CO2-Induced Changes in Wheat Grain Composition: Meta-Analysis and Response Functions
Abstract
:1. Introduction
- Are the negative effects of eCO2 on N concentration and N content independent of the experimental setup, such as exposure system, rooting environment, and concentration level of CO2 treatment?
- Is the negative effect of eCO2 on N concentration saturating at high CO2?
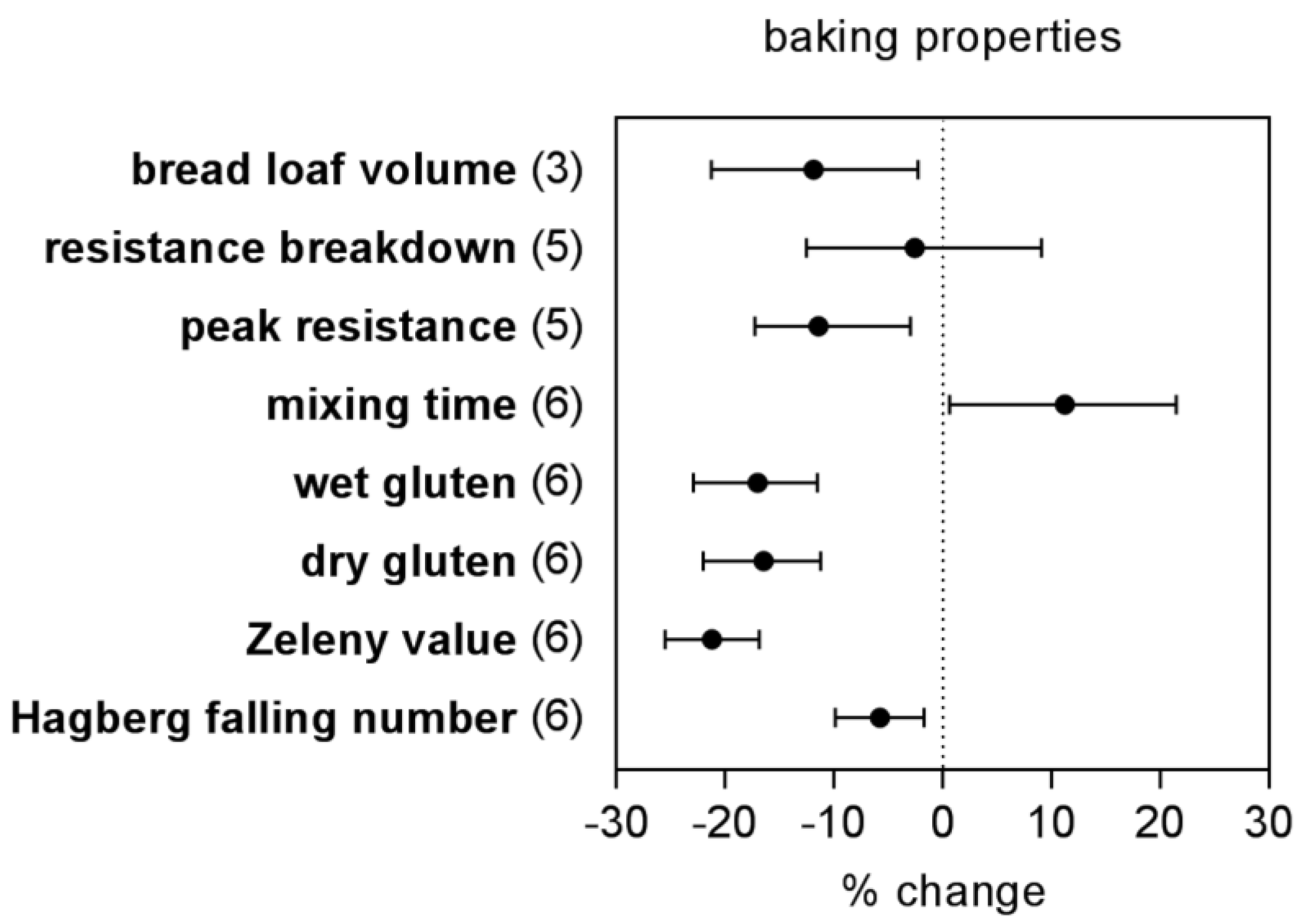
- To what extent are the nutritional and baking quality of wheat grain negatively affected by eCO2?
- Can starch dilution explain the reduction in concentration of N and minerals under eCO2?
