The Effectiveness of Mixed Food Attractant for Managing Helicoverpa armigera (Hübner) and Agrotis ipsilon (Hufnagel) in Peanut Fields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Locations
2.2. Method for Population Dynamics Monitoring and Dissecting the Internal Reproductive System for the Adults Attracted and Killed by the Mixed Food Attractant in Peanut Fields
2.3. Population Dynamics Monitoring Method for Eggs and Larvae in Peanut Fields
2.4. Damage Rate of New Leaves in Peanut Fields
2.5. The Costs of Conventional Chemical Control and Biological Food Attractant Control
2.6. Data Analyses
3. Results
3.1. The Attract-and-Kill Effect of Toxicant-Infused Food Bait on Noctuid Pests in Peanut Fields
3.1.1. The Species and Ratio of the Pests Attracted by the Biological Food Attractants
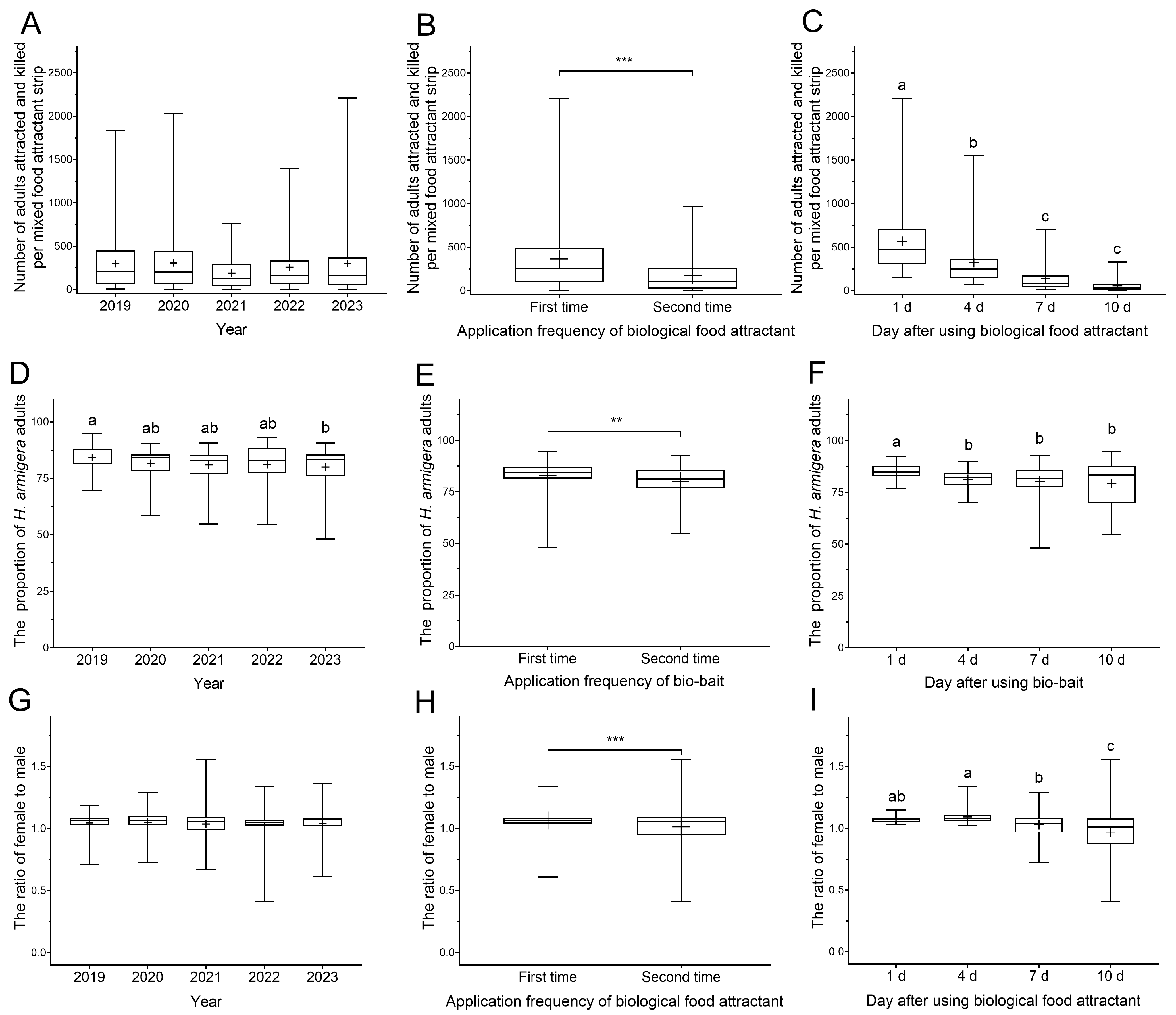
3.1.2. The Attract-and-Kill Effect of Toxicant-Infused Food Bait on Cotton Bollworm Adults
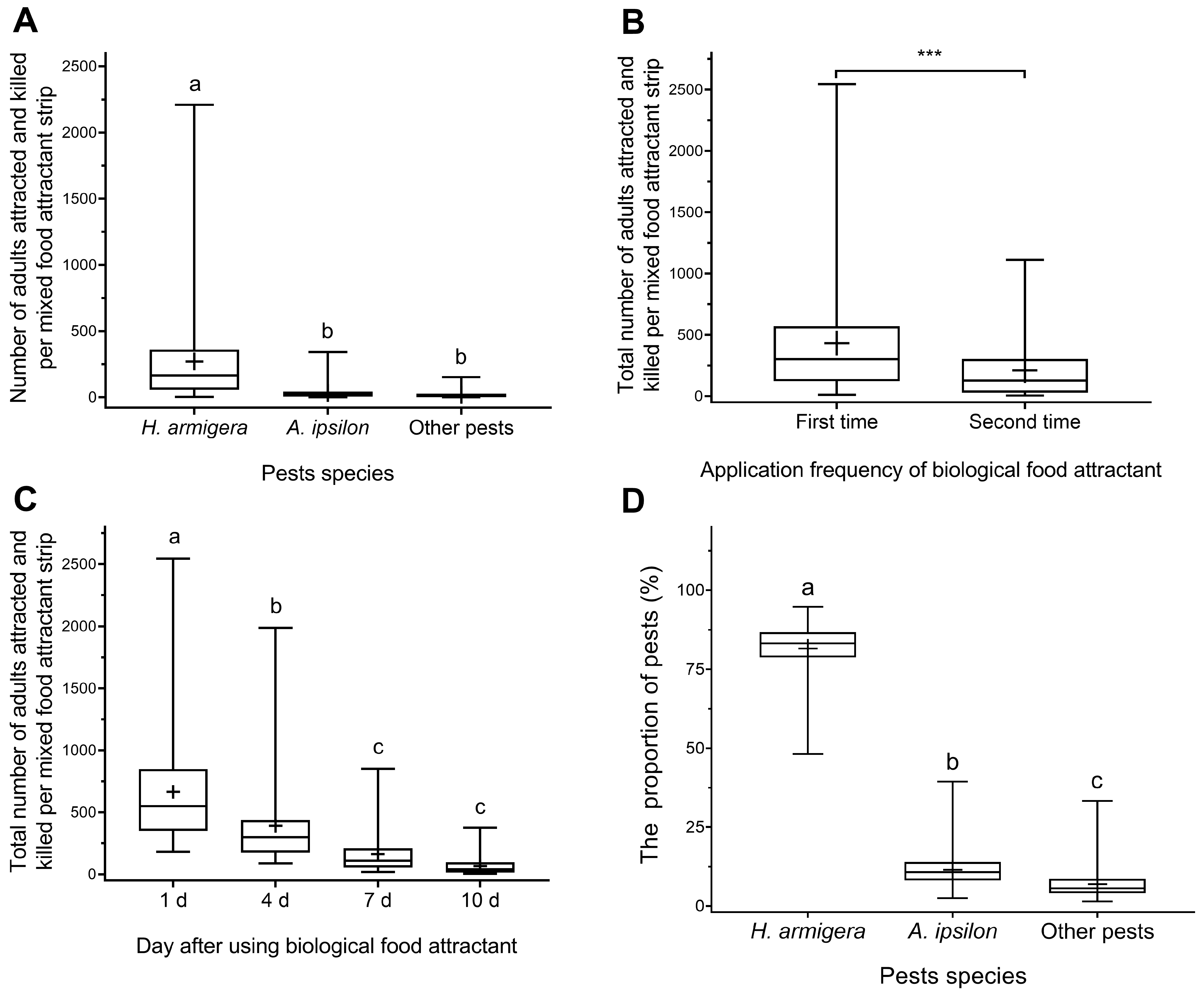
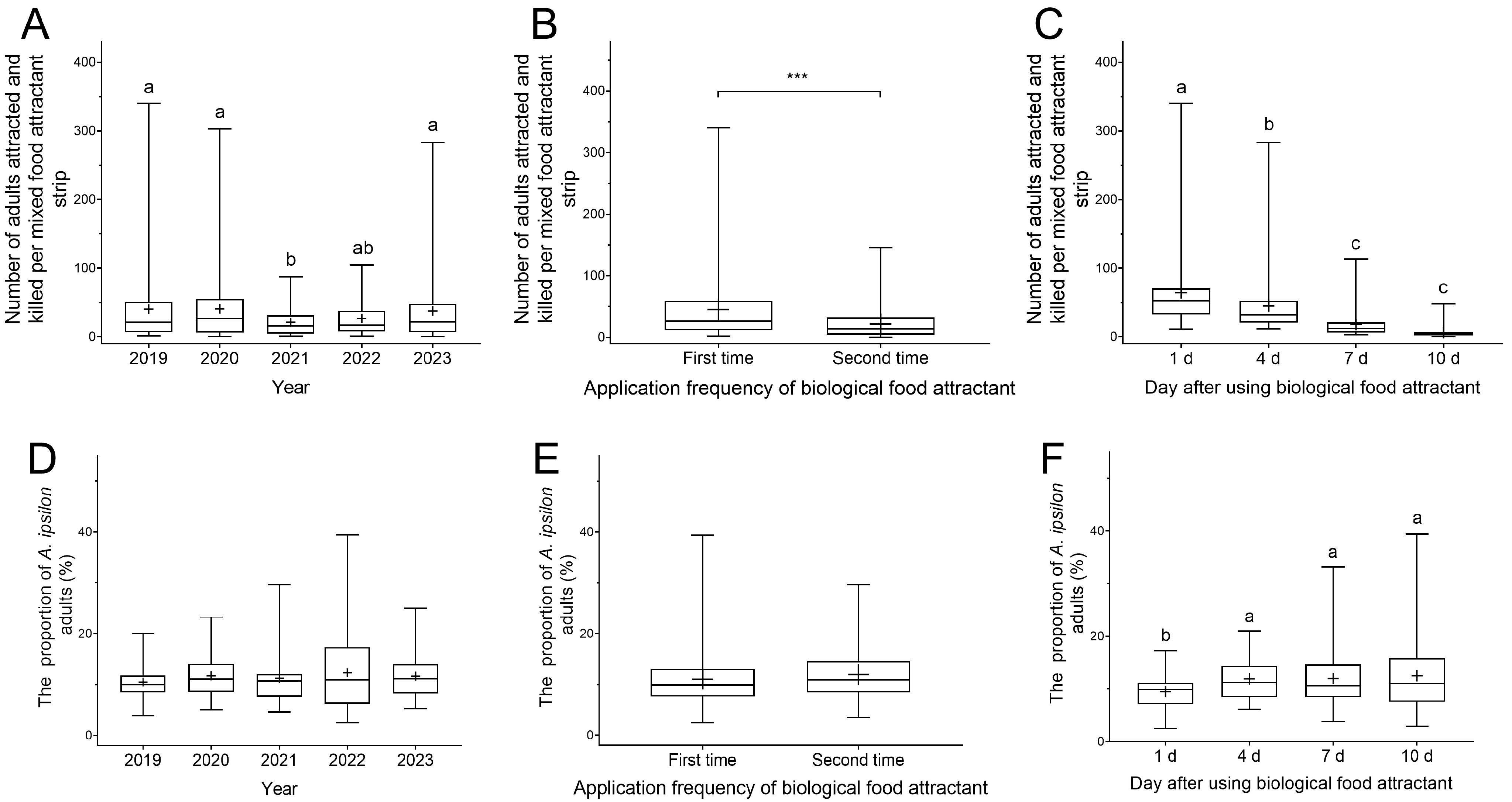
3.1.3. The Attract-and-Kill Effect of Toxicant-Infused Food Bait on Black Cutworm Adults
3.2. The Control Effect of Toxicant-Infused Food Baits on Cotton Bollworm and Black Cutworm Eggs and Larvae in Peanut Fields
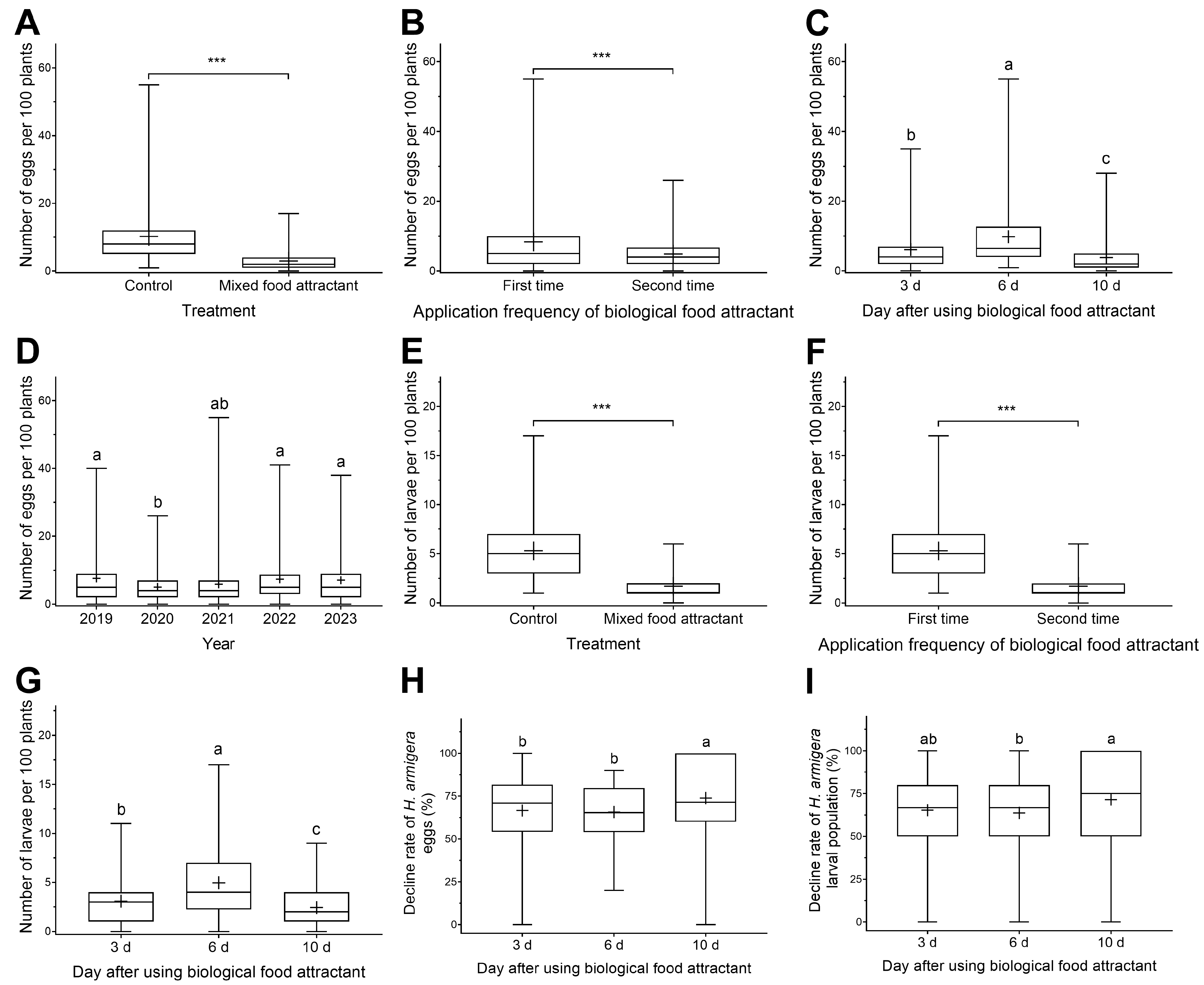
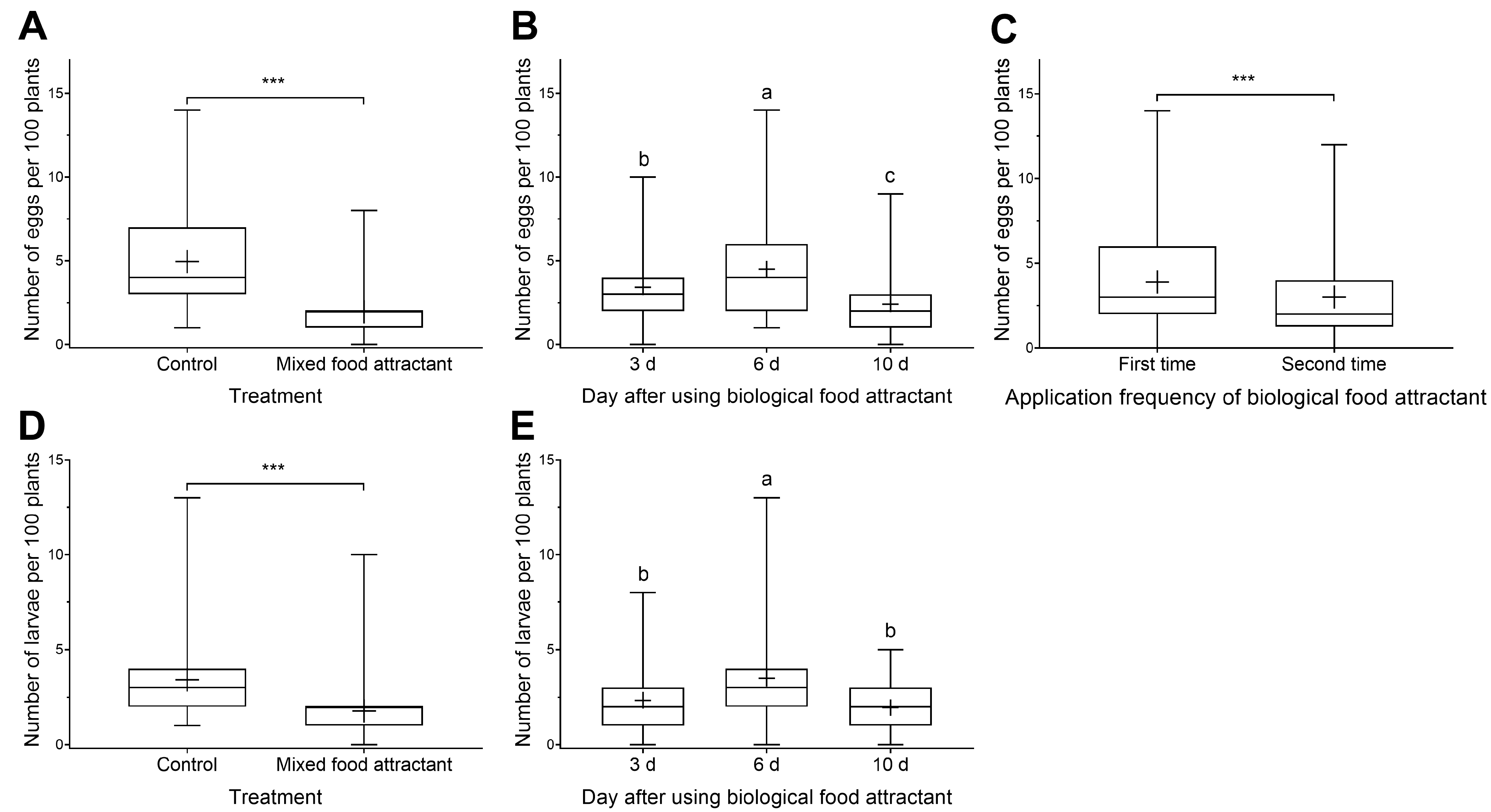
3.2.1. The Control Effect of Toxicant-Infused Food Baits on Cotton Bollworm Eggs and Larvae in Peanut Fields
