Addition of Biochar to a Sandy Desert Soil: Effect on Crop Growth, Water Retention and Selected Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and Biochar
2.2. Experimental Design
2.2.1. Experiment1: Wheat Growth
2.2.2. Experiment2: Water Retention
2.2.3. Experiment3: Changes in Selected Physical and Chemical Properties
2.3. Statistical Analysis
3. Results
3.1. Wheat Yield and N and P Uptake Response to Biochar Addition
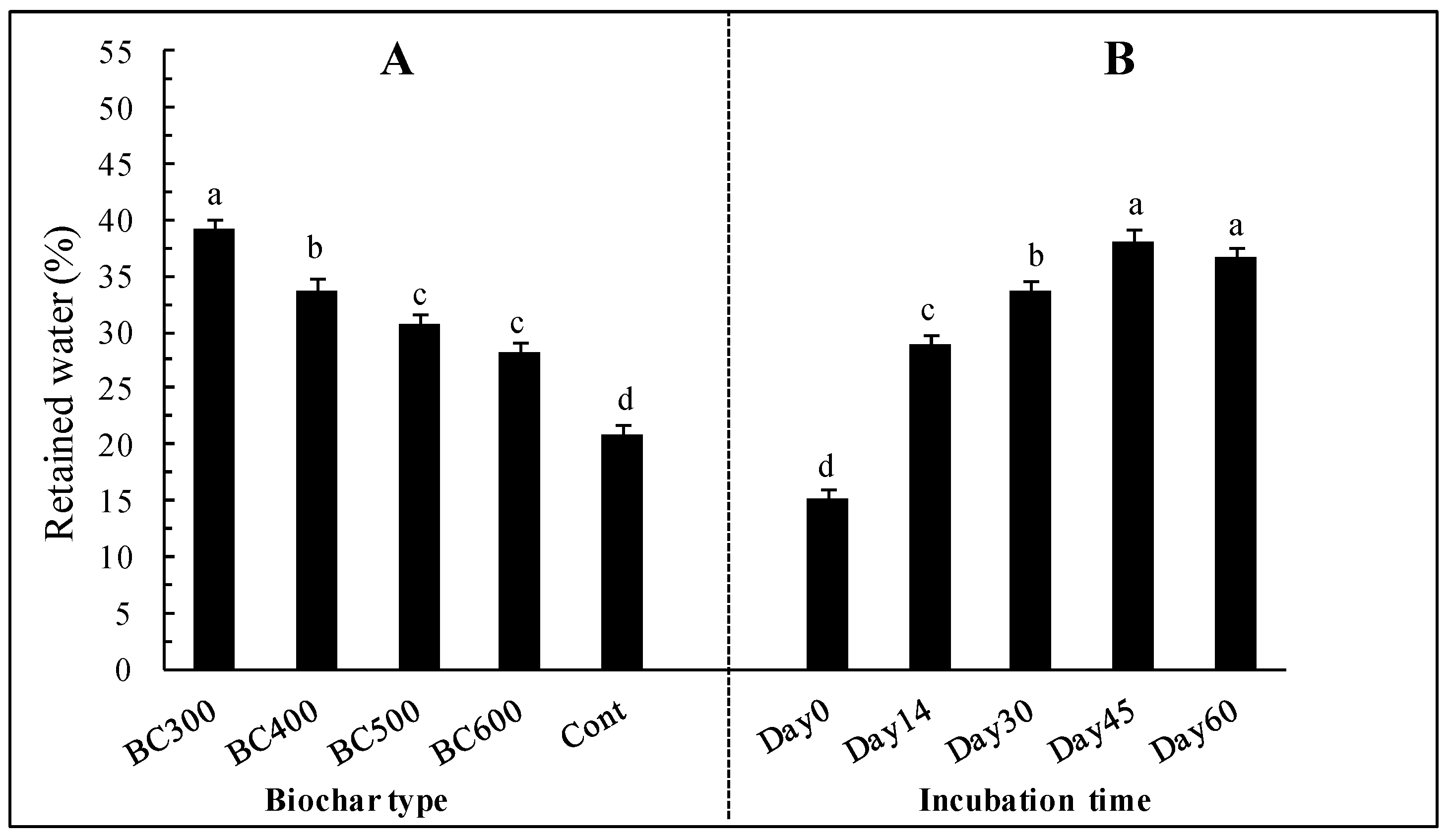
3.2. Impact of Date Palm Residues Biochars on Water Retention
3.3. Impact of Date Palm Residues Biochars on Soil Bulk Density and Total Porosity
3.4. Impact of Date Palm Residues Biochars on Selected Soil Chemical Properties
4. Discussion
4.1. Wheat Growth Response to Date Palm Residue Biochar Addition
4.2. Effect of Date Palm Residue Biochar Addition on Water Retention
4.3. Impact of Date Palm Residues Biochars on Selected Soil Physical and Chemical Properties
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, M.L.; Van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Xie, T.; Sadasivam, B.Y.; Reddy, K.R.; Wang, C.; Spokas, K. Review of the effects of biochar amendment on soil properties and carbon sequestration. J. Hazard. Toxic Radioact. Waste 2015, 20, 04015013. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Farooq, M.; Nawaz, A.; Al-Sadi, A.M.; Solaiman, Z.M.; Alghamdi, S.S.; Ammara, U.; Ok, Y.S.; Siddique, K.H.M. Biochar for crop production: Potential benefits and risks. J. Soils Sediments 2016, 17, 685–716. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Aust. J. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Nelson, P.F. Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum). Chemosphere 2010, 78, 1167–1171. [Google Scholar] [CrossRef]
- Major, J.; Rondon, M.; Molina, D.; Riha, S.J.; Lehmann, J. Maize yield and nutrition during 4 years after biochar application to a Colombian savanna Oxisol. Plant Soil 2010, 333, 117–128. [Google Scholar] [CrossRef]
- Deal, C.; Brewer, C.E.; Brown, R.C.; Okure, M.A.E.; Amoding, A. Comparison of kiln-derived and gasifier-derived biochars as soil amendments in the humid tropics. Biomass Bioenergy 2012, 37, 161–168. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Murphy, D.V.; Abbott, L.K. Biochars influence seed germination and early growth of seedlings. Plant Soil 2012, 353, 273–287. [Google Scholar] [CrossRef]
- Wang, J.; Pan, X.; Liu, Y.; Zhang, X.; Xiong, Z. Effects of biochar amendment in two soils on greenhouse gas emissions and crop production. Plant Soil 2012, 360, 287–298. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Salazar, P.; Barrón, V.; Torrent, J.; del Campillo, M.D.C.; Gallardo, A.; Villar, R. Enhanced wheat yield by biochar addition under different mineral fertilization levels. Agron. Sustain. Dev. 2013, 33, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, M.K.; Anwar, A.A. Ameliorating effects of biochar derived from poultry manure and white clover residues on soil nutrient status and plant growth promotion-greenhouse experiments. PLoS ONE 2015, 10, e0131592. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, K.D.; Schoenau, J.J. Application of Two Bioenergy Byproducts with Contrasting Carbon Availability to a Prairie Soil: Three-Year Crop Response and Changes in Soil Biological and Chemical Properties. Agronomy 2016, 6, 13. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Li, S.; Shangguan, Z. Positive effects of apple branch biochar on wheat yield only appear at a low application rate, regardless of nitrogen and water conditions. J. Soils Sediments 2018, 18, 3235–3243. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2011, 337, 1–18. [Google Scholar] [CrossRef]
