Comprehensive Screening of Some West and Central African Sesame Genotypes for Drought Resistance Probing by Agromorphological, Physiological, Biochemical and Seed Quality Traits
Abstract
:1. Introduction
2. Results
2.1. Overall Variability among Genotypes, Water Regimes and Studied Traits
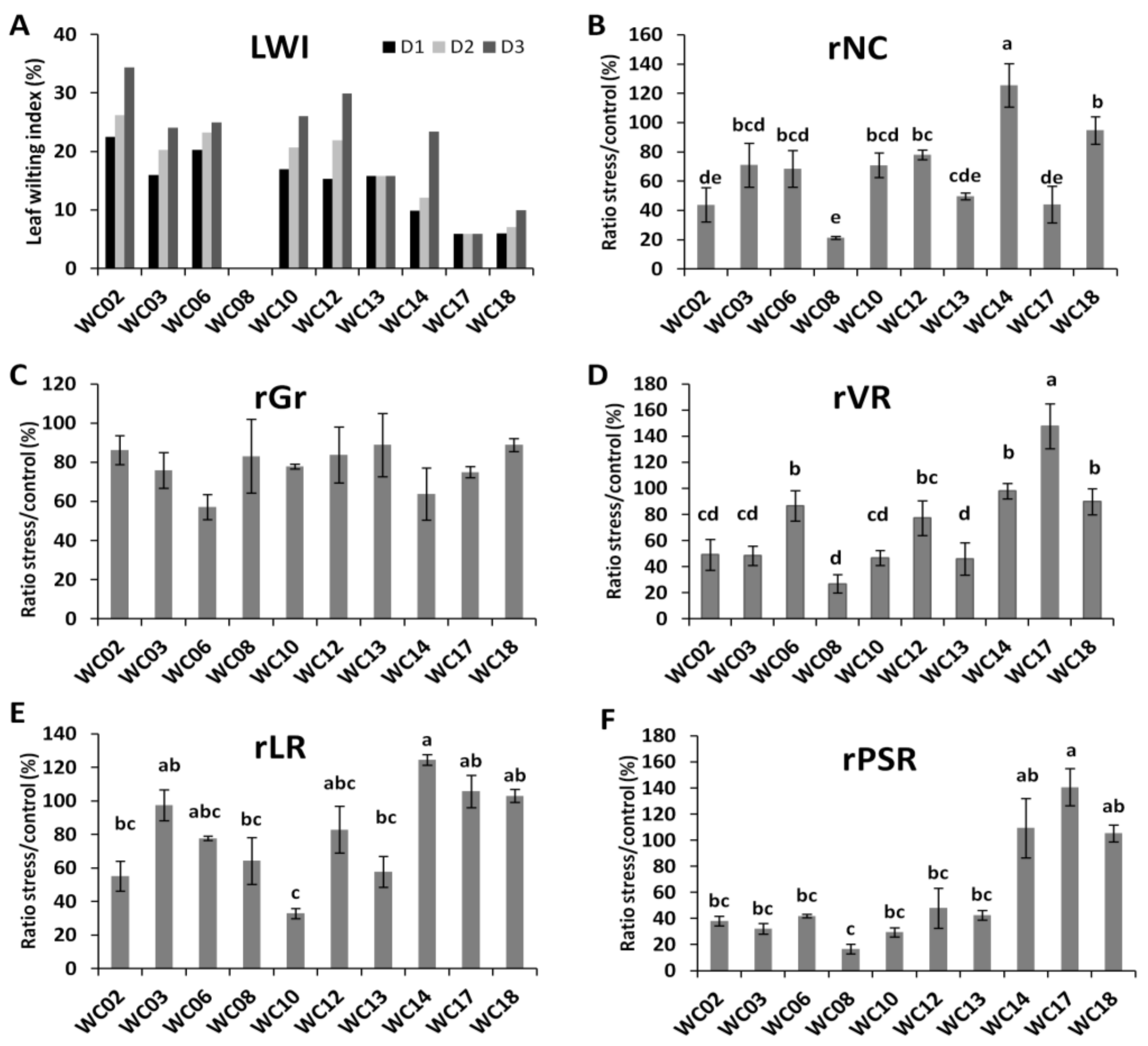
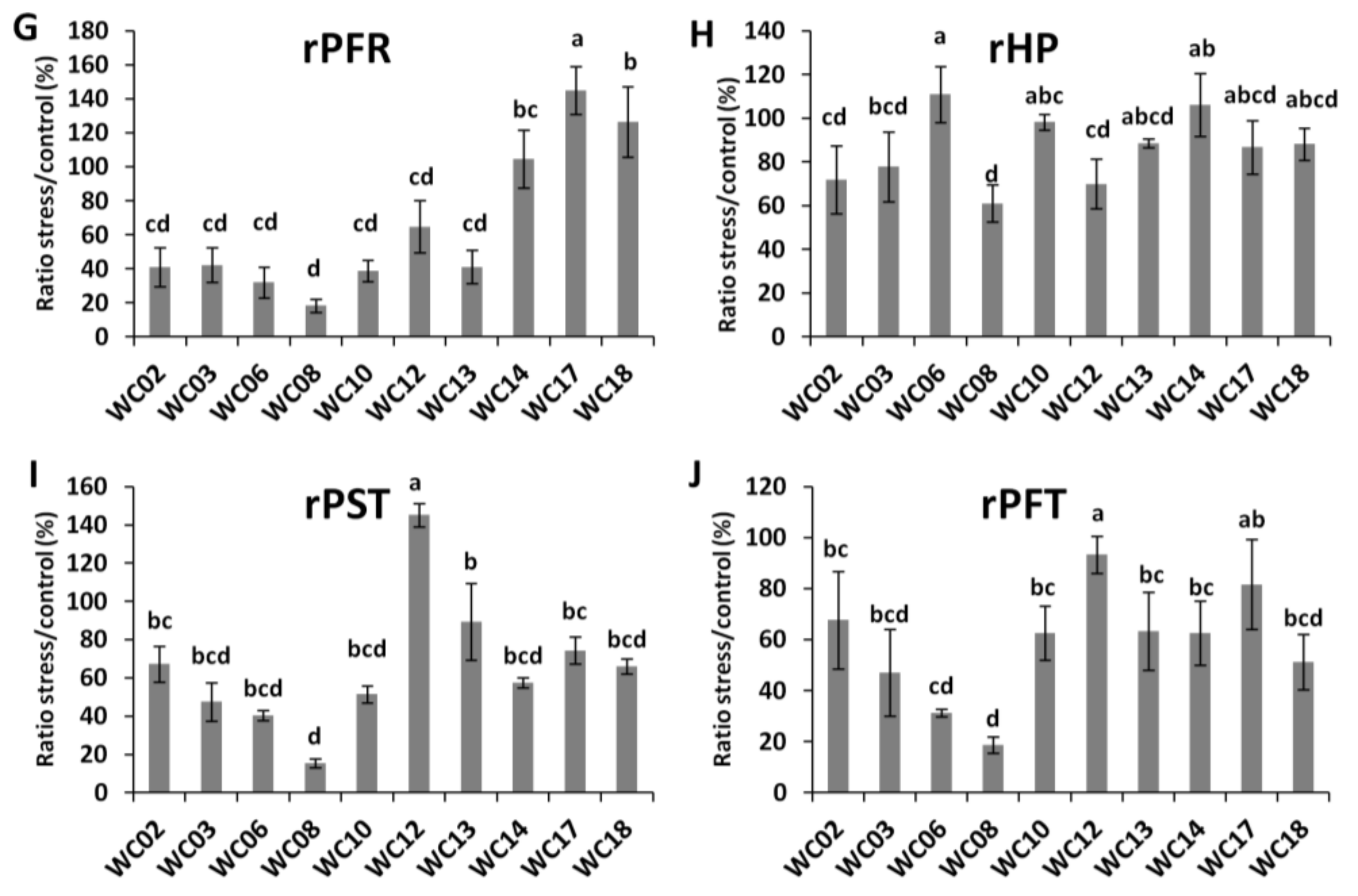
2.2. Effects of Water Deficit on Sesame Plant Growth and Yield Relative Indexes
2.3. Physiological and Biochemical Responses of Sesame Genotypes to Water Deficit
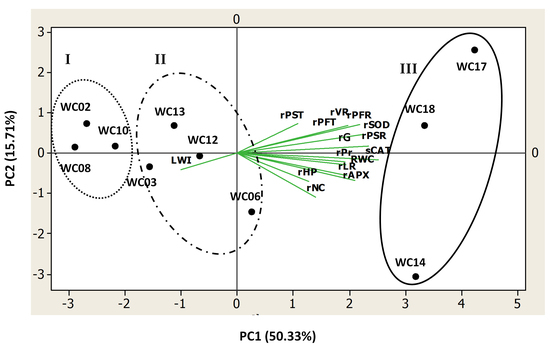
2.4. Relationships among the Studied Traits
2.5. Drought Resistance Ranking of the Ten Sesame Genotypes Using Integrated PCA Score Values
2.6. Seed Quality Traits under Well-Watered and Drought Stress Conditions in Five Sesame Genotypes
3. Discussion
3.1. Drought Avoidance and Tolerance Are Required for Resistance under Severe Water Deficit in Sesame
