The Effect of Phosphorus and Root Zone Temperature on Anthocyanin of Red Romaine Lettuce
Abstract
:1. Introduction
2. Materials and Methods
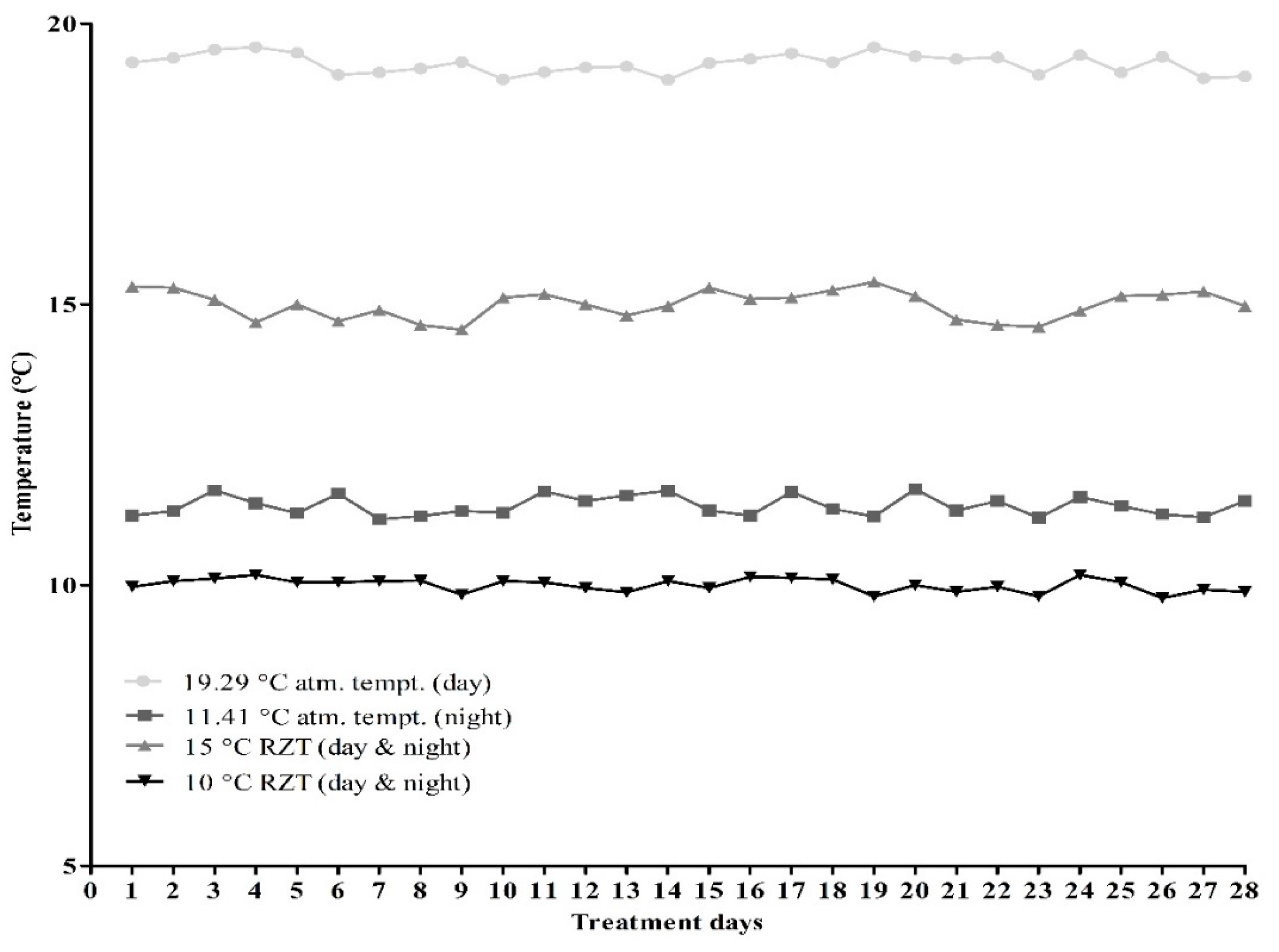
2.1. Lettuce Growing Environment
2.2. Lettuce and Treatments
2.3. Mineral Contents of Red Romaine Lettuce
2.4. Growth Parameters
2.5. Chlorophyll (chl a, chl b and chl Total) and Anthocyanin Contents
2.6. Statistical Analyses
3. Results
3.1. Mineral Contents of Red Romaine Lettuce
3.2. Plant Yield, Leaf Dry Matter, Fresh Root Weight, Root Length and Root Dry Matter
3.3. Leaf Number, Leaf Length, Leaf Width and Leaf Chlorophyll Content (SPAD)
3.4. Leaf Color Value (L*, a*, b*, Chrome and ºh)
3.5. Chlorophyll (chl a, chl b and chl Total) and Anthocyanin Content
4. Discussion
4.1. Mineral Contents of Red Romaine Lettuce
4.2. Plant Yield, Leaf Dry Matter, Fresh Root Weight, Root Length and Root Dry Matter
4.3. Leaf Number, Leaf Length, Leaf Width and Leaf Chlorophyll Content (SPAD)
4.4. Leaf Color Value (L*, a*, b*, Chrome and ºh)
4.5. Chlorophyll (chl a, chl b and chl Total) and Anthocyanin Content
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- AFRASTAT. Agriculture, Food and Rural Affairs Statistics Yearbook; Ministry of Agriculture, Food and Rural Affairs, Republic of Korea, Director-General for Policy Planning Bureau: Sejong, Korea, 2017; p. 104. [Google Scholar]
- Tsormpatsidis, E.; Henbest, R.G.C.; Davis, F.J.; Battey, N.H.; Hadley, P.; Wagstaffe, A. UV irradiance as a major influence on growth, development and secondary products of commercial importance in Lollo Rosso lettuce “Revolution” grown under polyethylene films. Environ. Exp. Bot. 2008, 63, 232–239. [Google Scholar] [CrossRef]
- Sublett, W.L.; Barickman, T.C.; Sams, C.E. The effect of environment and nutrients on hydroponic lettuce yield, quality, and phytonutrients. Horticulturae 2018, 4, 1–15. [Google Scholar]
- Fallovo, C.; Rouphael, Y.; Rea, E.; Battistelli, A.; Colla, G. Nutrient solution concentration and growing season affect yield and quality of Latuca sativa L. var. acephala in floating raft culture. J. Sci. Food Agric. 2009, 89, 1682–1689. [Google Scholar] [CrossRef]
- Nozzi, V.; Graber, A.; Schmautz, Z.; Mathis, A.; Junge, R. Nutrient management in aquaponics: comparison of three approaches for cultivating lettuce, mint and mushroom herb. Agronomy 2018, 8, 27. [Google Scholar] [CrossRef]
- Chen, R.; Song, S.; Li, X.; Liu, H.; Huang, D. Phosphorus deficiency restricts plant growth but induces pigment formation in the flower stalk of Chinese kale. Hortic. Environ. Biotechnol. 2013, 54, 243–248. [Google Scholar]