3.2.2. The Control Effect of Toxicant-Infused Food Baits on Black Cutworm Eggs and Larvae in Peanut Fields
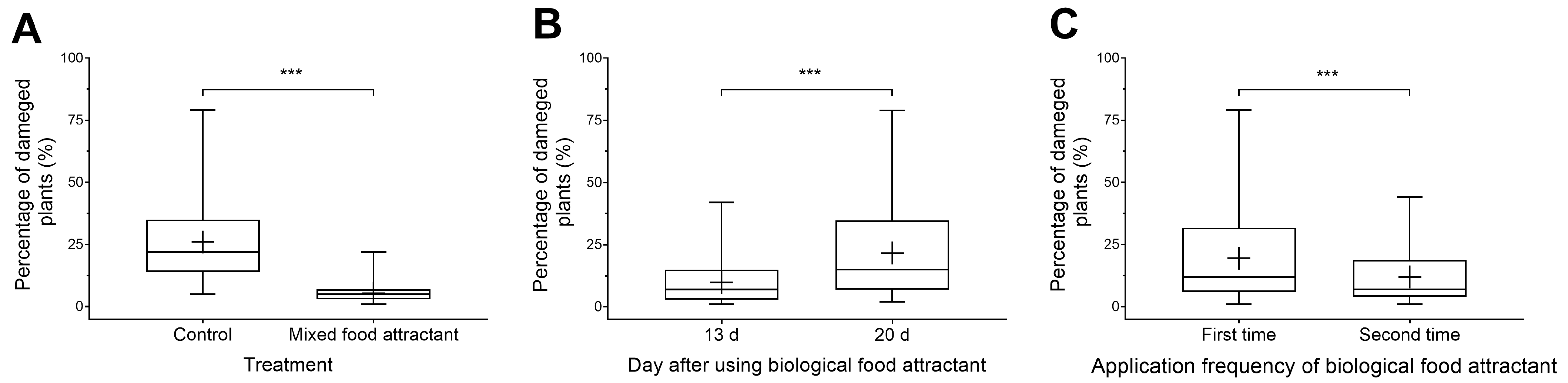
3.3. Impacts of Toxicant-Infused Food Baits on Damage Rate of New Leaves in Peanut Fields
3.4. Cost-Savings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO (Food and Agriculture Organization of the United Nations), Crops and Livestock Products. 2024. Available online: https://www.fao.org/faostat/zh/#data/QCL (accessed on 30 January 2024).
- NBS (National Bureau of Statistics), China Statistical Yearbook—2023. 2024. Available online: https://www.stats.gov.cn/sj/ndsj/2023/indexch.htm (accessed on 30 January 2024).
- Liao, B.S. A review on progress and prospects of peanut industry in China. Chin. J. Oil Crop. Sci. 2020, 42, 161–166. [Google Scholar]
- Li, J.C.; Li, X.Z.; Bai, Z.M. Evolution of global peanut trade pattern and policy implication. China Oils Fats 2022, 47, 8–15. [Google Scholar]
- Wan, S.B.; Zhang, J.L. Development strategy and countermeasure of peanut industry in Xinjiang. J. Peanut Sci. 2019, 48, 66–68. [Google Scholar]
- Qiang, G.; Yao, N.Q.; Wei, F. Identification and control method of peanut common above ground pests. J. Agr. Catastrophol. 2014, 4, 13–15. [Google Scholar]
- Wang, C.T.; Zhang, J.C. Genetic Improvement of Peanuts; Shanghai Science and Technology Press: Shanghai, China, 2013. [Google Scholar]
