associated with risk for malignancy but not for oral cancer. Anticancer Res. 2008, 28, 1675–1679. [Google Scholar] [PubMed]
- Vincent, F.; Bonnin, P.; Clemessy, M.; Contreres, J.-O.; Lamande, N.; Gasc, J.-M.; Vilar, J.; Hainaud, P.; Tobelem, G.; Corvol, P.; et al. Angiotensinogen delays angiogenesis and tumor growth of hepatocarcinoma in transgenic mice. Cancer Res. 2009, 69, 2853–2860. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Sun, Y.; Rong, X.; Bai, L. High glucose promotes breast cancer proliferation and metastasis by impairing angiotensinogen expression. Biosci. Rep. 2019, 39, BSR20190436. [Google Scholar] [CrossRef] [PubMed]
- Polakis, P. The many ways of Wnt in cancer. Curr. Opin. Genet. Dev. 2007, 17, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Volpert, O.V.; Ward, W.F.; Lingen, M.W.; Chesler, L.; Solt, D.B.; Johnson, M.D.; Molteni, A.; Polverini, P.J.; Bouck, N.P. Captopril inhibits angiogenesis and slows the growth of experimental tumors in rats. J. Clin. Investig. 1996, 98, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, R.; Yoshiji, H.; Kuriyama, S.; Yoshii, J.; Ikenaka, Y.; Yanase, K.; Namisaki, T.; Kitade, M.; Yamazaki, M.; Mitoro, A.; et al. Combination of interferon-β and the angiotensin-converting enzyme inhibitor, perindopril, attenuates murine hepatocellular carcinoma development and angiogenesis. Clin. Cancer Res. 2003, 9, 6038–6045. [Google Scholar]
- Yasumatsu, R.; Nakashima, T.; Masuda, M.; Ito, A.; Kuratomi, Y.; Nakagawa, T.; Komune, S. Effects of the angiotensin-I converting enzyme inhibitor perindopril on tumor growth and angiogenesis in head and neck squamous cell carcinoma cells. J. Cancer Res. Clin. Oncol. 2004, 130, 567–573. [Google Scholar] [CrossRef]
- Medeiros, R.; Vasconcelos, A.; Costa, S.; Pinto, D.; Lobo, F.; Morais, A.; Oliveira, J.; Lopes, C. Linkage of angiotensin I-converting enzyme gene insertion/deletion polymorphism to the progression of human prostate cancer. J. Pathol. 2004, 202, 330–335. [Google Scholar] [CrossRef]
- Koh, W.P.; Yuan, J.M.; Sun, C.L.; Van den Berg, D.; Seow, A.; Lee, H.P.; Yu, M.C. Angiotensin I-converting enzyme (ACE) gene polymorphism and breast cancer risk among Chinese women in Singapore. Cancer Res. 2003, 63, 573–578. [Google Scholar]
- Zhang, Y.; He, J.; Deng, Y.; Zhang, J.; Li, X.; Xiang, Z.; Huang, H.; Tian, C.; Huang, J.; Fan, H. The insertion/deletion (I/D) polymorphism in the Angiotensin-converting enzyme gene and cancer risk: A meta-analysis. BMC Med. Genet. 2011, 12, 159. [Google Scholar] [CrossRef]
- Santos, R.A.S.; Simoes e Silva, A.C.; Maric, C.; Silva, D.M.R.; Machado, R.P.; de Buhr, I.; Heringer-Walther, S.; Pinheiro, S.V.B.; Lopes, M.T.; Bader, M.; et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc. Natl. Acad. Sci. USA 2003, 100, 8258–8263. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fan, J.; Wu, F.; Huang, Q.; Guo, M.; Lv, Z.; Han, J.; Duan, L.; Hu, G.; Chen, L.; et al. The ACE2/angiotensin-(1-7)/Mas receptor axis: Pleiotropic roles in cancer. Front. Physiol. 2017, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Egami, K.; Murohara, T.; Shimada, T.; Sasaki, K.; Shintani, S.; Sugaya, T.; Ishii, M.; Akagi, T.; Ikeda, H.; Matsuishi, T.; et al. Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth. J. Clin. Investig. 2003, 112, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Deshayes, F.; Nahmias, C. Angiotensin receptors: A new role in cancer? Trends Endocrinol. Metab. 2005, 16, 293–299. [Google Scholar] [CrossRef]