- Luna, M.C.; Martınez-Sanchez, A.; Selma, M.V.; Tudela, J.A.; Baixaulib, C.; Gil, M.I. Influence of nutrient solutions in an open-field soilless system on the quality characteristics and shelf life of fresh-cut red and green lettuces (Lactuca sativa L.) in different seasons. J. Sci. Food Agric. 2013, 93, 93415–93421. [Google Scholar] [CrossRef]
- Goto, T.; Kondo, T. Structure and molecular stacking of anthocyanins-flower color variation. Angew. Chem. Int. Ed. 1991, 30, 17–33. [Google Scholar]
- Sublett, W.L.; Barickman, T.C.; Sams, C.E. Effects of elevated temperature and potassium on biomass and quality of dark red ‘Lollo Rosso’ lettuce. Horticulturae 2018, 4, 11. [Google Scholar] [CrossRef]
- Islam, M.Z.; Mele, M.A.; Choi, K.Y.; Kang, H.M. Nutrient and salinity concentrations effects on quality and storability of cherry tomato fruits grown by hydroponic system. Bragantia 2018, 77, 385–393. [Google Scholar]
- Islam, M.Z.; Mele, M.A.; Baek, J.P.; Kang, H.M. Cherry tomato qualities affected by foliar spraying with boron and calcium. Hortic. Environ. Biotechnol. 2016, 57, 46–52. [Google Scholar] [CrossRef]
- Mele, M.A.; Islam, M.Z.; Baek, J.P.; Kang, H.M. Quality, storability, and essential oil content of Ligularia fischeri during modified atmosphere packaging storage. J. Food Sci. Technol. 2017, 54, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Moran, R. Formulate for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Tachiban, S. Effect of root temperature on the rate of water and nutrient absorption in cucumber cultivars and figleaf gourd. J. Jpn. Soc. Hort. Sci. 1987, 55, 461–467. [Google Scholar] [CrossRef]
- Lee, S.; Jung, J.; Sung, J.; Ha, S.; Lee, D.; Kim, T.; Song, B. Responses of nutrient uptake, carbohydrates and antioxidants against low temperature in plants. C.N.U. J. Agric. Sci. 2014, 41, 75–83. [Google Scholar]
- Urrestarazu, M.; Salas, M.C.; Valera, D.; Go´me, A.; Mazuela, P.C. Effects of heating nutrient solution on water and mineral uptake and early yield of two cucurbits under soilless culture. J. Plant Nutr. 2008, 31, 527–538. [Google Scholar] [CrossRef]
- Yan, Q.; Duan, Z.; Mao, J.; Li, X.; Dong, F. Effects of root-zone temperature and N, P, and K supplies on nutrient uptake of cucumber (Cucumis sativus L.) seedlings in hydroponics. Soil. Sci. Plant Nutr. 2012, 58, 707–717. [Google Scholar] [CrossRef]
- Albornoz, F.; Lieth, J.H. Diurnal macronutrients uptake patterns by lettuce roots under various light and temperature levels. J. Plant Nutr. 2015, 38, 2028–2043. [Google Scholar] [CrossRef]
- Park, K.W.; Chiang, M.H.; Won, J.H.; Jang, K.H. The effect of nutrient solution temperature on the absorption of water and minerals in Chinese leafy vegetables. J. Kor. Soc. Hort. Sci. 1995, 36, 309–316. [Google Scholar]