- Lu, Y.F.; Kan, H.L.; Li, L.L.; Zhuang, Q.Y.; Men, X.Y.; Guo, W.X.; Yu, Y. Preliminary evaluation of the monitoring and trapping efficacy of biological food attractant on Noctuidae adults in peanut fields in Junan county, Shangdong province. Plant Prot. 2020, 46, 248–253. [Google Scholar]
- Wang, C.Y.; Zhao, W.X. Identification of Peanut Diseases and Pests and Green Prevention and Control; Henan Science and Technology Press: Zhengzhou, China, 2021. [Google Scholar]
- Guo, Y.Y. Studies on Cotton Bollworm; Agriculture Press: Beijing, China, 1998. [Google Scholar]
- Balamurugan, R.; Kandasamy, P. Effectiveness of portable solar-powered light-emitting diode insect trap: Experimental investigation in a groundnut field. J. Asia-Pac. Entomol. 2021, 24, 1024–1032. [Google Scholar] [CrossRef]
- Wu, K.M.; Lu, Y.H.; Feng, H.Q.; Jiang, Y.Y.; Zhao, J.Z. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin-containing cotton. Science 2008, 321, 1676–1678. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wyckhuys, K.A.; Yang, L.; Liu, B.; Zeng, J.; Jiang, Y.; Desneux, N.; Zhang, W.; Wu, K. Bt cotton area contraction drives regional pest resurgence, crop loss, and pesticide use. Plant Biotechnol. J. 2022, 20, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Li, Y.P.; Wu, Y.D. Current status of insecticide resistance in Helicoverpa armigera after 15 years of Bt cotton planting in China. J. Econ. Entomol. 2013, 106, 375–381. [Google Scholar] [CrossRef]
