Breeding of the Dormant Thermosensitive Genic Male-Sterile Lines of Early Rice to Overcome Pre-Harvest Sprouting of the Hybrid Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Germination Tests
2.2.2. Pollen Fertility Identification of the TGMS Lines and Seed Propagation Method of Male-sterile Lines
2.2.3. Measurement of ABA Content
2.2.4. Quantitative Real-Time PCR Analysis
3. Results
3.1. Breeding TGMS Lines “Yezao S” and “Yezhuzao S”
3.2. Comparison of Seed Dormancy Between the TGMS Lines “Yezao S” and “Yezhuzao S”
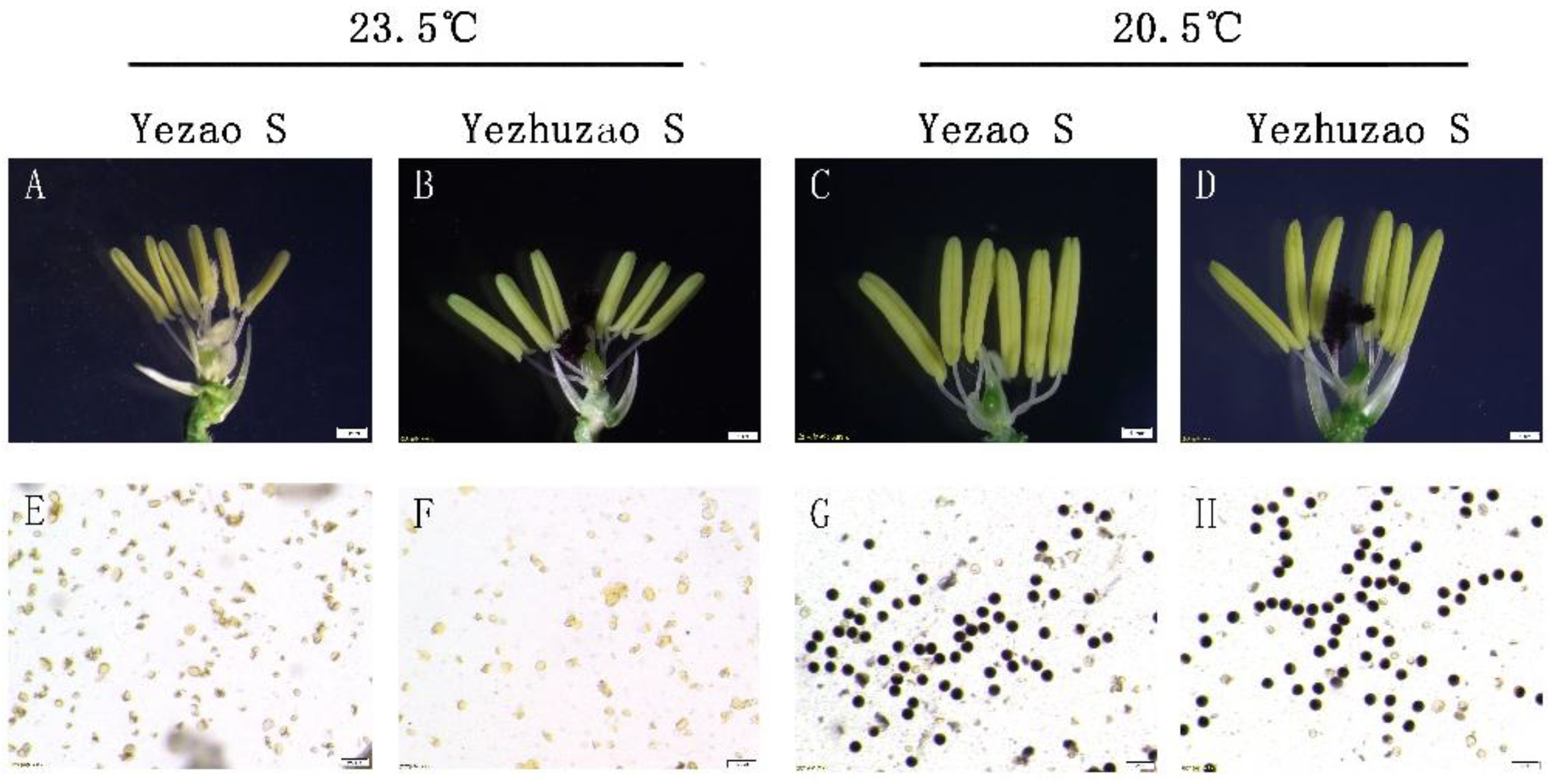
3.3. Fertility Identification of the Early Rice TGMS Lines “Yezao S” and “Yezhuzao S” with Seed Dormancy
3.4. Comparison of Stigma Exsertion Rate Between the Male-sterile Lines “Yezao S” and “Yezhuzao S”
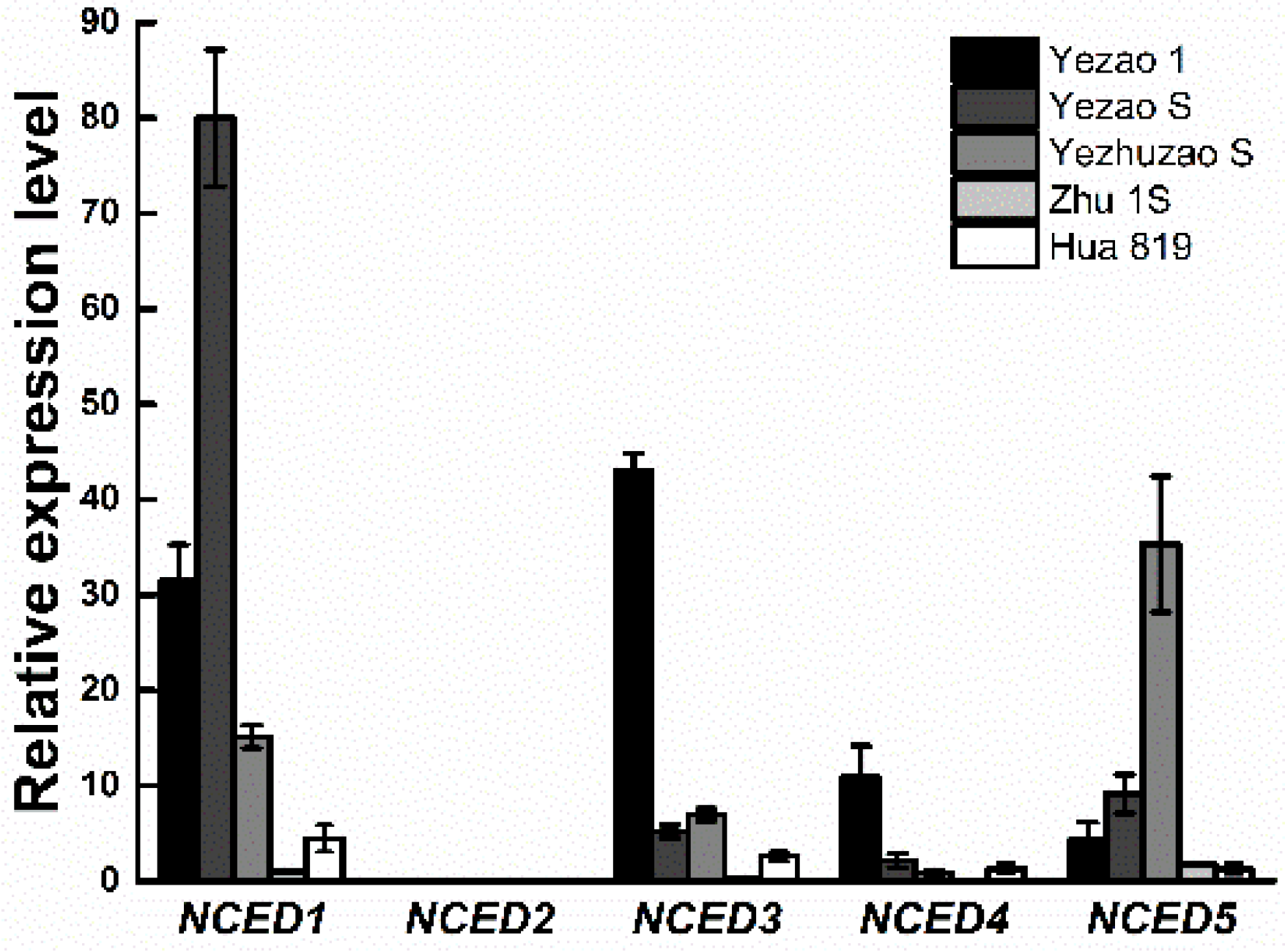
3.5. OsNCEDs Expression of Different Rice Lines in Seeds
3.6. Comparison of the Difference of ABA Content in Seeds
4. Discussion
4.1. Importance of Seed Dormancy of TGMS Lines on Improving the Quality of Hybrid Seeds
4.2. Relationship Between GA3 and ABA and the Occurrence of PHS on Hybrid Rice Seeds
4.3. Relationship Between OsNCED Regulating ABA Synthesis and Seed Dormancy
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B

References
- Lu, X.Y.; Yi, J.H.; Rao, L.Q.; Ma, H.B.; Luo, Z.M. Effects of Temperature and Daylength on Fertility of “ANNONGS-1” and the Mechanism of Its Spollen Abortion. J. Hunan Agric. Coll. 1991, 17, 427–433. [Google Scholar]
- Chen, L.B.; Li, X.Z.; Zhou, G.Q. Effects of Temperature on the Sterility of Rice in Photo-sensitive and Thermo-senstive Male Sterile Lines. Acta Agron. Sin. 1993, 19, 47–54. [Google Scholar]
- Si, H.M.; Fu, Y.P.; Liu, W.Z.; Sun, Z.X.; Hu, G.C. Pedigree Analysis of Photoperiod-thermo Sensitive Genic Male Sterile Rice. Acta Agron. Sin. 2012, 38, 394. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, M.; Yang, Y.Z.; Li, J.; Zhu, L.; Jiang, D.G.; Domh, J.F.; Liu, Q.J.; Gu, L.F.; Zhou, L.Y.; et al. RNase Z(S1) processes UbL40 mRNAs and controls thermosensitive genic male sterility in rice. Nat. Commun. 2014, 5, 4884–4893. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.F.; Li, S.; Zhong, X.C.; Xiao, C.L.; Yuan, Z.Z.; Yu, C.; Li, Z. Research Progress of Seed Dormancy and Pre-harvest Sprouting of Hybrid Rice. Hunan Agric. Sci. 2013, 5, 10–13. [Google Scholar]