- Smith, G.R.; Missailidis, S. Cancer, inflammation and the AT1 and AT2 receptors. J. Inflamm. 2004, 1, 3. [Google Scholar] [CrossRef][Green Version]
- Silvestre, J.-S.; Tamarat, R.; Senbonmatsu, T.; Icchiki, T.; Ebrahimian, T.; Iglarz, M.; Besnard, S.; Duriez, M.; Inagami, T.; Lévy, B.I. Antiangiogenic effect of angiotensin II Type 2 receptor in ischemia-induced angiogenesis in mice hindlimb. Circ. Res. 2002, 90, 1072–1079. [Google Scholar] [CrossRef]
- Featherston, T.; Yu, H.H.; Dunne, J.C.; Chibnall, A.M.; Brasch, H.D.; Davis, P.F.; Tan, S.T.; Itinteang, T. Cancer stem cells in moderately differentiated buccal mucosal squamous cell carcinoma express components of the renin–angiotensin system. Front. Surg. 2016, 3, 52. [Google Scholar] [CrossRef]
- Itinteang, T.; Dunne, J.C.; Chibnall, A.M.; Brasch, H.D.; Davis, P.F.; Tan, S.T. Cancer stem cells in moderately differentiated oral tongue squamous cell carcinoma express components of the renin–angiotensin system. J. Clin. Pathol. 2016, 69, 942–945. [Google Scholar] [CrossRef]
- Siljee, S.; Pilkington, T.; Brasch, H.D.; Bockett, N.; Patel, J.; Paterson, E.; Davis, P.F.; Tan, S.T. Cancer stem cells in head and neck metastatic malignant melanoma express components of the renin-angiotensin system. Life 2020, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, A.; Wickremesekera, S.K.; Van Schaijik, B.; Marsh, R.W.; Brasch, H.D.; Tan, S.T.; Itinteang, T. Cancer stem cells in liver metastasis from colon adenocarcinoma express components of the renin-angiotensin system. J. Cancer Metastasis Treat. 2019, 2019, 36. [Google Scholar] [CrossRef]
- Nallaiah, S.; Lee, V.M.Y.; Brasch, H.D.; de Jongh, J.; van Schaijik, B.; Marsh, R.; Tan, S.T.; Itinteang, T. Cancer stem cells within moderately differentiated head and neck cutaneous squamous cell carcinoma express components of the renin-angiotensin system. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Benicky, J.; Hafko, R.; Sanchez-Lemus, E.; Aguilera, G.; Saavedra, J.M. Six commercially available angiotensin II AT1 receptor antibodies are non-specific. Cell. Mol. Neurobiol. 2012, 32, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.J.; Kimura, K.; Eguchi, S. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type-1 receptor protein. Hypertension 2013, 61, e31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herrera, M.; Sparks, M.A.; Alfonso-Pecchio, A.R.; Harrison-Bernard, L.M.; Coffman, T.M. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type 1 receptor protein. Hypertension 2013, 61, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.C.; Wieland, T.; Tsujimoto, G. How reliable are G-protein-coupled receptor antibodies? Naunyn. Schmiedebergs. Arch. Pharmacol. 2009, 379, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Brasch, H.D.; van Schaijik, B.; Armstrong, J.R.; Marsh, R.W.; Davis, P.F.; Tan, S.T.; Itinteang, T. Expression and localization of cathepsins B, D, and G in Dupuytren’s disease. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1686. [Google Scholar] [CrossRef]
- George, A.J.; Thomas, W.G.; Hannan, R.D. The renin–angiotensin system and cancer: Old dog, new tricks. Nat. Rev. Cancer 2010, 10, 745–759. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Fu, X.; Yang, A. Identification of key genes and pathways in tongue squamous cell carcinoma using bioinformatics analysis. Med. Sci. Monit. 2017, 23, 5924–5932. [Google Scholar] [CrossRef]