- Mensah, R.K.; Gregg, P.C.; Del Socorro, A.P.; Moore, C.J.; Hawes, A.J.; Watts, N. Integrated pest management in cotton: Exploiting behaviour-modifying (semiochemical) compounds for managing cotton pests. Crop. Pasture Sci. 2013, 64, 763–773. [Google Scholar] [CrossRef]
- Gregg, P.C.; Del Socorro, A.P.; Landolt, P.J. Advances in attract-and-kill for agricultural pests: Beyond pheromones. Annu. Rev. Entomol. 2018, 63, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Utrio, P.; Eriksson, K. Volatile fermentation products as attractants for Macrolepidoptera. Ann. Zool. Fennici 1977, 14, 98–104. [Google Scholar]
- Bruce, T.J.; Cork, A. Electrophysiological and behavioral responses of female Helicoverpa armigera to compounds identified in flowers of African marigold, Tagetes erecta. J. Chem. Ecol. 2001, 27, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Gregg, P.C.; Del Socorro, A.P.; Henderson, G.S. Development of a synthetic plant volatile-based attracticide for female noctuid moths. II. Bioassays of synthetic plant volatiles as attractants for the adults of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 2010, 49, 21–30. [Google Scholar] [CrossRef]
- Del Socorro, A.P.; Gregg, P.C.; Alter, D.; Moore, C.J. Development of a synthetic plant volatile-based attracticide for female noctuid moths. I. Potential sources of volatiles attractive to Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 2010, 49, 10–20. [Google Scholar] [CrossRef]