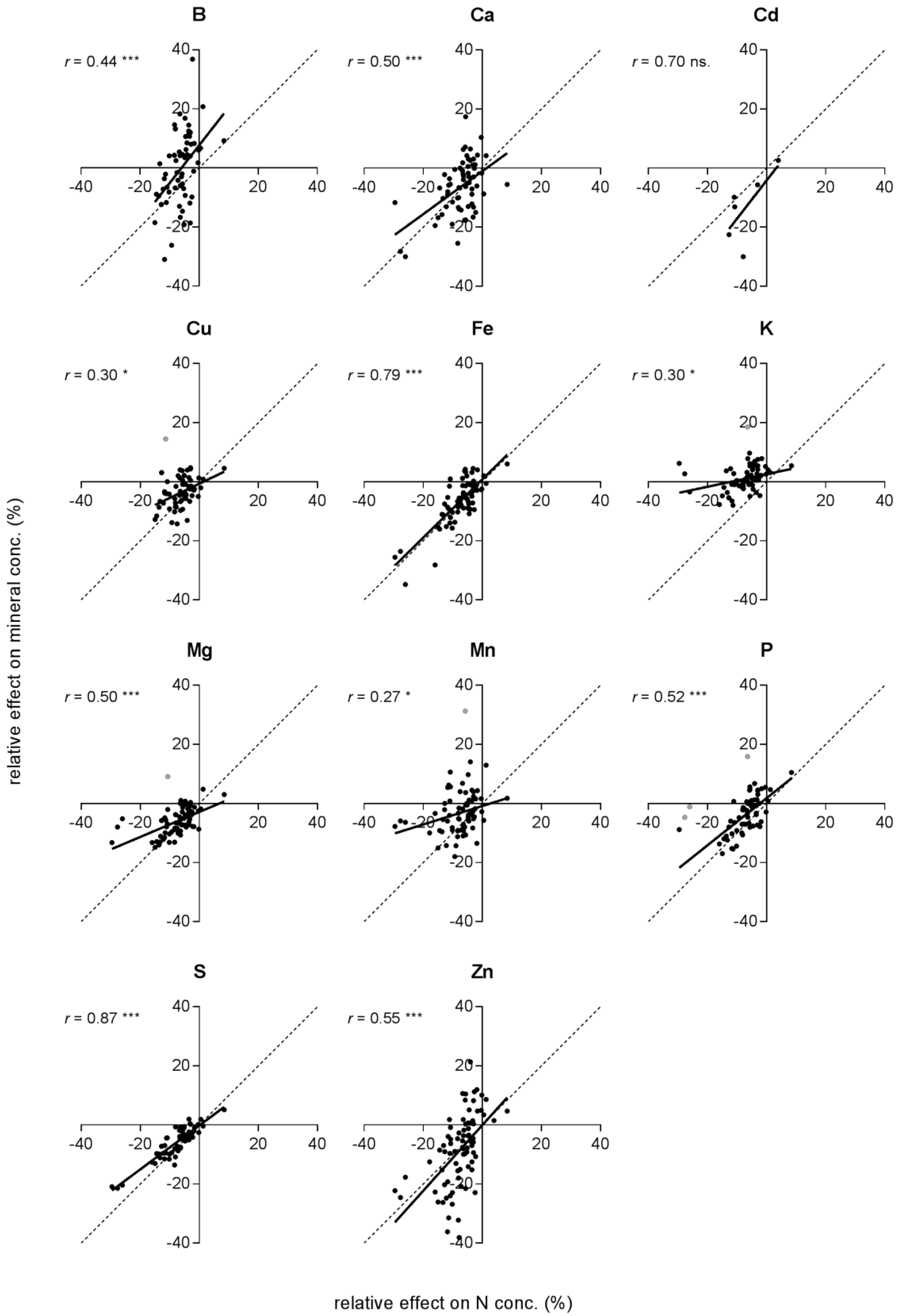
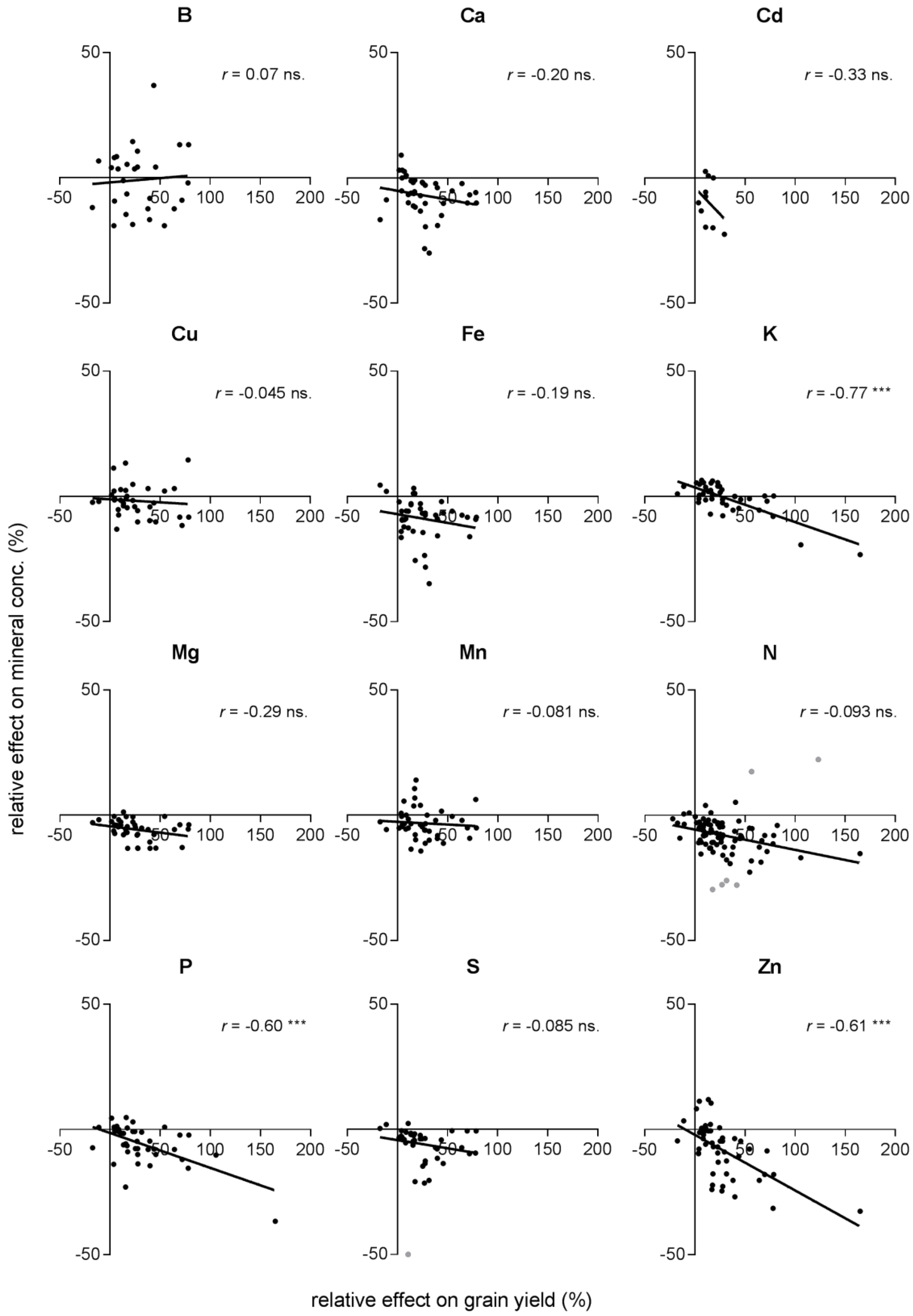
- Are effects of eCO2 on mineral concentration linked to the effect on N concentration and grain yield stimulation?
2. Results
2.1. Nitrogen and Starch
2.2. Minerals
2.3. Effects on Minerals in Relation to the Effects on N Concentration and Grain Yield
3. Discussion
4. Materials and Methods
4.1. Database
4.2. Meta-Analysis
4.3. Response Functions
4.4. Comparison of CO2 Effects on Different Response Variables
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis; World Meteorological Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants face the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef] [PubMed]
- Parry, M.L.; Rosenzweig, C.; Iglesias, A.; Livermore, M.; Fischer, G. Effects of climate change on global food production under sres emissions and socio-economic scenarios. Glob. Environ. Chang. 2004, 14, 53–67. [Google Scholar] [CrossRef]
- Manderscheid, R.; Weigel, H.J. Photosynthetic and growth responses of old and modern spring wheat cultivars to atmospheric CO2 enrichment. Agric. Ecosyst. Environ. 1997, 64, 65–73. [Google Scholar] [CrossRef]
- Schmid, I.; Franzaring, J.; Muller, M.; Brohon, N.; Calvo, O.C.; Hogy, P.; Fangmeier, A. Effects of CO2 enrichment and drought on photosynthesis, growth and yield of an old and a modern barley cultivar. J. Agron. Crop Sci. 2016, 202, 81–95. [Google Scholar] [CrossRef]
- Amthor, J.S. Effects of atmospheric CO2 concentration on wheat yield: Review of results from experiments using various approaches to control CO2 concentration. Field Crop. Res. 2001, 73, 1–34. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Leakey, A.D.B.; Morgan, P.B. Global food insecurity. Treatment of major food crops with elevated carbon dioxide or ozone under large-scale fully open-air conditions suggests recent models may have overestimated future yields. Phil. Trans. R. Soc. B 2005, 360, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Schimel, D. Climate change and crop yields: Beyond cassandra. Science 2006, 312, 1889–1890. [Google Scholar] [CrossRef] [PubMed]
- Ziska, L.H.; Bunce, J.A. Predicting the impact of changing CO2 on crop yields: Some thoughts on food. New Phytol. 2007, 175, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Korner, C. Plant CO2 responses: An issue of definition, time and resource supply. New Phytol. 2006, 172, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. FAO Cereal Supply and Demand Brief. Available online: http://www.fao.org/worldfoodsituation/csdb/en/ (aceessed on 12 December 2016).