- Abel, S.; Peters, A.; Trinks, S.; Schonsky, H.; Facklam, M.; Wessoolek, G. Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 2013, 202, 183–191. [Google Scholar] [CrossRef]
- Ulyett, J.; Sakrabani, R.; Kibbilewhite, M.; Hann, M. Impact of biochar addition on water retention, nitrification and carbon dioxide evolution from sandy loam soils. Eur. J. Soil Sci. 2014, 65, 96–104. [Google Scholar] [CrossRef]
- Głąb, T.; Palmowska, J.; Zaleski, T.; Gondek, K. Effect of biochar application on soil hydrological properties and physical quality of sandy soil. Geoderma 2016, 281, 11–20. [Google Scholar] [CrossRef]
- Omondi, M.O.; Xia, X.; Nahayo, A.; Liu, X.; Korai, P.K.; Pan, G. Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 2016, 274, 28–34. [Google Scholar] [CrossRef]
- Aller, D.; Rathke, S.; Laird, D.; Cruse, R.; Hatfield, J. Impacts of fresh and aged biochars on plant available water and water use efficiency. Geoderma 2017, 307, 114–121. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and soil physical properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Baiamonte, G.; Crescimanno, G.; Parrino, F.; De Pasquale, C. Effect of biochar on the physical and structural properties of a desert sandy soil. Catena 2019, 175, 294–303. [Google Scholar] [CrossRef]
- Stewart, C.E.; Zheng, J.; Botte, J.; Cotrufo, F. Co-generated fast pyrolysis biochar mitigates greenhouse gas emissions and increases carbon sequestration intemperate soils. Glob. Chang. Biol. Bioenergy 2013, 5, 153–164. [Google Scholar] [CrossRef]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Malo, D.D.; Julson, J.L. Effect of biochar on chemical properties of acidic soil. Arch. Agron. Soil Sci. 2014, 60, 393–404. [Google Scholar] [CrossRef]
- Xu, G.; Sun, J.; Shao, H.; Chang, S.X. Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol. Eng. 2014, 62, 54–60. [Google Scholar] [CrossRef]
- Jeffery, S.; Abalos, D.; Prodana, M.; Bastos, A.C.; Van Groenigen, J.W.; Hungate, B.A.; Verheijen, F. Biochar boosts tropical but not temperate crop yields. Environ. Res. Lett. 2017, 12, 053001. [Google Scholar] [CrossRef]
- Raboin, L.-M.; Razafmahafaly, A.H.D.; Rabenjarisoa, M.B.; Rabary, B.; Dusserre, J.; Becquer, T. Improving the fertility of tropical acid soils: Liming versus biochar application? A long term comparison in the highlands of Madagascar. Field Crops Res. 2016, 199, 99–108. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, A.; Ji, C.; Joseph, S.; Bian, R.; Li, L.; Pan, G.; Paz-Ferreiro, J. Biochar’s effect on crop productivity and the dependence on experimental conditions—A meta-analysis of literature data. Plant Soil 2013, 373, 583–594. [Google Scholar] [CrossRef]