- Lee, E.H.; Kim, B.Y.; Lee, K.D.; Lee, J.W.; Kwon, Y.S. Nitrate content and activities of nitrate reductase and glutamine synthetase as affected by temperature and pH of nutrient solution in leaf lettuce and water dropwort. Kor. J. Soc. Hort. Sci. 1998, 39, 157–160. [Google Scholar]
- Syrett, P.J. Nitrogen metabolism of microalgae. Can. Bull. Fish Aquati. Sci. 1981, 210, 182–210. [Google Scholar]
- Clarkson, D.T.; Hopper, M.J.; Jones, L.H.P. The effect of root temperature on the uptake of nitrogen and the relative size of the root system in Lolium perenne. I. Solutions containing both NH4+ and NO3−. Plant Cell Environ. 1986, 9, 535–545. [Google Scholar]
- Pedersen, A.; Kraemer, G.; Yarish, C. The effects of temperature and nutrient concentrations on nitrate and phosphate uptake in different species of Porphyra from Long Island Sound (USA). J. Exp. Mar. Biol. Ecol. 2004, 312, 235–252. [Google Scholar] [CrossRef]
- Mackay, A.D.; Barber, S.A. Soil temperature effects on root growth and phosphorous uptake by com. Soil Sci. Soc. Amer. J. 1984, 48, 818–823. [Google Scholar] [CrossRef]
- Fredeen, A.L.; Raab, T.K.; Rao, I.M.; Terry, N. Effects of phosphorus nutrition on photosynthesis in Glycine max (L.) Merr. Planta 1990, 181, 399–405. [Google Scholar] [PubMed]
- Akhtar, M.S.; Oki, Y.; Adachi, T. Genetic diversity in Brassica cultivars under deficiently buffered P-stress environment: I. Biomass accumulation, P-concentration, P-uptake, and related growth parameters. J. Am. Sci. 2007, 3, 55–63. [Google Scholar]
- Hurd, R.G.; Graves, C.J. Controlling the water supply. Grower 1981, 96, 21–22. [Google Scholar]
- Boo, H.O.; Heo, B.G.; Gorinstein, S.; Chon, S.U. Positive effects of temperature and growth conditions on enzymatic and antioxidant status in lettuce plants. Plant Sci. 2011, 181, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Singh, A.P.; Chung, G.C.; Ahn, S.J.; Noh, E.K.; Steudle, E. Exposure of roots of cucumber (Cucumis sativus) to low temperature severely reduces root pressure, hydraulic conductivity and active transport of nutrients. Physiol. Plant. 2004, 120, 413–420. [Google Scholar]
- Becker, C.; Klaering, H.-P.; Kroh, L.W.; Krumbein, A. Cool-cultivated red leaf lettuce accumulates cyaniding-3-O-(6˝-O-malonyl)-glucoside and caffeoylmalic acid. Food Chem. 2014, 146, 404–411. [Google Scholar]
- Bordonaba, J.G.; Terry, L.A. Manipulating the taste-related composition of strawberry fruits (Fragaria ananassa) from different cultivars using deficit irrigation. Food Chem. 2010, 122, 1020–1026. [Google Scholar]