- Loladze, I. Hidden shift of the ionome of plants exposed to elevated co2 depletes minerals at the base of human nutrition. Elife 2014, 3, e02245. [Google Scholar] [CrossRef] [PubMed]
- Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from face. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Lambers, H.; Dalling, M.J. Nitrogen redistribution during grain-growth in wheat (Triticum aestivum L.): 4. Development of a quantitative model of the translocation of nitrogen to the grain. Plant Physiol. 1983, 71, 7–14. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2014: Impacts, Adaptation and Vulnerability; World Meteorological Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Loladze, I. Rising atmospheric CO2 and human nutrition: Toward globally imbalanced plant stoichiometry? Trends Ecol. Evol. 2002, 17, 457–461. [Google Scholar] [CrossRef]
- Taub, D.R.; Miller, B.; Allen, H. Effects of elevated CO2 on the protein concentration of food crops: A meta-analysis. Glob. Chang. Biol. 2008, 14, 565–575. [Google Scholar] [CrossRef]
- McGrath, J.M.; Lobell, D.B. Reduction of transpiration and altered nutrient allocation contribute to nutrient decline of crops grown in elevated CO2 concentrations. Plant Cell Environ. 2013, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.J. Photorespiration and nitrate assimilation: A major intersection between plant carbon and nitrogen. Photosynth. Res. 2015, 123, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Pleijel, H.; Uddling, J. Yield vs. Quality trade-offs for wheat in response to carbon dioxide and ozone. Glob. Chang. Biol. 2012, 18, 596–605. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising (CO2): Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Högy, P.; Wieser, H.; Kohler, P.; Schwadorf, K.; Breuer, J.; Franzaring, J.; Muntifering, R.; Fangmeier, A. Effects of elevated CO2 on grain yield and quality of wheat: Results from a 3-year free-air CO2 enrichment experiment. Plant Biol. 2009, 11, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.S.; Wessells, K.R.; Kloog, I.; Zanobetti, A.; Schwartz, J. Effect of increased concentrations of atmospheric carbon dioxide on the global threat of zinc deficiency: A modelling study. Lancet Glob. Health 2015, 3, E639–E645. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Cadmium in Food. EFSA J. 2009, 980, 1–139. [Google Scholar]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Högy, P.; Fangmeier, A. Effects of elevated atmospheric CO2 on grain quality of wheat. J. Cereal Sci. 2008, 48, 580–591. [Google Scholar] [CrossRef]