3.2. Does Drought Resistance Maintain Good Seed Quality in Sesame?
3.3. Important Traits for Efficient Screening for Drought Resistance in Sesame
4. Materials and Methods
4.1. Plant Materials
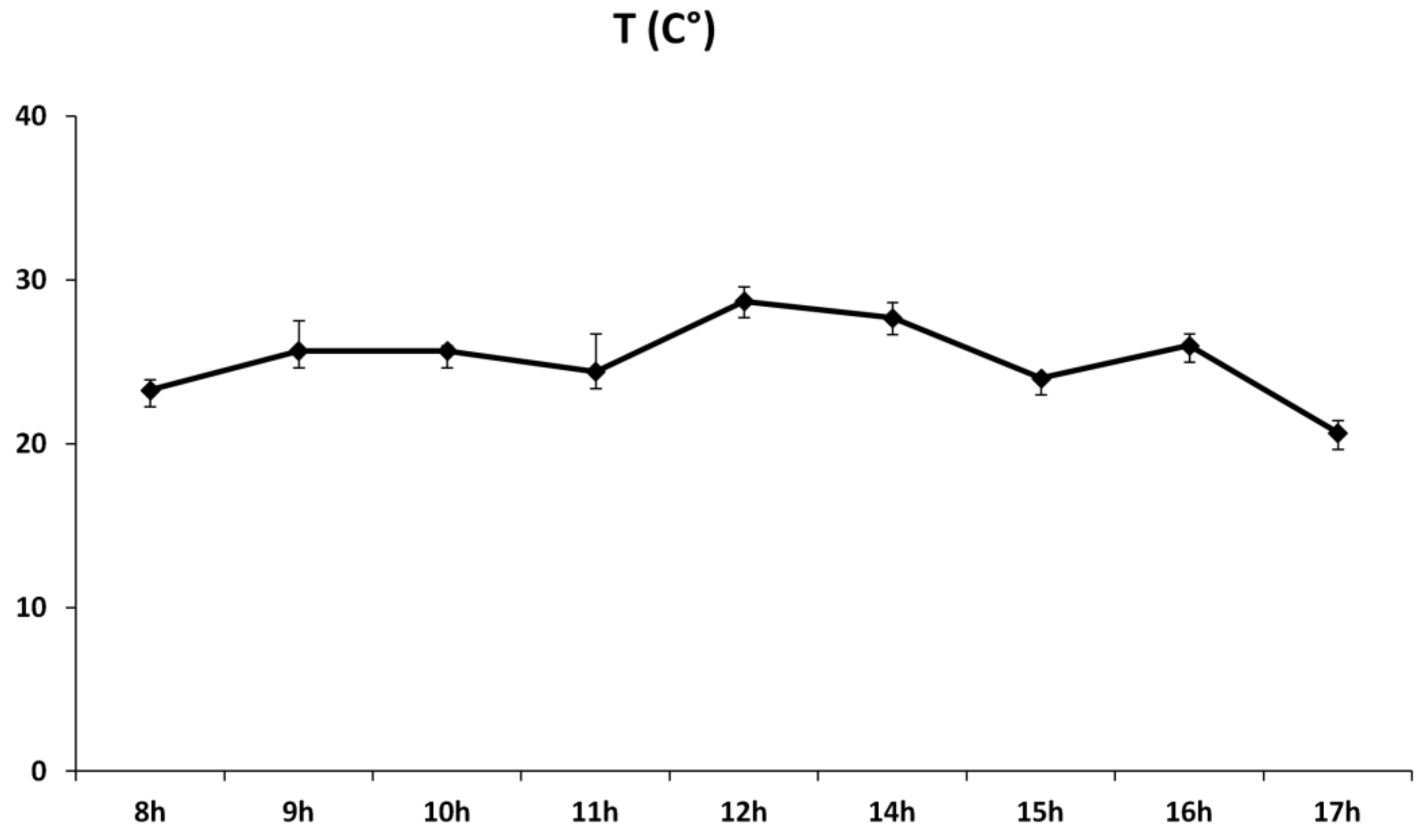
4.2. Experiment and Stress Treatment
4.3. Data Collection
4.3.1. Plant Growth and Yield Component Traits
4.3.2. Physiological and Biochemical Traits
4.3.3. Seed Quality Traits
4.4. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
References
- Uzun, B.; Arslan, C.; Furat, S. Variation in fatty acid compositions, oil content and oil yield in germplasm collection of sesame (Sesamum indicum L.). J. Am. Oil Chem. Soc. 2008, 85, 1135–1142. [Google Scholar] [CrossRef]
- Faostat. Food and Agriculture Organization Statistical Databases 2015. Available online: http://faostat.fao.org/ (accessed on 12 December 2016).