- Hu, W.M.; Ma, H.S.; Fan, L.J.; Ruan, S.L. Characteristics of Pre-harvest Sprouting in Sterile Lines in Hybrid Rice Seeds Production. Acta Agron. Sin. 2003, 3, 441–446. [Google Scholar]
- Yang, Y.Z.; Tang, P.L.; Yang, W.C.; Liu, A.M.; Chen, Y.Q.; Ling, W.B.; Shi, T.B. Breeding and Utilization of TGMS Line Zhu1S in Rice. Hybrid Rice 2000, 15, 6–9. [Google Scholar]
- Liu, A.M.; Ling, W.B.; Shi, T.L. The Major Characteristics of the TGMS Lines Zhu 1S and Lu 18S in Rice. Hybrid Rice 2003, 18, 28–30. [Google Scholar]
- Zhou, K.; Fu, L.M.; Wang, Y.H.; Huang, W. Study on Relationship between YieldI and Agronomic Characters of Zhu 1S Series Combinations. Zhongguo Daomi 2014, 20, 86–89. [Google Scholar]
- Bewley, J.D. Seed Germination and Dormancy. Plant Cell. 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, P.S.; Meyer, S.E. Ecological aspects of seed dormancy loss. Seed Sci. Res. 1998, 8, 183–192. [Google Scholar] [CrossRef]
- Vleeshouwers, L.M.; Bouwmeester, H.J. A simulation model for seasonal changes in dormancy and germination of weed seeds. Seed Sci. Res. 2001, 11, 77–92. [Google Scholar] [CrossRef]
- Misro, B.; Misra, P.K. Certain considerations on seed dormancy in rice. Oryza 1969, 15, 18–22. [Google Scholar]
- Li, H.; Yang, Y.Z.; Yao, C.; Liang, M.Z.; Chen, L.B. Studies on Fertility Characteristics of Sensitive to Photoperiod and Temperature of TGMS Lines Zhu 1S and Conditions for it’s Multiply. Life Sci. Res. 2008, 12, 158–162. [Google Scholar]
- Lin, S.C.; Yuan, L.P. Hybrid rice breeding in China. In Innovative Approaches to Rice Breeding; International Rice Research Institute: Los Baños, Laguna, Philippines, 1980. [Google Scholar]