- Uzoma, K.C.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag. 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Foster, E.J.; Hansen, N.; Wallenstein, M.; Cotrufo, M.F. Biochar and manure amendments impact soil nutrients and microbial enzymatic activities in a semi-arid irrigated maize cropping system. Agric. Ecosyst. Environ. 2016, 233, 404–414. [Google Scholar] [CrossRef] [Green Version]
- Mulcahy, D.N.; Mulcahy, D.L.; Dietz, D. Biochar soil amendment increases tomato seedling resistance to drought in sandy soils. J. Arid Environ. 2013, 88, 222–225. [Google Scholar] [CrossRef]
- Laghari, M.; Mirjat, M.S.; Hu, Z.; Fazal, S.; Xiao, B.; Hu, M.; Chen, Z.; Guo, D. Effects of biochar application rate on sandy desert soil properties and sorghum growth. Catena 2015, 135, 313–320. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Al-Wabel, M.I.; Usman, A.R.; Al-Omran, A. Effect of Conocarpus biochar application on the hydraulic properties of a sandy loam soil. Soil Sci. 2013, 178, 165–173. [Google Scholar] [CrossRef]
- Khalifa, N.; Yousef, L.F. A short report on changes of quality indicators for a sandy textured soil after treatment with biochar produced from fronds of date palm. Energy Procedia 2015, 74, 960–965. [Google Scholar] [CrossRef]
- El-Naggar, A.H.; Usman, A.R.; Al-Omran, A.; Ok, Y.S.; Ahmad, M.; Al-Wabel, M.I. Carbon mineralization and nutrient availability in calcareous sandy soils amended with woody waste biochar. Chemosphere 2015, 138, 67–73. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Al-Wabel, M.I.; Ok, Y.S.; Al-Harbi, A.; Wahb-Allah, M.; El-Naggar, A.H.; Ahmad, M.; Al-Faraj, A.; Al-Omran, A. Conocarpus biochar induces changes in soil nutrient availability and tomato growth under saline irrigation. Pedosphere 2016, 26, 27–38. [Google Scholar] [CrossRef]
- Bashour, I.I.; Al-Mashhady, A.S.; Prasad, J.D.; Miller, T.; Mazroa, M. Morphology and composition of some soils under cultivation in Saudi Arabia. Geoderma 1983, 29, 327–340. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 3. Chemical Methods; SSSA Book Series; Sparks, D.L., Ed.; SSSA and ASA: Madison, WI, USA, 1996; No. 5; pp. 961–1010. [Google Scholar]
- Loeppert, R.H.; Suarez, D. Carbonate and gypsum. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Bigham, J.M., Eds.; SSSA and ASA: Madison, WI, USA, 1996; pp. 437–474. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; SSSA and ASA: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Usman, A.R.; Abduljabbar, A.; Vithanage, M.; Ok, Y.S.; Ahmad, M.; Ahmad, M.; Elfaki, J.; Abdulazeem, S.S.; Al-Wabel, M.I. Biochar production from date palm waste: Charring temperature induced changes in composition and surface chemistry. J. Anal. Appl. Pyrolysis 2015, 115, 392–400. [Google Scholar] [CrossRef]
- Thomas, R.L.; Sheard, R.W.; Moyer, J.R. Comparision of conventional and automated procedures for nitrogen, phosphorus and potassium analysis of plant material using a single digestion. Agron. J. 1967, 59, 240–243. [Google Scholar] [CrossRef]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Ahmendna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; et al. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis. Part 1, 2nd ed.; Klute, A., Ed.; SSSA: Madison, WI, USA, 1986; pp. 363–376. [Google Scholar]