- Gildemacher, P.; Audet-Bélanger, G.; Mangnus, E.; Van de Pol, F.; Tiombiano, D.; Sanogo, K. Sesame Sector Development; Lessons Learned in Burkina Faso and Mali; KIT & CFC: Amsterdam, The Netherlands, 2015; Available online: https://commonfunds.sharepoint.com/Projects/FIGOOF/FIGOOF-27/1%20Sesame%20Sector%20Development.pdf (accessed on 16 March 2017).
- Langham, D.R. Phenology of sesame. In Issues in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2007; pp. 144–182. [Google Scholar]
- Dossa, K.; Konteye, M.; Niang, M.; Doumbia, Y.; Cissé, N. Enhancing sesame production in West Africa’s Sahel: A comprehensive insight into the cultivation of this untapped crop in Senegal and Mali. Agric. Food Secur. 2017, in press. [Google Scholar]
- Boureima, S.; Diouf, M.; Amoukou, A.I.; Damme, V.P. Screening for sources of tolerance to drought in sesame induced mutants: Assessment of indirect selection criteria for seed yield. Int. J. Pure Appl. Biosci. 2016, 4, 45–60. [Google Scholar] [CrossRef]
- Betram, K.; Janssens, M.J.J.; Abdalwahab, A. Breeding for drought tolerance in sesame (Sesamum indicum). In Proceedings of the Conference on Technological and Institutional Innovations for Sustainable Rural Development, Deutscher Tropentag, Gottingen, Germany, 8–10 October 2003; p. 135. [Google Scholar]
- Kim, K.S.; Park, S.H.; Jenks, M.A. Changes in leaf cuticular waxes of sesame (Sesamum indicum L.) plants exposed to water deficit. J. Plant Physiol. 2006, 164, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, M.; Asghari, A.; Jamaati-e-Somarin, S.; Saeidi, M.; Zabihi-e-Mahmoodabad, R.; Hokmalipour, S. Effects of water deficit on drought tolerance indices of sesame (Sesamum indicum L.) genotypes in moghan region. Res. J. Environ. Sci. 2009, 3, 116–121. [Google Scholar] [CrossRef]
- Bahrami, H.; Razmjoo, J.; Jafari, A.O. Effect of drought stress on germination and seedling growth of sesame cultivars (Sesamum indicum L.). Int. J. Agric. Sci. 2012, 2, 423–428. [Google Scholar]
- Boureima, S.; Oukarroum, A.; Diouf, M.; Cissé, N.; Van Damme, P. Screening for drought tolerance in mutant germplasm of sesame (Sesamum indicum) probing by chlorophyll a fluorescence. Environ. Exp. Bot. 2012, 81, 37–43. [Google Scholar] [CrossRef]
- Kadkhodaie, A.; Razmjoo, J.; Zahedi, M.; Pessarakli, M. Selecting sesame genotypes for drought tolerance based on some physiochemical traits. Agron. J. 2014, 106, 111–118. [Google Scholar] [CrossRef]
- Kadkhodaie, A.; Razmjoo, J.; Zahedi, M.; Pessarakli, M. Oil content and composition of sesame (Sesamum indicum L.) genotypes as affected by irrigation regimes. J. Am. Oil Chem. Soc. 2014, 91, 1737–1744. [Google Scholar] [CrossRef]
- Pathak, N.; Rai, A.K.; Kumari, R.; Thapa, A.; Bhat, K.V. Sesame crop: An underexploited oilseed holds tremendous potential for enhanced food value. Agric. Sci. 2014, 5, 519–529. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought stress in plants: An overview. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar]
- Bänziger, M.; Lafitte, H.R. Efficiency of secondary traits for improving maize for low-nitrogen target environments. Crop Sci. 1997, 37, 1110–1117. [Google Scholar] [CrossRef]
- Lata, C.; Yadav, A.; Prasad, M. Role of plant transcription factors in abiotic stress tolerance. In Abiotic Stress Response in Plants—Physiological, Biochemical and Genetic Perspectives; Shanker, A., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-672-0. Available online: http://www.intechopen.com/books/abiotic-stress-response-in-plants-physiological-biochemical-and-genetic-perspectives/role-of-plant-transcription-factors-in-abiotic-stress-tolerance (accessed on 2 June 2017).