- Costa, L.D.; Tomasi, N.; Gottardi, S.; Iacuzzo, F. The effect of growth medium temperature on corn salad [Valerianella locusta (L.) Laterr] baby leaf yield and quality. HortScience 2011, 46, 1619–1625. [Google Scholar]
- Hoque, M.M.; Ajwa, H.; Othman, M.; Smith, R.; Cahn, M. Yield and postharvest quality of lettuce in response to nitrogen, phosphorus and potassium fertilizers. HortScience 2010, 45, 1539–1544. [Google Scholar]
- Fernandez-Falcon, M.; Hernandez, M.; Alvarez, C.E.; Borges, A.A. Variation in nutrition along time and relative chlorophyll content of Leucospermum cordifolium cv. ‘High Gold’, and their relationship with chlorotic symptoms. Sci. Hortic. 2006, 107, 373–379. [Google Scholar]
- Mengel, K.; Kirkby, E.H. Principles of Plant Nutrition; Kluwer Academic Publishers: Boston, MA, USA, 2001. [Google Scholar]
- Mampholo, B.M.; Maboko, M.M.; Soundy, P.; Sivakumar, D. Phytochemicals and overall quality of leafy lettuce (Lactica sativa L.) varieties grown in closed hydroponic system. J. Food Qual. 2016, 39, 805–815. [Google Scholar] [CrossRef]
- Gazula, A.; Kleinhenz, M.D.; Scheerens, J.C.; Ling, P.P. Anthocyanin levels in nine lettuce (Lactuca sativa) cultivars: influence of planting date and relations among analytic, instrumented, and visual assessments of color. HortScience 2007, 42, 232–238. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Gazula, A.; Kleinhenz, M.D.; Streeter, J.G.; Miller, A.R. Temperature and cultivar effects on anthocyanin and chlorophyll b concentrations in three related Lollo Rosso lettuce cultivars. HortScience 2005, 40, 1731–1733. [Google Scholar]
- Sánchez-Calderón, L.; López-Bucio, J.; Chacón-López, A.; Gutiérrez-Ortega, A.; Hernández-Abreu, E.; Herrera-Estrella, L. Characterization of low phosphorus insensitive mutants reveals a crosstalk between low phosphorus-induced determinate root development and the activation of genes involved in the adaptation of Arabidopsis to phosphorus deficiency. Plant Physiol. 2006, 140, 879–889. [Google Scholar] [CrossRef]
- Mele, M.A.; Islam, M.Z.; Kang, H.M.; Giuffrè, A.M. Pre-and post-harvest factors and their impact on oil composition and quality of olive fruit. Emir. J. Food Agric. 2018, 30, 592–603. [Google Scholar]
| Ion Concentration | ||||||
|---|---|---|---|---|---|---|
| Stage | Germination stage in plug tray | Seedling stage in plug tray | Hardening stage in DFT | Growth and development stage in DFT system | ||
| Strength | - | 0.4× (low) | 0.4× (low) | 1×-P (full) | 0.5×-P (half) | 0×-P (none) |
| Treatment days | Days 0–15 (15 days) | Days 16–30 (15 days) | Days 31–45 (15 days) | Days 46–73 (28 days) | Days 46–73 days (28 days) | Days 46–73 (28 days) |
| pH | - | 6.0–6.5 | 6.0–6.5 | 6.0–6.5 | 6.0–6.5 | 6.0–6.5 |