- Blumenthal, C.; Rawson, H.M.; McKenzie, E.; Gras, P.W.; Barlow, E.W.R.; Wrigley, C.W. Changes in wheat grain quality due to doubling the level of atmospheric CO2. Cereal Chem. 1996, 73, 762–766. [Google Scholar]
- Högy, P.; Wieser, H.; Kohler, P.; Schwadorf, K.; Breuer, J.; Erbs, M.; Weber, S.; Fangmeier, A. Does elevated atmospheric CO2 allow for sufficient wheat grain quality in the future? J. Appl. Bot. Food Qual. 2009, 82, 114–121. [Google Scholar]
- Kimball, B.A.; Morris, C.F.; Pinter, P.J.; Wall, G.W.; Hunsaker, D.J.; Adamsen, F.J.; LaMorte, R.L.; Leavitt, S.W.; Thompson, T.L.; Matthias, A.D.; et al. Elevated CO2, drought and soil nitrogen effects on wheat grain quality. New Phytol. 2001, 150, 295–303. [Google Scholar] [CrossRef]
- Piikki, K.; De Temmerman, L.; Ojanpera, K.; Danielsson, H.; Pleijel, H. The grain quality of spring wheat (Triticum aestivum L.) in relation to elevated ozone uptake and carbon dioxide exposure. Eur. J. Agron. 2008, 28, 245–254. [Google Scholar] [CrossRef]
- Fernando, N.; Panozzo, J.; Tausz, M.; Norton, R.M.; Neumann, N.; Fitzgerald, G.J.; Seneweera, S. Elevated CO2 alters grain quality of two bread wheat cultivars grown under different environmental conditions. Agric. Ecosyst. Environ. 2014, 185, 24–33. [Google Scholar] [CrossRef]
- Wrigley, C.W.; Békés, F.; Bushuk, W. Chapter 1 gluten: A balance of gliadin and glutenin. In Gliadin and Glutenin: The Unique Balance of Wheat Quality; AACC International, Inc.: St. Paul, MN, USA, 2006; pp. 3–32. [Google Scholar]
- Kindred, D.R.; Gooding, M.J.; Ellis, R.H. Nitrogen fertilizer and seed rate effects on hagberg failing number of hybrid wheats and their parents are associated with alpha-amylase activity, grain cavity size and dormancy. J. Sci. Food Agric. 2005, 85, 727–742. [Google Scholar] [CrossRef]
- Hruskova, M.; Skodova, V.; Blazek, J. Wheat sedimentation values and falling number. Czech J. Food Sci. 2004, 22, 51–57. [Google Scholar]
- Agren, G.I.; Weih, M. Plant stoichiometry at different scales: Element concentration patterns reflect environment more than genotype. New Phytol. 2012, 194, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Waters, B.M.; Uauy, C.; Dubcovsky, J.; Grusak, M.A. Wheat (Triticum aestivum) nam proteins regulate the translocation of iron, zinc, and nitrogen compounds from vegetative tissues to grain. J. Exp. Bot. 2009, 60, 4263–4274. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (face)? A meta-analytic review of the responses of photosynthesis, canopy. New Phytol. 2005, 165, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Frei, M. Stressed food—The impact of abiotic environmental stresses on crop quality. Agric. Ecosyst. Environ. 2011, 141, 271–286. [Google Scholar] [CrossRef]