- Farooq, M.; Basra, S.M.A.; Wahid, A.; Cheema, Z.A.; Cheema, M.A.; Khaliq, A. Physiological role of exogenously applied glycinebetaine to improve drought tolerance in fine grain aromatic rice (Oryza sativa L.). J. Agron. Crop Sci. 2008, 194, 325–333. [Google Scholar] [CrossRef]
- Yordanova, R.Y.; Alexieva, V.S.; Popova, L.P. Influence of root oxygen deficiency on photosynthesis and antioxidant status in barley plants. Russ. J. Plant Physiol. 2003, 50, 163–167. [Google Scholar] [CrossRef]
- Sergi, M.; Alegre, L. Drought-induced changes in redox state of α-tocopherol, ascorbate and the diterpene carnosic acid in chloroplasts of Labiatae species differing in carnosic acid contents. Plant Physiol. 2003, 131, 1816–1825. [Google Scholar]
- Agarwal, S.; Pandey, V. Antioxidant enzyme responses to NaCl stress in Cassia angustifolia. Biol. Plant. 2004, 48, 555–560. [Google Scholar] [CrossRef]
- Pinhero, R.G.; Pao, M.V.; Palyath, G.; Murr, D.P.; Fletcher, R.A. Changes in the activities of antioxidant enzymes and their relationship to genetic and paclobutrazol induced chilling tolerance of maize seedlings. J. Plant Physiol. 2000, 114, 695–704. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Dossa, K.; Wei, X.; Li, D.; Zhang, Y.; Fonceka, D.; Yang, W.; Diouf, D.; Cissé, N.; Liao, B.; Zhang, X. Analysis of genetic diversity and population structure of sesame accessions from Africa and Asia as major centers of its cultivation. Genes 2016, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dossa, K.; Niang, M.; Assogbadjo, A.E.; Cissé, N.; Diouf, D. Whole genome homology-based identification of candidate genes for drought tolerance in sesame (Sesamum indicum L.). Afr. J. Biotechnol. 2016, 15, 1464–1475. [Google Scholar]
- Dossa, K.; Wei, X.; Li, D.; Zhang, Y.; Wang, L.; Fonceka, D.; Yu, J.; Diouf, D.; Liao, B.; Cissé, N.; et al. Insight into the AP2/ERF transcription factor superfamily in sesame (Sesamum indicum) and expression profiling of the DREB subfamily under drought stress. BMC Plant Biol. 2016, 16, 171. [Google Scholar] [CrossRef] [PubMed]
- Dossa, K.; Diouf, D.; Cissé, N. Genome-wide investigation of Hsf genes in sesame reveals their segmental duplication expansion and their active role in drought stress response. Front. Plant Sci. 2016, 7, 1522. [Google Scholar] [CrossRef] [PubMed]
- De Santos, I.C.; de Almeida, A.A.F.; Anhert, D.; de Conceiçao, A.S.; Pirovani, C.P.; Pires, J.L.; Valle, R.R.; Baligar, V.C. Molecular, physiological and biochemical responses of Theobroma cacao L. genotypes to soil water deficit. PLoS ONE 2014, 9, e115746. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhou, G.; Shimizu, H. Plant responses to drought and rewatering. Plant Signal. Behav. 2010, 5, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Pradhan, S.; Singh, A.; Singh, O. Marker validation in recombinant inbred lines and random varieties of rice for drought tolerance. Aust. J. Crop Sci. 2012, 6, 606–612. [Google Scholar]
- Subashri, M.; Robin, S.; Vinod, K.K.; Rajeswari, S.; Mohanasundaram, K.; Raveendran, T.S. Trait identification and QTL validation for reproductive stage drought resistance in rice using selective genotyping of near flowering RILs. Euphytica 2008, 166, 291–305. [Google Scholar] [CrossRef]