| EC (dS m −1) | - | 0.75 | 0.75 | 1.80 | 1.80 | 1.80 |
| NO3–N | - | 5.20 | 5.20 | 13 | 13 | 13 |
| NH4–N | - | 0.46 | 0.46 | 1.17 | 0.60 [NH4Cl] | 0.60 [NH4Cl] and 0.60 [(NH4)2SO4] |
| PO4–P | - | 1.40 | 1.40 | 3.5 [NH4H2PO4] | 1.75 [NH4H2PO4] | 0 |
| K | - | 3.60 | 3.60 | 9 | 9 | 9 |
| Ca | - | 1.60 | 1.60 | 4 | 4 | 4 |
| Mg | - | 0.80 | 0.80 | 2 | 2 | 2 |
| SO4–S | - | 0.80 | 0.80 | 2 | 2 | 2 |
| Fe | - | 0.02 | 0.02 | 0.07 | 0.07 | 0.07 |
| B | - | 0.05 | 0.05 | 0.14 | 0.14 | 0.14 |
| Mn | - | 0.0072 | 0.0072 | 0.018 | 0.018 | 0.018 |
| Zn | - | 0.0008 | 0.0008 | 0.002 | 0.002 | 0.002 |
| Cu | - | 0.00024 | 0.00024 | 0.0006 | 0.0006 | 0.0006 |
| Mo | - | 0.00052 | 0.00052 | 0.0013 | 0.0013 | 0.0013 |
| P Strength | Temp. | pH Value | |
|---|---|---|---|
| Nutrient Solution Adding Time | Nutrient Solution before Adding Time | ||
| 1×-P (Full) | 15 °C | 6.45a z | 5.75a |
| 10 °C | 6.40ab | 5.68a | |
| 0.5×-P (Half) | 15 °C | 6.33ab | 5.73a |
| 10 °C | 6.35ab | 5.65a | |
| 0×-P (None) | 15 °C | 6.38ab | 5.50a |
| 10 °C | 6.25b | 5.05b | |
| p-value of St. & Temp. | * | *** | |
| p-value at 15 °C | NS | * | |
| p-value at 10 °C | NS | *** | |
| P Strength | Temp. | % DW | µg/gm DW | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N–NO3 | N–NH4 | P–PO4 | K2O | CaO | MgO | Fe | Cl | ||
| 1×-P (Full) | 15 °C | 0.92d z | 0.28cd | 0.63a | 0.63a | 0.09a | 0.013ab | 6.09bc | 6.72ab |
| 10 °C | 0.72d | 0.22d | 0.41b | 0.57ab | 0.08ab | 0.010b | 5.23b | 6.40b | |
| 0.5×-P (Half) | 15 °C | 1.19cd | 0.43bc | 0.37bc | 0.54abc | 0.07abc | 0.017abc | 8.77abc | 7.66ab |
| 10 °C | 1.59bc | 0.32cd | 0.24cd | 0.40bcd | 0.06bcd | 0.020abc | 7.12abc | 7.15ab | |
| 0×-P (None) | 15 °C | 2.52a | 0.67a | 0.13d | 0.38cd | 0.05cd | 0.023bc | 10.31a | 9.15a |
| 10 °C | 1.95ab | 0.55ab | 0.10d | 0.29d | 0.04d | 0.027c | 9.49ab | 8.81ab | |
| p-value of St. & Temp. | *** | *** | *** | *** | *** | ** | *** | *** | |
| p-value at 15 °C | *** | *** | *** | *** | *** | * | ** | ** | |
| p-value at 10 °C | *** | *** | *** | *** | *** | ** | *** | * | |
| P Strength | Temp. | Yield/Plant (g) | Leaf Dry Matter (g) | Root Fresh Weight (g) | Root Length (cm) | Root Dry Matter (g) |
|---|---|---|---|---|---|---|
| 1×-P (Full) | 15 °C | 47.68a z | 5.80d | 4.76ab | 35.06b | 7.81c |
| 10 °C | 26.25b | 8.03c | 2.89c | 23.38d | 10.19b | |
| 0.5×-P (Half) | 15 °C | 45.40a | 5.94d | 4.75ab | 36.06ab | 7.80c |
| 10 °C | 27.22b | 9.00c | 3.10bc | 25.75cd | 11.25ab | |
| 0×-P (None) | 15 °C | 13.94c | 11.29b | 4.85a | 40.94a | 7.91c |
| 10 °C | 11.23c | 13.25a | 3.29abc | 30.75bc | 12.25a | |
| p-value of St. & Temp. | *** | *** | *** | *** | *** | |
| p-value at 15 °C | *** | *** | *** | *** | * | |