- Cheng, S.H.; Zhuang, J.Y.; Fan, Y.Y.; Du, J.H.; Cao, L.Y. Progress in research and development on hybrid rice: A super-domesticate in China. Ann. Bot. 2007, 100, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Normile, D. Reinventing rice to feed the world. Science 2008, 321, 330–333. [Google Scholar] [CrossRef] [PubMed]
- He, G.M.; Zhu, X.P.; Elling, A.A.; Chen, L.B.; Wang, X.F.; Guo, L.; Deng, X.W. Global Epigenetic and Transcriptional Trends among Two Rice Subspecies and Their Reciprocal Hybrids. Plant Cell 2010, 22, 17–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si, H.M.; Liu, W.Z.; Fu, Y.P.; Sun, Z.X.; Hu, G.C. Current situation and suggestions for development of two-line hybrid rice in China. Chin. J. Rice Sci. 2011, 25, 544–552. [Google Scholar]
- Su, N.; Hu, M.L.; Wu, D.X.; Wu, F.G.; Fei, G.L.; Lan, Y.; Chen, X.L.; Shu, X.L.; Zhang, X.; Guo, X.P.; et al. Disruption of a rice pentatricopeptide repeat protein causes a seedling-specific albino phenotype and its utilization to enhance seed purity in hybrid rice production. Plant Physiol. 2012, 159, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.H.; Li, C.Y.; Deng, Q.Y.; Wang, B. Rapid constructing a genetic linkage map by AFLP technique and mapping a new gene tms5. Acta Bot. Sin. 2003, 45, 614–620. [Google Scholar]
- Wang, Y.G.; Xing, Q.H.; Deng, Q.Y.; Liang, F.S.; Yuan, L.P.; Meng, M.L.; Wang, B. Fine mapping of the rice thermo-sensitive genic male-sterile gene tms5. Theor. Appl. Genet. 2003, 107, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, C.; Zhuang, W.; Li, J.; Deng, H.; Deng, Q.; Wang, B. Characterization and identification of the candidate gene of rice thermo-sensitive genic male sterile gene tms5 by mapping. Planta 2007, 225, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.F.; Chen, X.H.; Lu, Y.P.; Peng, Y.F.; Wan, B.H.; Chen, N.D.; Wu, B.; Xin, S.P.; Zhang, G.Q. Fine mapping of a gene for non-pollen type thermosensitive genic male sterility in rice (Oryza sativa L.). Theor. Appl. Genet. 2010, 120, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.M.; Li, B.H. Analysis on the Correlation Between Fertility of TGMS Rice and Temperature. Hybrid Rice 1996, 11, 25–28. [Google Scholar]