- Salunkhe, A.S.; Poornima, R.; Prince, K.S.J.; Kanagaraj, P.; Sheeba, J.A.; Amudha, K.; Suji, K.K.; Senthil, A.; Babu, R.C. Fine mapping QTL for drought resistance traits in rice (Oryza sativa L.) using bulk segregant analysis. Mol. Biotechnol. 2011, 49, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Mitra, J. Genetics and genetic improvement of drought resistance in crop plants. Curr. Sci. 2001, 80, 758–763. [Google Scholar]
- Courtois, B.; McLaren, G.; Sinha, P.K.; Prasad, K.; Yadav, R.; Shen, L. Mapping QTLs associated with drought avoidance in upland rice. Mol. Breed. 2000, 6, 55–66. [Google Scholar] [CrossRef]
- Tatrai, Z.A.; Sanoubar, R.; Pluhar, Z.; Mancarella, S.; Orsini, F.; Gianquinto, G. Morphological and physiological plant responses to drought stress in Thymus citriodorus. Int. J. Agron. 2016, 2016, 14165750. [Google Scholar] [CrossRef]
- Fazeli, F.; Ghorbanli, M.; Niknam, V. Effect of drought on biomass, protein content, lipid peroxidation and antioxidant enzymes in two sesame cultivars. Biol. Plant. 2007, 51, 98–103. [Google Scholar] [CrossRef]
- Kadkhodaie, A.; Zahedi, M.; Razmjoo, J.; Pessarakli, M. Changes in some anti-oxidative enzymes and physiological indices among sesame genotypes (Sesamum indicum L.) in response to soil water deficits under field conditions. Acta Physiol. Plant. 2013, 36, 641–650. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Jensen, R.G. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996, 14, 89–97. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 11, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Agbicodo, E.M.; Fatokun, C.A.; Muranaka, S.; Visser, R.G.F. Breeding drought tolerant cowpea: Constraints, accomplishments, and future prospects. Euphytica 2009, 167, 353–370. [Google Scholar] [CrossRef]
- Nezhadahmadi, A.; Prodhan, Z.H.; Faruq, G. Drought tolerance in wheat. Sci. World J. 2013, 2013, 610721. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Drought resistance, water-use efficiency, and yield potential—Are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Eskandari, H.; Zehtabsalmasi, S.; Ghassemigolezani, K.; Gharineh, M.H. Effects of water limitation on grain and oil yields of sesame cultivars. J. Food Agric. Environ. 2009, 7, 339–342. [Google Scholar]
- Ozkan, A.; Kulak, M. Effects of water stress on growth, oil yield, fatty acid composition and mineral content of Sesamum indicum. J. Anim. Plant Sci. 2013, 23, 1686–1690. [Google Scholar]
- Aslam, M.N.; Nelson, M.N.; Kailis, S.G.; Bayliss, K.L.; Speijers, J.; Cowling, W.A. Canola oil increases in polyunsaturated fatty acids and decreases in oleic acid in drought-stressed Mediterranean type environments. Plant Breed. 2009, 128, 348–355. [Google Scholar] [CrossRef]
- Dornbos, D.L.; Mullen, R.E. Soybean seed protein and oil contents and fatty acid composition adjustments by drought and temperature. J. Am. Oil Chem. Soc. 1992, 69, 228–231. [Google Scholar] [CrossRef]
- Ashrafi, E.O.; Razmjoo, K.O. Effect of irrigation regimes on oil content and composition of safflower (Carthamus tinctorius L.) cultivars. J. Am. Oil Chem. Soc. 2010, 87, 499–506. [Google Scholar] [CrossRef]
- Dossa, K.; Diouf, D.; Wang, L.; Wei, X.; Zhang, Y.; Niang, M.; Fonceka, D.; Yu, J.; Mmadi, A.M.; Yehouessi, L.W.; et al. The emerging oilseed crop Sesamum indicum enters the “Omics” era. Front. Plant Sci. 2017, 8, 1154. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Serna, R.; Ramírez-Vallejo, P.; Acosta-Gallegos, J.A.; Castillo-González, F.; Kelly, J.D. Grain yield and drought tolerance of common bean under field conditions. Agrociencia 2000, 34, 153–165. [Google Scholar]