- Nguyen, G. Renin, (pro)renin and receptor: An update. Clin. Sci. 2011, 120, 169–178. [Google Scholar] [CrossRef]
- Shibayama, Y.; Fujimori, T.; Nguyen, G.; Hirose, T.; Totsune, K.; Ichihara, A.; Kitada, K.; Nakano, D.; Kobori, H.; Kohno, M.; et al. (Pro)renin receptor is crucial for Wnt/β-catenin-dependent genesis of pancreatic ductal adenocarcinoma. Sci. Rep. 2015, 5, 8854. [Google Scholar] [CrossRef]
- Katwa, L.C.; Tyagi, S.C.; Campbell, S.E.; Lee, S.J.; Cicila, G.T.; Weber, K.T. Valvular interstitial cells express angiotensinogen and cathepsin D, and generate angiotensin peptides. Int. J. Biochem. Cell Biol. 1996, 28, 807–821. [Google Scholar] [CrossRef]
- Hackenthal, E.; Hackenthal, R.; Hilgenfeldt, U. Isorenin, pseudorenin, cathepsin D and renin. A comparative enzymatic study of angiotensin-forming enzymes. Biochim. Biophys. Acta Enzymol. 1978, 522, 574–588. [Google Scholar] [CrossRef]
- Kawada, A.; Hara, K.; Kominami, E.; Kobayashi, T.; Hiruma, M.; Ishibashi, A. Cathepsin B and D expression in squamous cell carcinoma. Br. J. Dermatol. 1996, 135, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Featherston, T.; Marsh, R.W.; van Schaijik, B.; Brasch, H.D.; Tan, S.T.; Itinteang, T. Expression and localization of cathepsins B, D, and G in two cancer stem cell subpopulations in moderately differentiated oral tongue squamous cell carcinoma. Front. Med. 2017, 4, 100. [Google Scholar] [CrossRef] [PubMed]
- Ikeguchi, M.; Sakatani, T.; Ueta, T.; Fukuda, K.; Oka, S.; Hisamitsu, K.; Yamaguchi, K.; Tsujitani, S.; Kaibara, N. Correlation between cathepsin D expression and p53 protein nuclear accumulation in oesophageal squamous cell carcinoma. J. Clin. Pathol. 2002, 55, 121–126. [Google Scholar] [CrossRef][Green Version]
- Fan, Y.-L.; Zheng, M.; Tang, Y.-L.; Liang, X.-H. A new perspective of vasculogenic mimicry: EMT and cancer stem cells (Review). Oncol. Lett. 2013, 6, 1174–1180. [Google Scholar] [CrossRef]
- Nguyen, L.; Ager, E.I.; Neo, J.; Christophi, C. Regulation of colorectal cancer cell epithelial to mesenchymal transition by the renin angiotensin system. J. Gastroenterol. Hepatol. 2016, 31, 1773–1782. [Google Scholar] [CrossRef]
- Urata, H.; Kinoshita, A.; Misono, K.S.; Bumpus, F.M.; Husain, A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J. Biol. Chem. 1990, 265, 22348–22357. [Google Scholar] [CrossRef]
- Sugimoto, M.; Yamaoka, Y.; Shirai, N.; Furuta, T. Role of renin-angiotensin system in gastric oncogenesis. J. Gastroenterol. Hepatol. 2012, 27, 442–451. [Google Scholar] [CrossRef]
- Ibaraki, T.; Muramatsu, M.; Takai, S.; Jin, D.; Maruyama, H.; Orino, T.; Katsumata, T.; Miyazaki, M. The relationship of tryptase- and chymase-positive mast cells to angiogenesis in stage I non-small cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2005, 28, 617–621. [Google Scholar] [CrossRef]