- Del Socorro, A.P.; Gregg, P.C.; Hawes, A.J. Development of a synthetic plant volatile-based attracticide for female noctuid moths. III. Insecticides for adult Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 2010, 49, 31–39. [Google Scholar] [CrossRef]
- Gregg, P.C.; Del Socorro, A.P.; Hawes, A.J.; Binns, M.R. Developing bisexual attract-and-kill for polyphagous insects: Ecological rationale versus pragmatics. J. Chem. Ecol. 2016, 42, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Gregg, P.C.; Del Socorro, A.P.; Binns, M.R. Non-target impacts of an attract-and-kill formulation based on plant volatiles: Responses of some generalist predators. J. Chem. Ecol. 2016, 42, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Justiniano, W.; Fernandes, M.G. Effect of food attractants and insecticide toxicity for the control of Spodoptera frugiperda (Lepidoptera: Noctuidae) adults. J. Agr. Sci-Cambridge 2019, 12, 129–137. [Google Scholar] [CrossRef]
- Justiniano, W.; Fernandes, M.G.; Raizer, J. Toxic bait as an alternative tool in the management of Spodoptera frugiperda in second corn crops. J. Agr. Sci-Cambridge 2021, 13, 102–112. [Google Scholar] [CrossRef]
- Gregg, P.C.; Del Socorro, A.P.; Wilson, S.; Knight, K.M.; Binns, M.R.; Armytage, P. Bisexual attract-and-kill: A novel component of resistance management for transgenic cotton in Australia. J. Econ. Entomol. 2022, 115, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Xiu, C.L.; Li, A.L.; Lu, W.; Liu, Z.; Lu, Y.H. The effectiveness of using food attractant to lure cotton bollworm moths into traps under field conditions. Chin. J. Appl. Entomol. 2018, 55, 44–48. [Google Scholar]
- He, W.; Zhao, X.; Ali, A.; Ge, S.; Zhang, H.; He, L.; Wu, K. Population dynamics and reproductive developmental analysis of Helicoverpa armigera (Lepidoptera: Noctuidae) trapped using food attractants in the field. J. Econ. Entomol. 2021, 114, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, L.; Zhu, X.; Zhang, J.; Li, N.; Fan, J.; Li, H.; Sun, X.; Zhang, L.; Lin, Y.; et al. Large-area field application confirms the effectiveness of toxicant-infused food bait for managing Helicoverpa armigera (Hübner) in maize fields. Pest Manag. Sci. 2023, 79, 5405–5417. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.S.; Sun, M.H.; Hui, X.H.; Cui, W.N.; Zhao, Y.L.; Xu, L.; Wang, H.M.; Hu, L.B. Study on the effect of using a combination of biological food attractants and methomyl to trap and kill major pests in peanut and corn fields. China Plant Prot. 2016, 36, 38–41. [Google Scholar]
- Lu, Y.H.; Wang, L.Y.; Wu, K.M.; Su, M.; Li, J.J. A Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) Attractant. China Patent CN10812 4869B, 2020.