- Rosielle, A.A.; Hamblin, J. Theoretical aspects of selection for yield in stress and non-stress environment. Crop Sci. 1981, 21, 943–946. [Google Scholar] [CrossRef]
- Teran, H.; Singh, S.P. Comparison of sources and lines selected for drought resistance in common bean. Crop Sci. 2002, 42, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Lafitte, R.; Blum, A.; Atlin, G. Using secondary traits to help identify drought-tolerant genotypes. In Breeding Rice for Drought-Prone Environments; Fischer, K.S., Lafitte, R., Fukai, S., Atlin, G., Hardy, B., Eds.; International Rice Research Institute: Los Banos, PH, USA, 2003; pp. 37–61. [Google Scholar]
- Dossa, K.; Li, D.; Wang, L.; Zheng, X.; Liu, A.; Yu, J.; Wei, X.; Zhou, R.; Fonceka, D.; Diouf, D.; et al. Transcriptomic, biochemical and physio-anatomical investigations shed more light on responses to drought stress in two contrasting sesame genotypes. Sci. Rep. 2017, 7, 8755. [Google Scholar] [CrossRef] [PubMed]
- Vadez, V. Root hydraulics: The forgotten side of roots in drought adaptation. Field Crop. Res. 2014, 165, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Passot, S.; Gnacko, F.; Moukouanga, D.; Lucas, M.; Guyomarc’h, S.; Ortega, M.B.; Atkinson, J.A.; Belko, M.N.; Bennett, M.J.; Gantet, P.; et al. Characterization of pearl millet root architecture and anatomy reveals three types of lateral roots. Front. Plant Sci. 2016, 7, 829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amato, M.; Ritchie, J.T. Spatial distribution of roots and water uptake of maize (Zea mays L.) as affected by soil structure. Crop Sci. 2002, 42, 773–780. [Google Scholar] [CrossRef]
- Henry, A.; Gowda, V.R.P.; Torres, R.O.; McNally, K.L.; Serraj, R. Variation in root system architecture and drought response in rice (Oryza sativa): Phenotyping of the OryzaSNP panel in rainfed lowland fields. Field Crop. Res. 2011, 120, 205–214. [Google Scholar] [CrossRef]
- Liao, M.T.; Palta, J.A.; Fillery, I.R.P. Root characteristics of vigorous wheat improve early nitrogen uptake. Aust. J. Agric. Res. 2006, 57, 1097–1107. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Yin, D.M.; Chen, S.M.; Chen, F.D.; Guan, Z.Y.; Fang, W.M. Morpho-anatomical and physiological responses of two Dendranthema species to waterlogging. Environ. Exp. Bot. 2010, 68, 122–130. [Google Scholar] [CrossRef]
- Wei, W.; Li, D.; Wang, L.; Ding, X.; Zhang, Y.; Gao, Y.; Zhang, X. Morpho-anatomical and physiological responses to waterlogging of sesame (Sesamum indicum L.). Plant Sci. 2013, 208, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, Y.; Li, D.; Wang, L.; Gao, Y.; Zhou, R.; Zhang, X.; Wei, X. Establishment of near infrared reflectance spectroscopy model for sesame oil and protein content detection and variation analysis of the core collection of sesame (Sesamum indicum L.). Chin. J. Oil Crop Sci. 2016, 38, 722–729. [Google Scholar]
- Dossa, K.; Wei, X.; Niang, M.; Liu, P.; Zhang, Y.; Wang, L.; Liao, B.; Cissé, N.; Zhang, X.; Diouf, D. Near-infrared reflectance spectroscopy reveals wide variation in major components of sesame seeds from Africa and Asia. Crop J. 2017, in press. [Google Scholar] [CrossRef]