- Chen, G.H.; Jiang, L.C.; Wei, M.; Wang, G.M.; He, C.N.; Cai, Z.G.; Qiao, Y.X. A preliminary study on the hybrid seed production techniques for rice TGMS lines. Hybrid Rice 1997, 12, 45–46. [Google Scholar]
- Chen, G.H.; Jiang, L.C.; Wei, M.; Wang, G.M. A Preliminary Study on Correlation Between Fertility of Thermo-sensitive Genic Male Sterile (TGMS) Rice and Temperature. Agric. Meteorol. 2004, 25, 51–53. [Google Scholar]
- He, Q.; Cai, Y.D.; Xu, Y.W.; Chen, L.Y. Problems and Countermeasures in Utilization of PTGMS Lines in Rice. Hybrid Rice 2004, 19, 1–5. [Google Scholar]
- Chen, L.B.; Xu, M.L.; Zhou, G.Q. Selection of PTGMS Lines with Double Low Critical Values in Temperature in Rice. Hybrid Rice 1999, 14, 3–4. [Google Scholar]
- Xu, M.L.; Chen, L.B.; Zhou, G.Q.; Liang, M.Z. Temperature Effects on Fertility of Rice TGMS Line Between 96-5-2S with Double Low Critical Temperature Values and Peiai 64S. Acta Ecol. Sin. 2002, 22, 541–547. [Google Scholar]
- Pan, W.J. Effect of GA 3 on Elongation of the First Top Internode of M S Lines in Rice. Hybrid Rice 1999, 14, 20–21. [Google Scholar]
- Yang, Y.S.; Tang, L.; Liao, F.M.; Li, X.Q.; Liu, A.M.; Wu, X.J. Response of Heading Related Traits to Exogenous Gibberellic Acid (GA3) in Different Rice PTGMS Lines. Hybrid Rice 2012, 27, 57–64. [Google Scholar]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant. Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Qian, L.C.; Nibau, C.; Duan, Q.H.; Kita, D.; Levasseur, K.; Li, X.Q.; Lu, C.Q.; Hui, L.; Hou, C.C.; et al. FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase. Proc. Natl. Acad. Sci. USA 2012, 109, 14693–14698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.Y.; Kim, D.H.; Hwang, I. ABA homeostasis and signaling involving multiple subcellular compartments and multiple receptors. Plant Cell Rep. 2013, 32, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Taylor, I.B.; Burbidge, A.; Thompson, A.J. Control of abscisic acid synthesis. J. Exp. Bot. 2000, 51, 1563–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, F.; Waadtl, R.; Schroeder, J.I. Evolution of abscisic acid synthesis and signaling mechanisms. Curr. Biol. 2011, 21, R346–R355. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.C.; Schwartz, S.H.; Zeevaart, J.A.; McCarty, D.R. Genetic control of abscisic acid biosynthesis in maize. Proc. Natl. Acad. Sci. USA 1997, 94, 12235–12240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milborrow, B.V. The pathway of biosynthesis of abscisic acid in vascular plants: a review of the present state of knowledge of ABA biosynthesis. J. Exp. Bot. 2001, 52, 1145–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, S.H.; Tan, B.C.; Gage, D.A.; Zeevaart, J.A.; McCarty, D.R. Specific oxidative cleavage of carotenoids by VP14 of maize. Science 1997, 276, 1872–1874. [Google Scholar] [CrossRef] [PubMed]
- Priya, R.; Siva, R. Analysis of phylogenetic and functional diverge in plant nine-cis epoxycarotenoid dioxygenase gene family. J. Plant Res. 2015, 128, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zeevaart, J.A.D. Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance. Plant Physiol. 2002, 128, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Andújar, C.; Ordiz, M.I.; Huang, Z.; Nonogaki, M.; Beachy, R.N.; Nonogaki, H. Induction of 9-cis-epoxycarotenoid dioxygenase in Arabidopsis thaliana seeds enhances seed dormancy. Proc. Natl. Acad. Sci. USA 2011, 108, 17225–17229. [Google Scholar] [CrossRef] [Green Version]
- Ye, N.; Zhu, G.; Liu, Y.; Li, Y.; Zhang, J. ABA controls H2O2 accumulation through the induction of OsCATB in rice leaves under water stress. Plant Cell Physiol. 2011, 52, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, Y.; Liu, Y.; Zhang, F.; Wang, Z.; Wang, H.; Wang, F.; Li, D.; Mao, D.; Luan, S.; et al. 9-cis-Epoxycarotenoid Dioxygenase 3 Regulates Plant Growth and Enhances Multi-Abiotic Stress Tolerance in Rice. Front. Plant Sci. 2018, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]



| Primer Name | Primer Sequence |
|---|---|
| ACTIN F | 5′-CAATGTGCCAGCTATGTATGTCGCC-3′ |
| ACTIN R | 5′-TTCCCGTTCAGCAGTGGTAGTGAAG-3′ |