- Zhang, Y.M.; Mou, J.Y. Studies on histochemistry and prediction grading of ovarian development for Helicoverpa armigera. Shandong Agr. Sci. 1994, 3, 7–9. [Google Scholar]
- Wu, Y.J.; Wang, B.J.; Wang, M.R.; Peng, Y.C.; Cao, H.Q.; Sheng, C.W. Control efficacy and joint toxicity of metaflumizone mixed with chlorantraniliprole or indoxacarb against the fall armyworm, Spodoptera frugiperda. Pest Manag. Sci. 2023, 79, 1094–1101. [Google Scholar] [CrossRef]
- Landolt, P.J.; Phillips, T.W. Host plant influences on sex pheromone behavior of phytophagous insects. Annu. Rev. Entomol. 1997, 42, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Beyaert, I.; Hilker, M. Plant odour plumes as mediators of plant-insect interactions. Biol. Rev. 2014, 89, 68–81. [Google Scholar] [CrossRef]
- Knolhoff, L.M.; Heckel, D.G. Behavioral assays for studies of host plant choice and adaptation in herbivorous insects. Annu. Rev. Entomol. 2014, 59, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Meiners, T. Chemical ecology and evolution of plant-insect interactions: A multitrophic perspective. Curr. Opin. Insect Sci. 2015, 8, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.Q.; Wu, X.F.; Wu, B.; Wu, K.M. Seasonal migration of Helicoverpa armigera (Lepidoptera: Noctuidae) over the Bohai Sea. J. Econ. Entomol. 2009, 102, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Fu, X.W.; Feng, H.Q.; Liu, Z.F.; Wu, K.M. Trans-regional migration of Agrotis ipsilon (Lepidoptera: Noctuidae) in north-east Asia. Ann. Entomol. Soc. Am. 2015, 108, 519–527. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Z.; Cai, X.; Cui, L.; Zhang, H.; Xiao, H.; Li, Z.; Zhang, L.; Zeng, J. Progresses on integrated pest management (IPM) of agricultural insect pests in China. Chin. J. Appl. Entomol. 2017, 54, 349–363. [Google Scholar]
- Wyckhuys, K.A.; Lu, Y.; Zhou, W.; Cock, M.J.; Naranjo, S.E.; Fereti, A.; Williams, F.E.; Furlong, M.J. Ecological pest control fortifies agricultural growth in Asia-Pacific economies. Nat. Ecol. Evol. 2020, 4, 1522–1530. [Google Scholar] [CrossRef]
- Gould, F.; Brown, Z.S.; Kuzma, J. Wicked evolution: Can we address the sociobiological dilemma of pesticide resistance? Science 2018, 360, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Daraban, G.M.; Hlihor, R.M.; Suteu, D. Pesticides vs. biopesticides: From pest management to toxicity and impacts on the environment and human health. Toxics 2023, 11, 983. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Abbas, S.; Abbas, A.; Abbas, M.; Hafeez, F.; Shakeel, M.; Xiao, F.; Zhao, C.R. Emerging trends in insect sex pheromones and traps for sustainable management of key agricultural pests in Asia: Beyond insecticides-a comprehensive review. Int. J. Trop. Insect Sci. 2023, 43, 1867–1882. [Google Scholar] [CrossRef]
- Ivey, V.; Hillier, N.K. Hybridization in heliothine moths: Impacts on reproduction, pheromone communication, and pest management. Front. Ecol. Evol. 2023, 11, 1208079. [Google Scholar] [CrossRef]
- Blas, L.I.; Wratten, S.D.; Didham, R.K.; Gurr, G. Increasing floral diversity for selective enhancement of biological control agents: A double-edged sword? Basic-Appl. Ecol. 2006, 7, 236–243. [Google Scholar]
- Zhou, Y.; Zhao, S.Y.; Wang, M.L.; Yu, W.H.; Wyckhuys, K.A.G.; Wu, K.M. Floral visitation can enhance fitness of Helicoverpa armigera (Lepidoptera: Noctuidae) long-distance migrants. J. Econ. Entomol. 2019, 112, 2655–2662. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, W.J.; Xu, C.L.; Chen, H.P.; Xu, H.L.; Huang, Q.N.; Cao, L.D. Research progress on the mode of action and formulation application of food attractants. Modern Agrochem. 2022, 21, 12–17. [Google Scholar]
- Deng, J.Y.; Wei, H.Y.; Huang, Y.P.; Du, J.W. Enhancement of attraction to sex pheromones of Spodoptera exigua by volatile compounds produced by host plants. J. Chem. Ecol. 2004, 30, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.M.; Li, Z.Q.; Pan, H.S.; Lu, Y.H. Research and application of food-based attractants of herbivorous insect pests. Chin. J. Biol. Control 2018, 34, 8–35. [Google Scholar]