| p-value at 10 °C | *** | *** | *** | *** | *** | |
| P Strength | Temp. | Leaf Number | Leaf Length (cm) | Leaf Width (cm) | Leaf Chlorophyll Content (SPAD) |
|---|---|---|---|---|---|
| 1×-P (Full) | 15 °C | 8.70a z | 19.30a | 12.20a | 27.21a |
| 10 °C | 8.30ab | 16.60ab | 11.25ab | 26.55ab | |
| 0.5×-P (Half) | 15 °C | 8.60a | 18.50a | 13.70a | 27.03ab |
| 10 °C | 8.40ab | 17.15a | 11.50ab | 25.59abc | |
| 0×-P (None) | 15 °C | 7.80b | 14.00b | 9.30ab | 24.35ab |
| 10 °C | 7.70b | 13.80b | 8.25b | 23.39c | |
| p-value of St. & Temp. | * | *** | *** | *** | |
| p-value at 15 °C | * | *** | ** | ** | |
| p-value at 10 °C | * | *** | ** | *** | |
| P Strength | Temp. | Color (L*) | Color (a*) | Color (b*) | Color (chrome) | Color (ºh) |
|---|---|---|---|---|---|---|
| 1×-P (Full) | 15 °C | 38.90a z | −2.52b | 5.44a | 6.86a | 111.87a |
| 10 °C | 37.42ab | −1.21ab | 3.29bc | 3.55ab | 106.80ab | |
| 0.5×-P (Half) | 15 °C | 38.37ab | −1.40ab | 4.23ab | 4.38ab | 109.34ab |
| 10 °C | 37.34ab | −1.06ab | 2.70bc | 2.76b | 104.83ab | |
| 0×-P (None) | 15 °C | 36.88ab | −0.88ab | 2.15c | 2.23b | 102.07b |
| 10 °C | 36.12b | −0.43a | 1.81c | 2.04b | 100.77b | |
| p-value of St. & Temp. | * | ** | *** | *** | *** | |
| p-value at 15 °C | NS | NS | *** | ** | ** | |
| p-value at 10 °C | NS | * | *** | ** | * | |
| P Strength | Temp. | Chlorophyll a (µg/mL) | Chlorophyll b (µg/mL) | Chlorophyll Total (µg/mL) | Anthocyanin (mg/100 g FW) |
|---|---|---|---|---|---|
| 1×-P (Full) | 15 °C | 9.99a z | 13.62a | 23.61a | 0.64d |
| 10 °C | 8.04ab | 11.77ab | 19.80abc | 1.44cd | |
| 0.5×-P (Half) | 15 °C | 8.93ab | 12.77ab | 21.70ab | 0.77cd |
| 10 °C | 7.87ab | 11.30ab | 19.17abc | 2.15c | |
| 0×-P (None) | 15 °C | 7.78ab | 10.54ab | 18.32bc | 4.80b |
| 10 °C | 7.59b | 9.31b | 16.90c | 6.65a | |
| p-value of St. & Temp. | * | *** | *** | *** | |
| p-value at 15 °C | NS | * | *** | *** | |
| p-value at 10 °C | NS | * | NS | *** | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.Z.; Lee, Y.-T.; Mele, M.A.; Choi, I.-L.; Kang, H.-M. The Effect of Phosphorus and Root Zone Temperature on Anthocyanin of Red Romaine Lettuce. Agronomy 2019, 9, 47. https://doi.org/10.3390/agronomy9020047
Islam MZ, Lee Y-T, Mele MA, Choi I-L, Kang H-M. The Effect of Phosphorus and Root Zone Temperature on Anthocyanin of Red Romaine Lettuce. Agronomy. 2019; 9(2):47. https://doi.org/10.3390/agronomy9020047
Chicago/Turabian StyleIslam, Mohammad Zahirul, Young-Tack Lee, Mahmuda Akter Mele, In-Lee Choi, and Ho-Min Kang. 2019. "The Effect of Phosphorus and Root Zone Temperature on Anthocyanin of Red Romaine Lettuce" Agronomy 9, no. 2: 47. https://doi.org/10.3390/agronomy9020047