| OsNCED1F | 5′-CTCACCATGAAGTCCATGAGGCTT-3′ |
| OsNCED1R | 5′-GTTCTCGTAGTCTTGGTCTTGGCT-3′ |
| OsNCED2F | 5′-TGCGAATCTGACCTTCCCTACT-3′ |
| OsNCED2R | 5′-TGCGACCTTGCTGCCTGCTC-3′ |
| OsNCED3F | 5′-CCCCTCCCAAACCATCCAAACCGA-3′ |
| OsNCED3R | 5′-TGTGAGCATATCCTGGCGTCGTGA-3′ |
| OsNCED4F | 5′-CGTGTCCAAGCCGTACCT-3′ |
| OsNCED4R | 5′-CGACGCCTTCTCCCTGT-3′ |
| OsNCED5F | 5′-ACATCCGAGCTCCTCGTCGTGAA-3′ |
| OsNCED5R | 5′-TTGGAAGGTGTTTTGGAATGAACCA-3′ |
| Lines | Sowing Date (Day/Month) | Initial Heading Date (Day/Month) | The Duration from Sowing to Heading (day) | Maturity Date (Day/Month) | Growth Period (day) |
|---|---|---|---|---|---|
| Yezao 1 | 25/3 | 29/5 | 65 | 2/7 | 99 |
| Yezao S | 25/3 | 7/6 | 74 | 9/7 | 106 |
| Yezhuzao S | 25/3 | 6/6 | 73 | 8/7 | 105 |
| Zhu 1S | 25/3 | 10/6 | 77 | 12/7 | 109 |
| Hua 819 | 25/3 | 7/6 | 74 | 8/7 | 105 |
| Lines | Germination Rate (%) | Dormancy Level |
|---|---|---|
| Yezao 1 | 0 | Very strong dormancy |
| Yezao S | 0 | Very strong dormancy |
| Yezhuzao S | 10.3 ± 1.7 | Strong dormancy |
| Zhu 1S | 95.3 ± 2.5 | No dormancy |
| Hua 819 | 85.0 ± 2.0 | No dormancy |
| Lines | Single Exerted Stigma (PSES) (%) | Dual Exerted Stigma (PDES) (%) | Total Exerted Stigma (PES) (%) |
|---|---|---|---|
| Yezao S | 42.3 | 37.6 | 79.9 |
| Yezhuzao S | 46.3 | 30.5 | 76.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Tian, Y.; Lu, X. Breeding of the Dormant Thermosensitive Genic Male-Sterile Lines of Early Rice to Overcome Pre-Harvest Sprouting of the Hybrid Seeds. Agronomy 2018, 8, 191. https://doi.org/10.3390/agronomy8090191
Chen X, Tian Y, Lu X. Breeding of the Dormant Thermosensitive Genic Male-Sterile Lines of Early Rice to Overcome Pre-Harvest Sprouting of the Hybrid Seeds. Agronomy. 2018; 8(9):191. https://doi.org/10.3390/agronomy8090191
Chicago/Turabian StyleChen, Xiaoyu, Yun Tian, and Xiangyang Lu. 2018. "Breeding of the Dormant Thermosensitive Genic Male-Sterile Lines of Early Rice to Overcome Pre-Harvest Sprouting of the Hybrid Seeds" Agronomy 8, no. 9: 191. https://doi.org/10.3390/agronomy8090191