- Wójcik-Jagła, M.; Rapacz, M.; Tyrka, M.; Kościelniak, J.; Crissy, K.; Żmuda, K. Comparative QTL analysis of early short-time drought tolerance in Polish fodder and malting spring barleys. Theor. Appl. Genet. 2013, 126, 3021–3034. [Google Scholar] [CrossRef] [PubMed]
- Dray, S.; Dufour, A.; Leeuw, J.D.; Zeileis, A. The ade4 Package: Implementing the duality diagram for ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- De Mendiburu, F. Agricolae: Statistical procedures for agricultural research. R Package 2014, 1, 1–16. [Google Scholar]
- Hervé, M. GrapheR: A Multiplatform GUI for drawing customizable graphs in R. R J. 2011, 3, 99–103. [Google Scholar]
- Harell, F.E., Jr. The Hmisc Package. R Package 2006, 3, 12. [Google Scholar]
- Liu, Y.; Zhang, X.; Tran, H.; Shan, L.; Kim, J.; Childs, K.; Ervin, E.H.; Frazier, T.; Zhao, B. Assessment of drought tolerance of 49 switchgrass (Panicum virgatum) genotypes using physiological and morphological parameters. Biotechnol. Biofuels 2015, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.I.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A free, open-source system for microarray data management and analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [PubMed]
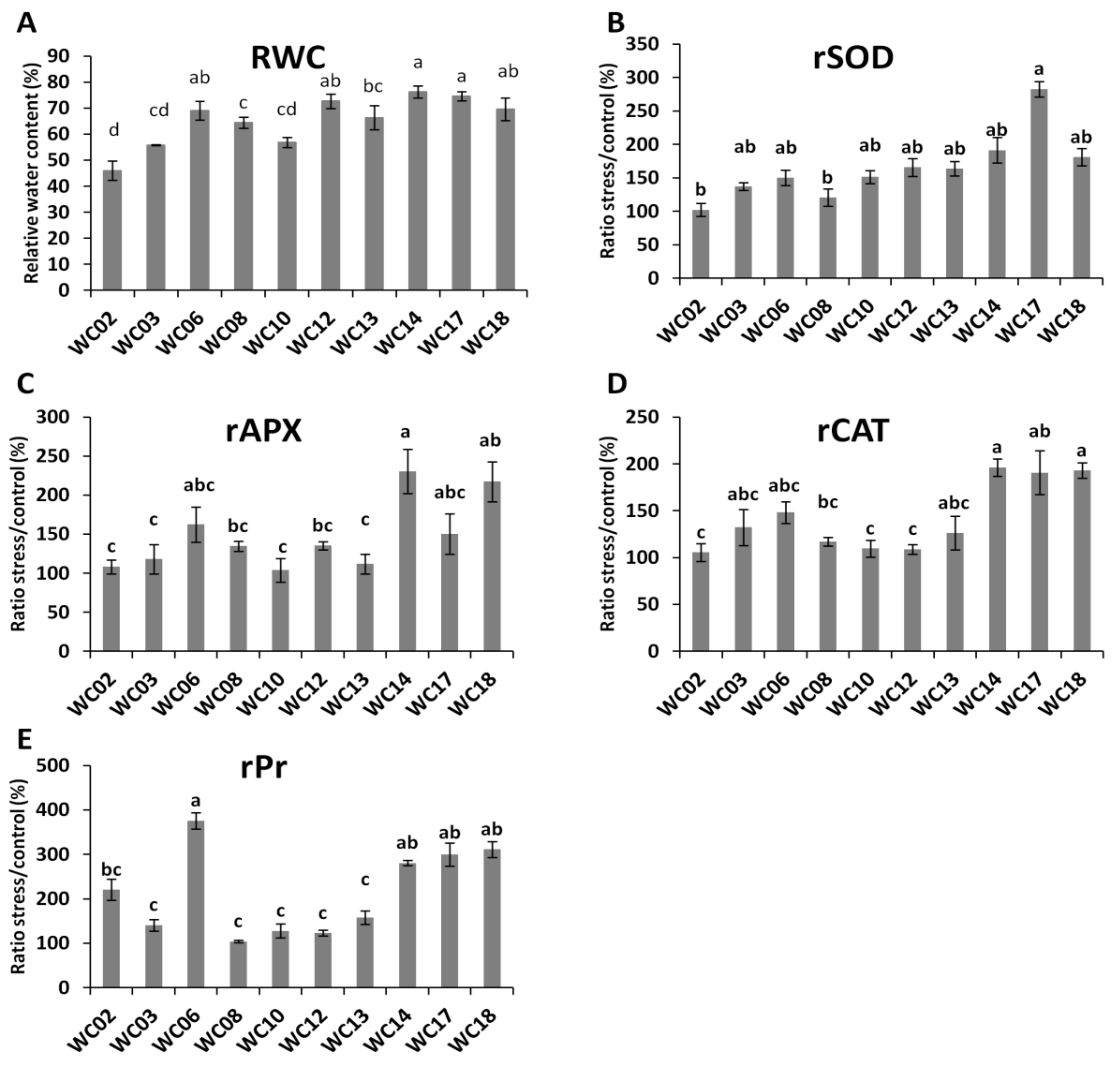
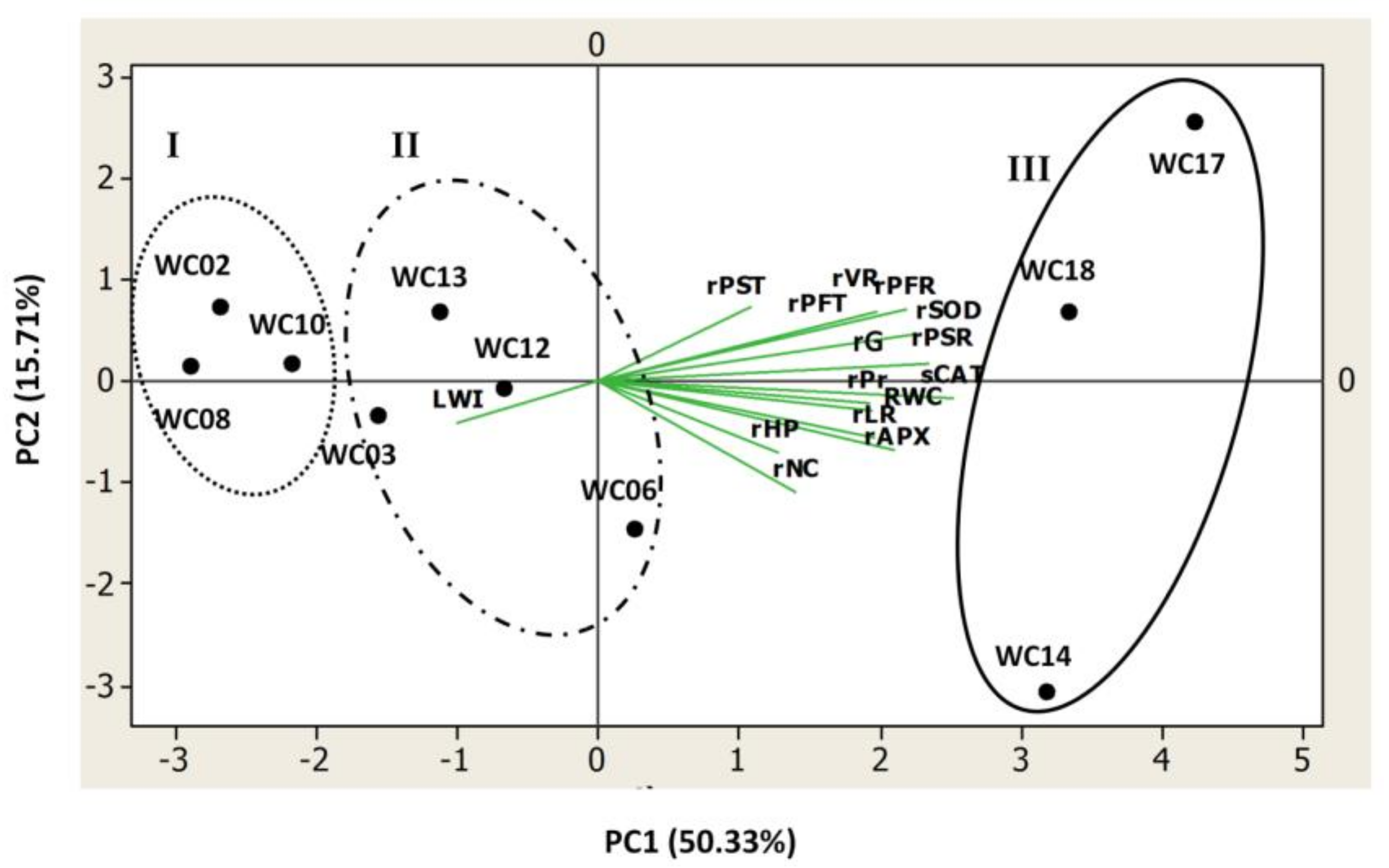
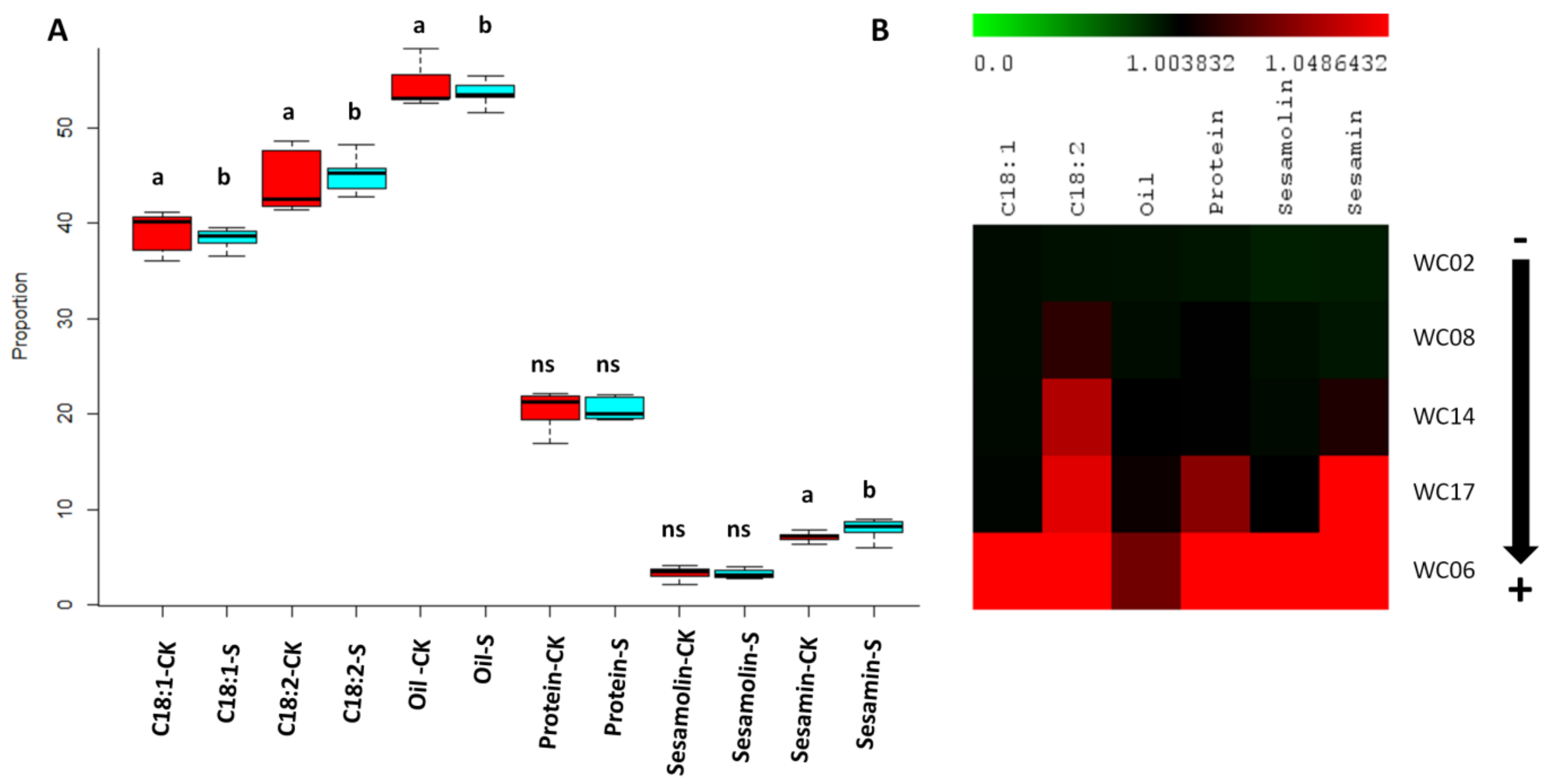
| Abbreviation | Definition | Unit |
|---|---|---|
| RWC | Relative water content | % |
| LWI | Leaf wilting index | - |
| Gr | Number of seeds per capsule | - |
| HP | Stem height | cm |
| NC | Number of capsules | - |
| VR | Root volume | cm3 |
| LR | Primary root length | cm |
| PFR | Root fresh weight | g |
| PSR | Root dry weight | g |
| PFT | Stem fresh weight | g |
| PST | Stem dry weight | g |
| SOD | Superoxide dismutase | (U·g−1·min−1) |
| CAT | Catalase | (U·g−1·min−1) |
| APX | Ascorbate peroxidase | (U g−1·min−1) |
| Pr | Proline content | (μg·g−1·Fw) |
| LAC | Linoleic acid content of the seed | % |
| OC | Oil content of the seed | % |
| OAC | Oleic acid content of the seed | % |
| PC | Protein content of seed | % |
| SC | Sesamolin content of the seed | % |
| SeC | Sesamin content seed | % |
| R/S | Root to Shoot ratio | - |
| SV | df | RWC | Gr | VR | LR | PFR | PFT | NC | PST | PSR | HP | SOD | CAT | APX | Pr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G | 9 | * | ns | *** | *** | *** | *** | ** | *** | ** | *** | * | *** | *** | ** |
| T | 1 | - | ns | ** | ** | ** | *** | ** | *** | * | *** | * | ** | * | ** |
| G × T | 9 | - | ns | ns | *** | * | * | ** | ** | ns | ** | * | * | ** | * |
| SV | df | OAC | LAC | OC | PC | SeC | SC |
|---|---|---|---|---|---|---|---|
| G | 4 | *** | *** | *** | *** | *** | *** |
| T | 1 | *** | *** | *** | ns | *** | ns |
| G × T | 4 | *** | *** | *** | *** | *** | *** |
| R/S | ||
|---|---|---|
| Genotypes | Control | Stress |
| WC02 | 0.19 ± 0.00 abcd | 0.11 ± 0.00 bc |
| WC03 | 0.20 ± 0.00 abcd | 0.12 ± 0.01 bc |
| WC06 | 0.148 ± 0.001 bcd | 0.15 ± 0.02 abc |
| WC08 | 0.23 ± 0.04 abcd | 0.24 ± 0.01 ab |
| WC10 | 0.29 ± 0.06 abcd | 0.16 ± 0.04 abc |
| WC18 | 0.08 ± 0.00 cd | 0.23 ± 0.02 abc |
| WC13 | 0.33 ± 0.04 a | 0.17 ± 0.02 abc |
| WC14 | 0.06 ± 0.04 ad | 0.25 ± 0.03 abc |
| WC17 | 0.15 ± 0.02 bcd | 0.28 ± 0.04 a |
| WC12 | 0.16 ± 0.03 bcd | 0.09 ± 0.00 c |