- Rhoades, J.D. Cation exchange capacity. In Methods of Soil Analysis. Part 2: Chemical and Mineralogical Properties; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; No. 9; pp. 149–157. [Google Scholar]
- Asai, H.; Samson, B.K.; Stephan, H.M.; Songyikhangsuthor, K.; Homma, K.; Kiyono, Y.; Inoue, Y.; Shiraiwa, T.; Horie, T. Biochar amendment techniques for upland rice production in Northern Laos: 1. Soil physical properties, leaf SPAD and grain yield. Field Crops Res. 2009, 111, 81–84. [Google Scholar] [CrossRef]
- Schulz, H.; Glaser, B. Effects of biochar compared to organic and inorganic fertilizers on soil quality and plant growth in a greenhouse experiment. J. Plant Nutr. Soil Sci. 2012, 175, 410–422. [Google Scholar] [CrossRef]
- Kookana, R.S.; Sarmah, A.K.; Van Zwieten, L.; Krull, E.; Singh, B. Biochar application to soil: Agronomic and environmental benefits and unintended consequences. Adv. Agron. 2011, 112, 103–143. [Google Scholar]
- Nguyen, T.T.N.; Xu, C.Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef] [Green Version]
- Recous, S.; Robi, D.; Darwis, D.; Mary, B. Soil inorganic N availability: Effect on maize residue decomposition. Soil Biol. Biochem. 1995, 27, 1529–1538. [Google Scholar] [CrossRef]
- Sakala, W.D.; Cadisch, G.; Giller, K.E. Interactions between residues of maize and pigeonpea and mineral N fertilizers during decomposition and N mineralization. Soil Biol. Biochem. 2000, 32, 679–688. [Google Scholar] [CrossRef]
- Basso, A.S.; Miguez, F.E.; Laird, D.A.; Horton, R.; Westgate, M. Assessing potential of biochar for increasing water-holding capacity of sandy soils. GCB Bioenergy 2013, 5, 132–143. [Google Scholar] [CrossRef]
- Sun, Z.; Moldrup, P.; Elsgaard, L.; Artur, E.; Bruun, E.; Hauggaard-Nielsen, H.; de Jonge, L.W. Direct and indirect short-term effects of biochar on physical characteristics of an arable sandy loam. Soil Sci. 2013, 178, 465–473. [Google Scholar] [CrossRef]
- Hardie, M.; Clothier, B.; Bound, S.; Oliver, G.; Close, D. Does biochar influence soil physical properties and soil water availability? Plant Soil 2014, 376, 347–361. [Google Scholar] [CrossRef]
- Jeffery, S.; Meinders, M.B.J.; Stoof, C.R.; Bezemer, T.M.; van de Voorde, T.F.J.; Mommer, L.; van Groenigen, J.W. Biochar application does not improve the soil hydrological function of a sandy soil. Geoderma 2015, 251, 47–54. [Google Scholar] [CrossRef]
- Kinney, T.J.; Masiello, C.A.; Dugan, B.; Hockaday, W.C.; Dean, M.R.; Zygourakis, K.; Barnes, R.T. Hydrologic properties of biochars produced at different temperatures. Biomass Bioenergy 2012, 41, 34–43. [Google Scholar] [CrossRef]
- Gray, M.; Johnson, M.G.; Dragila, M.I.; Kleber, M. Water uptake in biochars: The roles of porosity and hydrophobicity. Biomass Bioenergy 2014, 61, 196–205. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.M.; Dallmeyer, I.; Garcia-Pérez, M. The role of biochar porosity and surface functionality in augmenting hydrologic properties of a sandy soil. Sci. Total Environ. 2017, 574, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.R. Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Miyamoto, T.; Iwata, Y.; Shiono, T. Effects of biochar produced from sugarcane bagasse at different pyrolysis temperatures on water retention of a calcaric dark red soil. Soil Sci. 2016, 181, 20–28. [Google Scholar] [CrossRef]