- Diaconu, N.-C.; Rummukainen, J.; Naukkarinen, A.; Mättö, M.; Harvima, R.J.; Pelkonen, J.; Harvima, I.T. Mast cell chymase is present in uterine cervical carcinoma and it detaches viable and growing cervical squamous carcinoma cells from substratum in vitro. Arch. Dermatol. Res. 2011, 303, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Kurihara-Shimomura, M.; Sasahira, T.; Shimomura, H.; Bosserhoff, A.K.; Kirita, T. Mast cell chymase promotes angiogenesis and lymphangiogenesis mediated by activation of melanoma inhibitory activity gene family members in oral squamous cell carcinoma. Int. J. Oncol. 2020, 56, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Gudiseva, S.; Santosh, A.B.R.; Chitturi, R.; Anumula, V.; Poosarla, C.; Baddam, V.R.R. The role of mast cells in oral squamous cell carcinoma. Contemp. Oncol. 2017, 21, 21–29. [Google Scholar] [CrossRef]
- Yadav, A.; Desai, R.S.; Bhuta, B.A.; Singh, J.S.; Mehta, R.; Nehete, A.P. Altered immunohistochemical expression of mast cell tryptase and chymase in the pathogenesis of oral submucous fibrosis and malignant transformation of the overlying epithelium. PLoS ONE 2014, 9, e98719. [Google Scholar] [CrossRef] [PubMed]
- De Souza, D.A.; Santana, A.C.; Da Silva, E.Z.M.; Oliver, C.; Jamur, M.C. The role of mast cell specific chymases and tryptases in tumor angiogenesis. BioMed Res. Int. 2015, 2015, 142359. [Google Scholar] [CrossRef]
- Schick, C.; Kamachi, Y.; Bartuski, A.J.; Çataltepe, S.; Schechter, N.M.; Pemberton, P.A.; Silverman, G.A. Squamous cell carcinoma antigen 2 is a novel serpin that inhibits the chymotrypsin-like proteinases cathepsin g and mast cell chymase. J. Biol. Chem. 1997, 272, 1849–1855. [Google Scholar] [CrossRef]
- Qian, Y.R.; Guo, Y.; Wan, H.Y.; Fan, L.; Feng, Y.; Ni, L.; Xiang, Y.; Li, Q.Y. Angiotensin-converting enzyme 2 attenuates the metastasis of non-small cell lung cancer through inhibition of epithelial-mesenchymal transition. Oncol. Rep. 2013, 29, 2408–2414. [Google Scholar] [CrossRef]
- Feng, Y.; Ni, L.; Wan, H.; Fan, L.; Fei, X.; Ma, Q.; Gao, B.; Xiang, Y.; Che, J.; Li, Q. Overexpression of ACE2 produces antitumor effects via inhibition of angiogenesis and tumor cell invasion in vivo and in vitro. Oncol. Rep. 2011, 26, 1157–1164. [Google Scholar] [CrossRef]
- Zong, H.; Yin, B.; Zhou, H.; Cai, D.; Ma, B.; Xiang, Y. Loss of angiotensin-converting enzyme 2 promotes growth of gallbladder cancer. Tumor Biol. 2015, 36, 5171–5177. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, R.; Zhang, L.; Yao, W.; Li, J.; Yuan, Y. Angiotensin-converting enzyme 2 acts as a potential molecular target for pancreatic cancer therapy. Cancer Lett. 2011, 307, 18–25. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, S.; Li, T.; Yu, L.; Zhang, Y.; Zeng, H.; Qian, X.; Bi, J.; Lin, Y. ACE2 inhibits breast cancer angiogenesis via suppressing the VEGFa/VEGFR2/ERK pathway. J. Exp. Clin. Cancer Res. 2019, 38, 173. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Tang, W.; Wang, Y.; Shen, Q.; Wang, B.; Cai, C.; Meng, X.; Zou, F. Downregulation of ACE2/Ang-(1-7)/Mas axis promotes breast cancer metastasis by enhancing store-operated calcium entry. Cancer Lett. 2016, 376, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, L.; Li, M.; Wang, X. The SARS-CoV-2 host cell receptor ACE2 correlates positively with immunotherapy response and is a potential protective factor for cancer progression. Comput. Struct. Biotechnol. J. 2020, 18, 2438–2444. [Google Scholar] [CrossRef] [PubMed]