- Gelang, J.; Pleijel, H.; Sild, E.; Danielsson, H.; Younis, S.; Sellden, G. Rate and duration of grain filling in relation to flag leaf senescence and grain yield in spring wheat (Triticum aestivum) exposed to different concentrations of ozone. Physiol. Plant. 2000, 110, 366–375. [Google Scholar] [CrossRef]
- Broberg, M.C.; Feng, Z.Z.; Xin, Y.; Pleijel, H. Ozone effects on wheat grain quality—A summary. Environ. Pollut. 2015, 197, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Salt, D.E.; Prince, R.C.; Pickering, I.J.; Raskin, I. Mechanisms of cadmium mobility and accumulation in indian mustard. Plant Physiol. 1995, 109, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.Z.; Kobayashi, K.; Ainsworth, E.A. Impact of elevated ozone concentration on growth, physiology, and yield of wheat (Triticum aestivum L.): A meta-analysis. Glob. Chang. Biol. 2008, 14, 2696–2708. [Google Scholar]
- Li, W.L.; Han, X.Z.; Zhang, Y.Y.; Li, Z.Z. Effects of elevated CO2 concentration, irrigation and nitrogenous fertilizer application on the growth and yield of spring wheat in semi-arid areas. Agric. Water Manag. 2007, 87, 106–114. [Google Scholar] [CrossRef]
- Tulchinsky, T.H. Micronutrient deficiency conditions: Global health issues. Publ. Health Rev. 2010, 32, 243–255. [Google Scholar]
- Federov, S. Getdata Graph Digitizer 2.26.0.20. Available online: http://www.getdata-graph-digitizer.com/ (accessed on 20 May 2015).
- NOAA. Available online: http://www.noaa.gov/ (accessed on 19 September 2014).
- Rosenberg, M.S.; Adams, D.C.; Gurevitch, J. Metawin: Statistical Software for Meta-Analysis; Version 2.0; Sinauer Associates, Inc.: Sunderland, MA, USA, 2000. [Google Scholar]
- Curtis, P.S.; Wang, X.Z. A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 1998, 113, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Koricheva, J.; Gurevitch, J.; Mengersen, K. Handbook of Meta-Analysis in Ecology and Evolution; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- Motulsky, H.J.; Brown, R.E. Detecting outliers when fitting data with nonlinear regression—A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinf. 2006, 7, 1–20. [Google Scholar] [CrossRef] [PubMed]


| Variable | Observations | Regression Model | B0 | B1 | B2 | r2 | Sign. | Preferred Model | |
|---|---|---|---|---|---|---|---|---|---|