| rVR | rLR | rPFR | rPFT | rNC | rPST | rPSR | rHP | RWC | rSOD | rAPX | rCAT | rPr | LWI | rGr | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rVR | 1 | ||||||||||||||
| rLR | 0.34 | 1 | |||||||||||||
| rPFR | 0.88 *** | 0.46 | 1 | ||||||||||||
| rPFT | 0.41 | 0.22 | 0.59 * | 1 | |||||||||||
| rNC | −0.03 | 0.67 * | 0.14 | 0.24 | 1 | ||||||||||
| rPST | 0.38 | 0.08 | 0.43 | 0.86 *** | −0.03 | 1 | |||||||||
| rPSR | 0.76 ** | 0.64 * | 0.90 * | 0.42 | 0.45 | 0.21 | 1 | ||||||||
| rHP | 0.24 | 0.21 | 0.11 | 0.27 | 0.58 * | 0.10 | 0.24 | 1 | |||||||
| RWC | 0.49 | 0.60 * | 0.50 | 0.02 | 0.43 | −0.01 | 0.69 * | 0.35 | 1 | ||||||
| rSOD | 0.83 ** | 0.45 | 0.92 *** | 0.44 | 0.21 | 0.27 | 0.89 *** | 0.33 | 0.71 * | 1 | |||||
| rAPX | 0.29 | 0.81 ** | 0.37 | 0.38 | 0.75 * | 0.22 | 0.57 | 0.45 | 0.66 * | 0.4 | 1 | ||||
| rCAT | 0.58 * | 0.79 ** | 0.68 * | 0.60 * | 0.56 | 0.38 | 0.74 * | 0.51 | 0.66 * | 0.73 * | 0.85 ** | 1 | |||
| rPr | 0.62 * | 0.48 | 0.44 | 0.44 | 0.34 | 0.35 | 0.48 | 0.66 * | 0.39 | 0.44 | 0.65 * | 0.74 * | 1 | ||
| LWI | −0.19 | −0.26 | −0.34 | −0.17 | 0.15 | −0.14 | −0.25 | 0.23 | −0.48 | −0.42 | −0.36 | −0.46 | −0.02 | 1 | |
| rGr | −0.21 | −0.26 | 0.09 | 0.31 | −0.32 | 0.44 | −0.09 | 0.42 * | −0.30 | −0.12 | −0.31 | −0.29 | −0.60 * | −0.22 | 1 |
| Genotype | PC1 | PC2 | PC3 | Score | Rank |
|---|---|---|---|---|---|
| WC17 | 520.24 | 30.87 | −45.51 | 260.98 | 1 |
| WC18 | 486.53 | −51.59 | −11.69 | 235.30 | 2 |
| WC14 | 475.46 | −172.23 | 27.02 | 215.63 | 3 |
| WC06 | 386.70 | −110.12 | −13.49 | 175.63 | 4 |
| WC12 | 318.26 | −55.67 | 5.80 | 152.16 | 5 |
| WC13 | 301.99 | −30.62 | −21.89 | 144.43 | 6 |
| WC03 | 290.14 | −28.12 | −2.64 | 141.27 | 7 |
| WC02 | 275.09 | −43.21 | −5.38 | 130.99 | 8 |
| WC10 | 266.28 | −47.73 | −19.17 | 124.12 | 9 |
| WC08 | 227.45 | −66.97 | −38.21 | 99.17 | 10 |
| Origin | Number of Genotypes | Number and Code (in Bracket) of Genotypes Kept for Drought Stress |
|---|---|---|
| Benin | 4 | 2 (WC14, WC17) |
| Burkina Faso | 1 | 1 (WC10) |
| Cameroon | 2 | 1 (WC18) |
| Ivory Coast | 1 | 1 (WC12) |
| Mali | 4 | 3 (WC02, WC03, WC08) |
| Senegal | 3 | 1 (WC06) |
| Togo | 5 | 1 (WC13) |
| Total | 20 | 10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dossa, K.; Yehouessi, L.W.; Likeng-Li-Ngue, B.C.; Diouf, D.; Liao, B.; Zhang, X.; Cissé, N.; Bell, J.M. Comprehensive Screening of Some West and Central African Sesame Genotypes for Drought Resistance Probing by Agromorphological, Physiological, Biochemical and Seed Quality Traits. Agronomy 2017, 7, 83. https://doi.org/10.3390/agronomy7040083
Dossa K, Yehouessi LW, Likeng-Li-Ngue BC, Diouf D, Liao B, Zhang X, Cissé N, Bell JM. Comprehensive Screening of Some West and Central African Sesame Genotypes for Drought Resistance Probing by Agromorphological, Physiological, Biochemical and Seed Quality Traits. Agronomy. 2017; 7(4):83. https://doi.org/10.3390/agronomy7040083
Chicago/Turabian StyleDossa, Komivi, Louis W. Yehouessi, Benoît C. Likeng-Li-Ngue, Diaga Diouf, Boshou Liao, Xiurong Zhang, Ndiaga Cissé, and Joseph M. Bell. 2017. "Comprehensive Screening of Some West and Central African Sesame Genotypes for Drought Resistance Probing by Agromorphological, Physiological, Biochemical and Seed Quality Traits" Agronomy 7, no. 4: 83. https://doi.org/10.3390/agronomy7040083