- Clara, J.A.; Monge, C.; Yang, Y.; Takebe, N. Targeting signalling pathways and the immune microenvironment of cancer stem cells—A clinical update. Nat. Rev. Clin. Oncol. 2020, 17, 204–232. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Huang, C.-H.; Lu, H.-I.; Chen, C.-H.; Huang, W.-T.; Hsieh, M.-J.; Rau, K.-M.; Chang, A.Y.W.; Lin, W.-C.; Li, S.-H. Prognostic impact of renin-angiotensin system blockade in esophageal squamous cell carcinoma. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 1185–1192. [Google Scholar] [CrossRef]
- Rodgers, K.E.; Xiong, S.; Steer, R.; DiZerega, G.S. Effect of angiotensin II on hematopoietic progenitor cell proliferation. Stem. Cells 2000, 18, 287–294. [Google Scholar] [CrossRef]
- Singh, K.D.; Karnik, S.S. Angiotensin receptors: Structure, function, signaling and clinical applications. J. Cell Signal. 2016, 1, 111. [Google Scholar] [CrossRef]
- Tadevosyan, A.; Xiao, J.; Surinkaew, S.; Naud, P.; Merlen, C.; Harada, M.; Qi, X.; Chatenet, D.; Fournier, A.; Allen, B.G.; et al. Intracellular angiotensin-II interacts with nuclear angiotensin receptors in cardiac fibroblasts and regulates RNA synthesis, cell proliferation, and collagen secretion. J. Am. Heart Assoc. 2017, 6, e004965. [Google Scholar] [CrossRef]
- Booz, G.W.; Conrad, K.M.; Hess, A.L.; Singer, H.A.; Baker, K.M. Angiotensin-II-binding sites on hepatocyte nuclei. Endocrinology 1992, 130, 3641–3649. [Google Scholar] [CrossRef]
- Re, R.N. Role of intracellular angiotensin II. Am. J. Physiol. Circ. Physiol. 2018, 314, H766–H771. [Google Scholar] [CrossRef]
- Katsuya, T.; Morishita, R. Gene polymorphism of angiotensin II type 1 and type 2 receptors. Curr. Pharm. Des. 2013, 19, 2996–3001. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Roth, I.; Wickremesekera, A.; Davis, P.; Kaye, A.; Mantamadiotis, T.; Stylli, S.; Tan, S. Therapeutic targeting of cancer stem cells in human glioblastoma by manipulating the renin-angiotensin system. Cells 2019, 8, 1364. [Google Scholar] [CrossRef] [PubMed]
- Roth, I.M.; Wickremesekera, A.C.; Wickremesekera, S.K.; Davis, P.F.; Tan, S.T. Therapeutic targeting of cancer stem cells via modulation of the renin-angiotensin system. Front. Oncol. 2019, 9, 745. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siljee, S.; Buchanan, O.; Brasch, H.D.; Bockett, N.; Patel, J.; Paterson, E.; Purdie, G.L.; Davis, P.F.; Itinteang, T.; Tan, S.T. Cancer Stem Cells in Metastatic Head and Neck Cutaneous Squamous Cell Carcinoma Express Components of the Renin-Angiotensin System. Cells 2021, 10, 243. https://doi.org/10.3390/cells10020243
Siljee S, Buchanan O, Brasch HD, Bockett N, Patel J, Paterson E, Purdie GL, Davis PF, Itinteang T, Tan ST. Cancer Stem Cells in Metastatic Head and Neck Cutaneous Squamous Cell Carcinoma Express Components of the Renin-Angiotensin System. Cells. 2021; 10(2):243. https://doi.org/10.3390/cells10020243
Chicago/Turabian StyleSiljee, Sam, Olivia Buchanan, Helen D. Brasch, Nicholas Bockett, Josie Patel, Erin Paterson, Gordon L. Purdie, Paul F. Davis, Tinte Itinteang, and Swee T. Tan. 2021. "Cancer Stem Cells in Metastatic Head and Neck Cutaneous Squamous Cell Carcinoma Express Components of the Renin-Angiotensin System" Cells 10, no. 2: 243. https://doi.org/10.3390/cells10020243
APA StyleSiljee, S., Buchanan, O., Brasch, H. D., Bockett, N., Patel, J., Paterson, E., Purdie, G. L., Davis, P. F., Itinteang, T., & Tan, S. T. (2021). Cancer Stem Cells in Metastatic Head and Neck Cutaneous Squamous Cell Carcinoma Express Components of the Renin-Angiotensin System. Cells, 10(2), 243. https://doi.org/10.3390/cells10020243