| N | concentration | 132 (4) | linear | 9.9 | −0.031 | 0.43 | * | ||
| quadratic | 49.9 | −0.198 | 1.66 × 10−4 | 0.57 | preferred | ||||
| content | 96 (11) | linear | −5.7 | 0.025 | 0.12 | * | |||
| quadratic | −51.9 | 0.216 | −1.85 × 10−4 | 0.18 | preferred | ||||
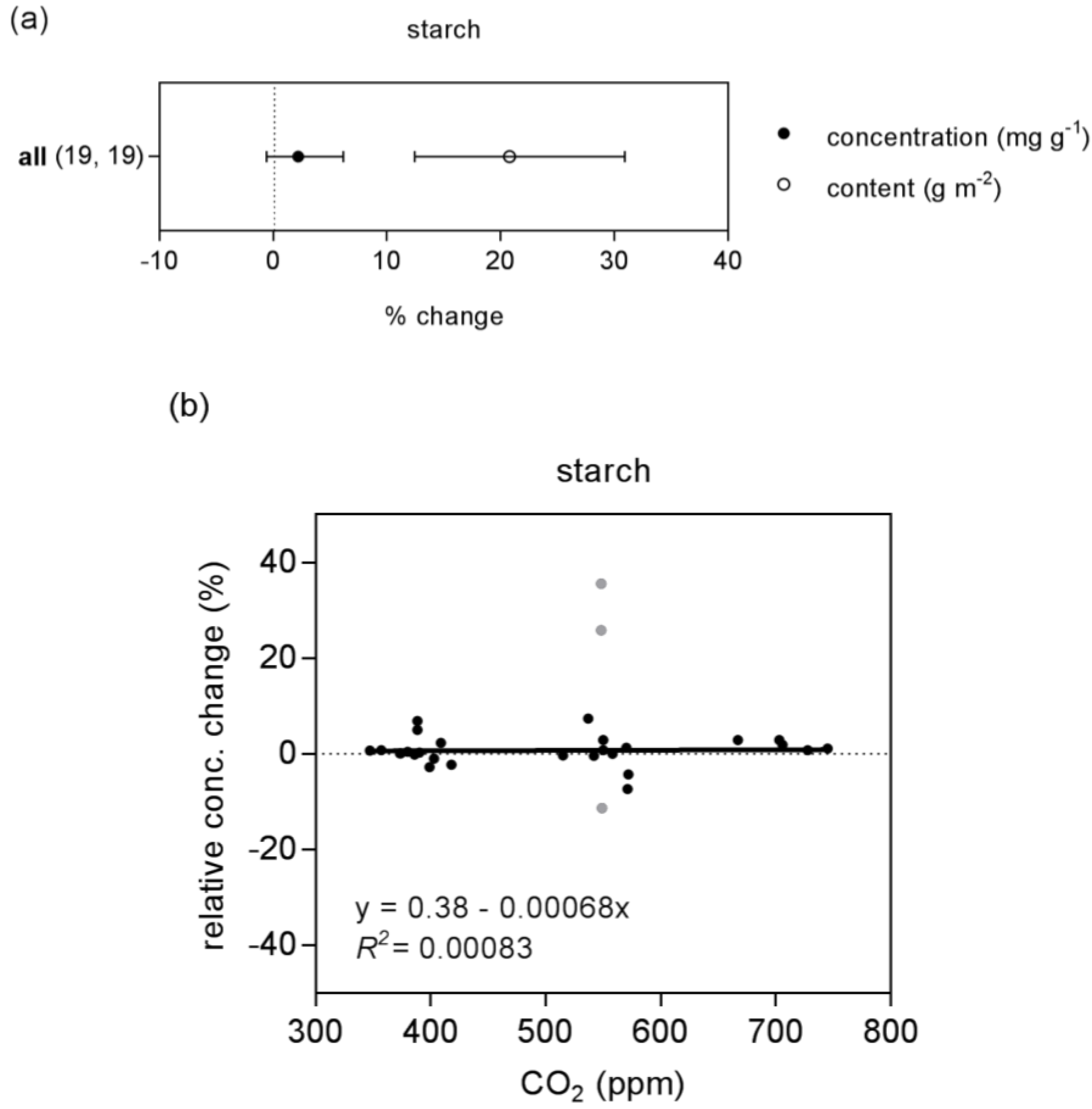
| starch | concentration | 30 (3) | linear | 0.4 | 0.001 | 0.00083 | ns | preferred | |
| quadratic | 10.3 | −0.039 | 3.72 × 10−5 | 0.028 | |||||
| content | 30 (2) | linear | −14.3 | 0.052 | 0.35 | * | preferred | ||
| quadratic | −7.7 | 0.026 | 2.46 × 10−5 | 0.35 | |||||
| B | concentration | 68 (2) | linear | 0.9 | −0.002 | 0.00046 | ns | preferred | |
| quadratic | n.a. | ||||||||
| content | 32 (4) | linear | −66.4 | 0.196 | 0.40 | * | preferred | ||
| quadratic | n.a. | ||||||||
| Ca | concentration | 83 (4) | linear | 12.9 | −0.037 | 0.32 | * | preferred | |
| quadratic | n.a. | ||||||||
| content | 47 (7) | linear | −16.1 | 0.056 | 0.16 | * | preferred | ||
| quadratic | n.a. | ||||||||
| Cd | concentration | 13 | linear | 12.4 | −0.039 | 0.31 | * | preferred | |
| quadratic | 64.0 | −0.253 | 2.07 × 10−4 | 0.39 | |||||
| content | 13 | linear | −1.4 | 0.003 | 0.0025 | ns | preferred | ||
| quadratic | 10.3 | −0.045 | 4.71 × 10−5 | 0.0068 | |||||
| Cu | concentration | 80 (2) | linear | 7.3 | −0.020 | 0.14 | * | preferred | |
| quadratic | n.a. | ||||||||
| content | 44 (5) | linear | −33.3 | 0.104 | 0.27 | * | preferred | ||
| quadratic | n.a. | ||||||||
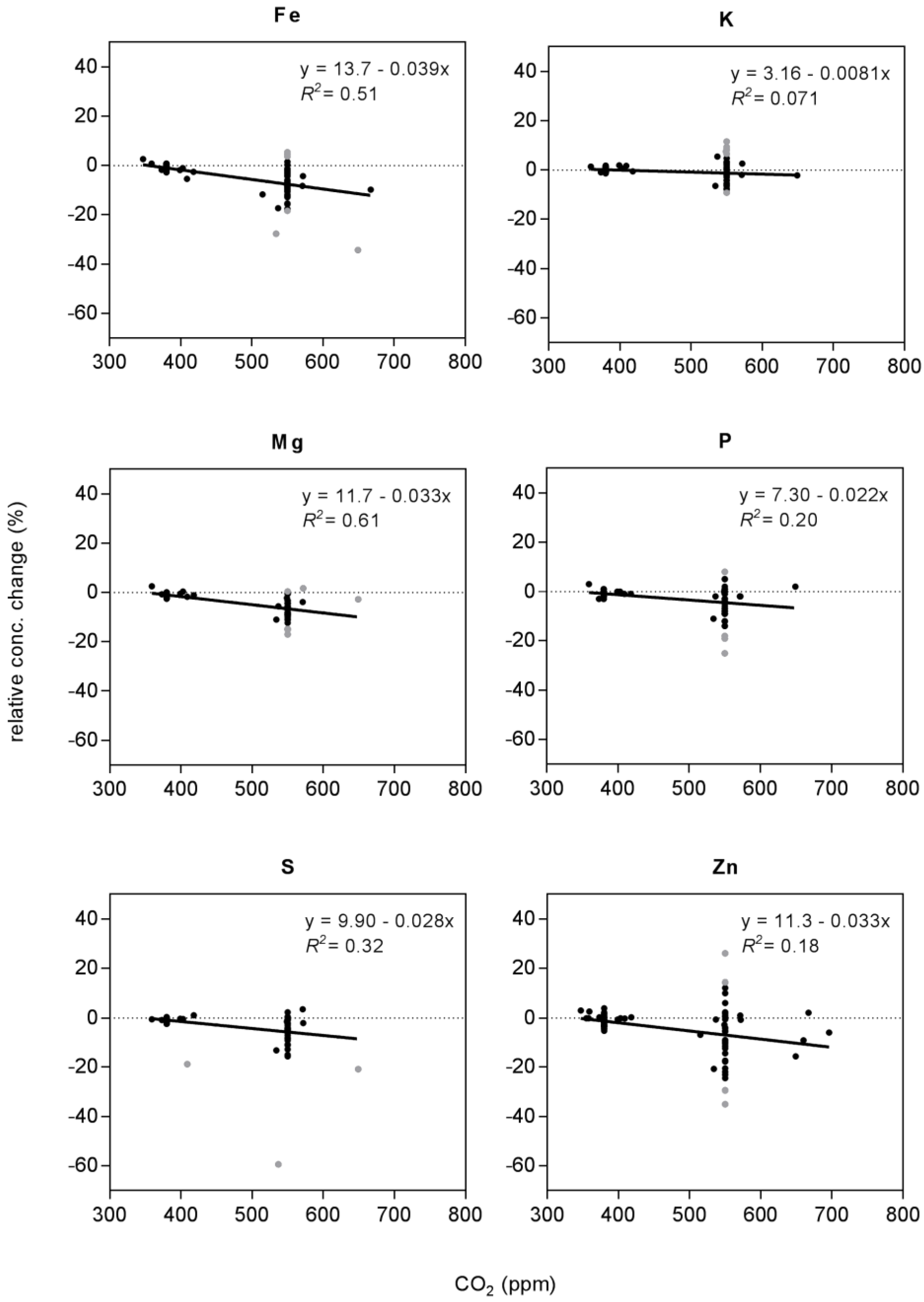
| Fe | concentration | 86 (7) | linear | 13.7 | −0.039 | 0.51 | * | preferred | |
| quadratic | n.a. | ||||||||
| content | 50 (4) | linear | −12.3 | 0.047 | 0.07 | ns | |||
| quadratic | −211.7 | 0.911 | −9.00 × 10−4 | 0.17 | preferred | ||||
| K | concentration | 83 (7) | linear | 3.2 | −0.008 | 0.07 | * | preferred | |
| quadratic | n.a. | ||||||||
| content | 47 (7) | linear | −37.6 | 0.116 | 0.51 | * | preferred | ||
| quadratic | n.a. | ||||||||
| Mg | concentration | 83 (8) | linear | 11.7 | −0.033 | 0.61 | * | preferred | |
| quadratic | n.a. | ||||||||
| content | 47 (3) | linear | 8.2 | −0.024 | 0.39 | * | preferred | ||
| quadratic | n.a. | ||||||||
| Mn | concentration | 84 (3) | linear | 6.4 | −0.019 | 0.13 | * | ||
| quadratic | 58.9 | −0.247 | 2.39 × 10−4 | 0.20 | preferred | ||||
| content | 48 (8) | linear | −20.1 | 0.067 | 0.36 | * | preferred | ||
| quadratic | −52.3 | 0.205 | −1.41 × 10−4 | 0.36 | |||||
| P | concentration | 83 (4) | linear | 7.3 | −0.022 | 0.20 | * | preferred | |