- Günal, E.; Erdem, H.; Çelik, İ. Effects of three different biochars amendment on water retention of silty loam and loamy soils. Agric. Water Manag. 2018, 208, 232–244. [Google Scholar] [CrossRef]
- Lei, O.; Zhang, R.D. Effects of biochars derived from different feedstocks and pyrolysis temperatures on soil physical and hydraulic properties. J. Soils Sediments 2013, 13, 1561–1572. [Google Scholar] [CrossRef]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.P.; Schoenau, J.J. Effects of biochar on yield, nutrient recovery, and soil properties in a canola (Brassica napus L)-wheat (Triticum aestivum L) rotation grown under controlled environmental conditions. Bioenergy Res. 2015, 8, 1183–1196. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhang, X.C. Effect of Biochar on pH of Alkaline Soils in the Loess Plateau: Results from Incubation Experiments. Int. J. Agric. Biol. 2012, 14, 745–750. [Google Scholar]
- Al-Wabel, M.I.; Usman, A.R.; Al-Farraj, A.S.; Ok, Y.S.; Abduljabbar, A.; Al-Faraj, A.I.; Sallam, A.S. Date palm waste biochars alter a soil respiration, microbial biomass carbon, and heavy metal mobility in contaminated mined soil. Environ. Geochem. Health 2017, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Yu, L.; Zhang, R. Effects of amendment of different biochars on soil carbon mineralization and sequestration. Soil Res. 2014, 52, 46–54. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Awad, Y.M.; Yang, X.; Ryu, C.; Rizwan, M.; Rinklebe, J.; Tsang, D.C.; Ok, Y.S. Influence of soil properties and feedstocks on biochar potential for carbon mineralization and improvement of infertile soils. Geoderma 2018, 332, 100–108. [Google Scholar] [CrossRef]





| FS/BC | Parameters | |||
|---|---|---|---|---|
| C (%) | N (%) | pH | EC (dS m−1) | |
| FS | 42.8 | 1.5 | 7.20 | 3.00 |
| BC300 | 61.4 | 0.75 | 7.67 | 5.61 |
| BC400 | 65 | 0.93 | 8.22 | 6.67 |
| BC500 | 71.7 | 1.0 | 8.40 | 7.53 |
| BC600 | 73.0 | 1.1 | 8.75 | 8.07 |
| Treatment | pH | EC | OM | CEC |
|---|---|---|---|---|
| dS m−1 | % | meq/100 | ||
| Control | 8.25 ± 0.01 a | 0.48 ± 0.03 c | 0.62 ± 0.00 b | 6.86 ± 0.42 b |
| FS | 8.07 ± 0.02 c | 1.51 ± 0.06 a | 0.69 ± 0.04 b | 8.24 ± 0.48 b |
| BC300 | 8.14 ± 0.04 b | 1.22 ± 0.18 a,b | 1.18 ± 0.12 a | 9.86 ± 0.40 a |
| BC400 | 8.23 ± 0.02 a | 1.09 ± 0.04 b | 0.70 ± 0.07 b | 7.09 ± 0.11 b |
| BC500 | 8.27 ± 0.01 a | 1.22 ± 0.03 a,b | 0.60 ± 0.04 b | 7.92 ± 0.15 b |
| BC600 | 8.19 ± 0.01 a,b | 1.54 ± 0.05 a | 0.57 ± 0.02 b | 7.72 ± 0.21 b |
| LSD (0.05) | 0.06 | 0.26 | 0.19 | 0.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, K.D.; Schoenau, J.J. Addition of Biochar to a Sandy Desert Soil: Effect on Crop Growth, Water Retention and Selected Properties. Agronomy 2019, 9, 327. https://doi.org/10.3390/agronomy9060327
Alotaibi KD, Schoenau JJ. Addition of Biochar to a Sandy Desert Soil: Effect on Crop Growth, Water Retention and Selected Properties. Agronomy. 2019; 9(6):327. https://doi.org/10.3390/agronomy9060327
Chicago/Turabian StyleAlotaibi, Khaled D., and Jeff J. Schoenau. 2019. "Addition of Biochar to a Sandy Desert Soil: Effect on Crop Growth, Water Retention and Selected Properties" Agronomy 9, no. 6: 327. https://doi.org/10.3390/agronomy9060327