| quadratic | n.a. | ||||||||
| content | 47 (7) | linear | −27.9 | 0.018 | 0.38 | * | preferred | ||
| quadratic | n.a. | ||||||||
| S | concentration | 83 (3) | linear | 9.9 | −0.028 | 0.32 | * | preferred | |
| quadratic | n.a. | ||||||||
| content | 47 (7) | linear | −19.9 | 0.065 | 0.20 | * | preferred | ||
| quadratic | n.a. | ||||||||
| Zn | concentration | 90 (5) | linear | 11.3 | −0.033 | 0.18 | * | preferred | |
| quadratic | 51.1 | −0.205 | 1.78 × 10−4 | 0.21 | |||||
| content | 54 (6) | linear | −18.0 | 0.062 | 0.28 | * | |||
| quadratic | −143.7 | 0.596 | −5.42 × 10−4 | 0.43 | preferred | ||||
| x | Element | Observations | r2 | B0 | B1 | Sign. |
|---|---|---|---|---|---|---|
| N | B | 64 | 0.20 | 7.65 | 1.28 | ns. |
| Ca | 69 | 0.46 | 1.80 | 0.80 | ns. | |
| Cd | 6 | 0.49 | −4.17 | 1.29 | ns. | |
| Cu | 65 (1) | 0.17 | −0.66 | 0.48 | * | |
| Fe | 70 | 0.63 | 0.80 | 0.99 | ns. | |
| K | 69 (1) | 0.11 | 2.57 | 0.21 | * | |
| Mg | 76 | 0.32 | −2.86 | 0.42 | * | |
| Mn | 74 (1) | 0.084 | −0.84 | 0.31 | * | |
| P | 69 (3) | 0.46 | 1.80 | 0.80 | ns. | |
| S | 68 | 0.75 | −0.18 | 0.74 | * | |
| Zn | 83 | 0.30 | −0.084 | 1.11 | ns. | |
| Grain yield | B | 28 | 0.0049 | −1.87 | 0.034 | ns. |
| Ca | 40 | 0.042 | −5.10 | −0.070 | ns. | |
| Cd | 10 | 0.11 | −3.90 | −0.42 | ns. | |
| Cu | 36 (1) | 0.0021 | −1.18 | −0.24 | ns. | |
| Fe | 42 | 0.038 | −7.08 | −0.069 | ns. | |
| K | 42 | 0.59 | 3.61 | −0.14 | * | |
| Mg | 40 | 0.087 | −4.42 | −0.51 | ns. | |
| Mn | 43 | 0.0065 | −2.63 | −0.022 | ns. | |
| P | 42 | 0.36 | −1.38 | −0.14 | * | |
| S | 40 (1) | 0.063 | −4.26 | −0.065 | ns. | |
| Zn | 50 | 0.37 | −21.1 | −0.22 | * | |
| N | 87 (6) | 0.18 | −5.70 | −0.081 | * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broberg, M.C.; Högy, P.; Pleijel, H. CO2-Induced Changes in Wheat Grain Composition: Meta-Analysis and Response Functions. Agronomy 2017, 7, 32. https://doi.org/10.3390/agronomy7020032
Broberg MC, Högy P, Pleijel H. CO2-Induced Changes in Wheat Grain Composition: Meta-Analysis and Response Functions. Agronomy. 2017; 7(2):32. https://doi.org/10.3390/agronomy7020032
Chicago/Turabian StyleBroberg, Malin C., Petra Högy, and Håkan Pleijel. 2017. "CO2-Induced Changes in Wheat Grain Composition: Meta-Analysis and Response Functions" Agronomy 7, no. 2: 32. https://doi.org/10.3390/agronomy7020032
APA StyleBroberg, M. C., Högy, P., & Pleijel, H. (2017). CO2-Induced Changes in Wheat Grain Composition: Meta-Analysis and Response Functions. Agronomy, 7(2), 32. https://doi.org/10.3390/agronomy7020032