- Staton, T.; Williams, D.T. A meta-analytic investigation of the potential for plant volatiles and sex pheromones to enhance detection and management of Lepidopteran pests. Bull. Entomol. Res. 2023, 113, 725–734. [Google Scholar] [CrossRef]



| Location | Application Area (ha) | Sampling Sites | Sampling Plots Area (ha) | Application Frequency | Application Date in Different Years | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Toxicant-Infused Food Bait | Control | 2019 | 2020 | 2021 | 2022 | 2023 | ||||
| Daming County, Handan City, Hebei Province (HD) | 667 | Zhengzai Village (115.312636° E, 36.292088° N) | Qianxiancheng Village (115.409660° E, 36.234650° N) | 5 | First time | 17 June | 13 June | 14 June | 16 June | 17 June |
| 667 | Zhengzai Village (115.312636° E, 36.292088° N) | Qianxiancheng Village (115.409660° E, 36.234650° N) | 5 | Second time | 19 July | 17 July | 16 July | 17 July | 19 July | |
| Jing County, Hengshui City, Hebei Province (HS) | 67 | Xingzhuang Village (116.049957° E, 37.733050° N | Xizhaozhuang Village (116.117763° E, 37.745064° N) | 5 | First time | 9 June | 10 June | 10 June | 13 June | 11 June |
| 67 | Xingzhuang Village (116.049957° E, 37.733050° N | Xizhaozhuang Village (116.117763° E, 37.745064° N) | 5 | Second time | 13 July | 12 July | 13 July | 15 July | 14 July | |
| Guan County, Langfang City, Heibei Province (LF) | 67 | Houying Village (116.249042° E, 39.361543° N) | Ligezhuang Village (116.192436° E, 39.344121° N) | 5 | First time | 24 June | 22 June | 24 June | 24 June | 26 June |
| 67 | Houying Village (116.249042° E, 39.361543° N) | Ligezhuang Village (116.192436° E, 39.344121° N) | 5 | Second time | 27 July | 24 July | 26 July | 27 July | 29 July | |
| Ningling County, Shangqiu City, Heinan Province (SQ) | 667 | Lilou Village (115.235574° E, 34.534178° N) | Hezhuang Village (115.203257° E, 34.555523° N) | 5 | First time | 12 June | 11 June | 10 June | 13 June | 11 June |
| 667 | Lilou Village (115.235574° E, 34.534178° N) | Hezhuang Village (115.203257° E, 34.555523° N) | 5 | Second time | 15 July | 14 July | 14 July | 15 July | 13 July | |
| Kalaqinzuoyi Mongolian Autonomous County, Chaoyang City, Liaoning Province (CY) | 667 | Baitazi Village (119.663944° E, 40.864848° N) | Zhangjiayao Village (119.670303° E, 40.672755° N) | 5 | First time | 25 June | 27 June | 27 June | 28 June | 30 June |
| 667 | Baitazi Village (119.663944° E, 40.864848° N) | Zhangjiayao Village (119.670303° E, 40.672755° N) | 5 | Second time | 27 July | 31 July | 28 July | 31 July | 2 August | |
| Laixi County, Yantai City, Shaodong Province (YT) | 67 | Taipingshan Village (120.559813° E, 36.781083° N) | Nanhouzai Village (120.562248° E, 36.766392° N) | 5 | First time | 19 June | 17 June | 16 June | 19 June | 15 June |
| 67 | Taipingshan Village (120.559813° E, 36.781083° N) | Nanhouzai Village (120.562248° E, 36.766392° N) | 5 | Second time | 21 July | 18 July | 17 July | 22 July | 17 July | |
| Experiment Sites | Variety | Row Spacing | Plant Spacing | Plants/ha. |
|---|---|---|---|---|
| Handan City, Hebei Province | Jihua 915 (Daming Xinxin Seed Co., Ltd., Handan, China) | 37.5 cm | 22 cm | 242,430 |
| Chaoyang City, Liaoning Province | Fuhua 12 (Peanut Research Institute, Liaoning Academy of Agricultural Sciences) | 15 cm | 45 cm | 148,000 |
| Shangqiu City, Henan province | Huayu 25, Shanghua 4, Luohua 4087 (Henan Huameng Seed Co.,Ltd, Shangqiu, China) | 33 cm | 20 cm | 270,000 |
| Hengshui City, Hebei province | Huayu 23 (Peanut Research Institute, Shandong Academy of Agricultural Sciences) | 60 cm | 12 cm | 135,000 |
| Langfang City, Hebei province | Jihua 5, Jiyou 4 (Hebei Haohaijianong Seed Co., Ltd., Shijiazhuang, China) | 40 cm | 15 cm | 150,000 |
| Yantai City, Shandong province | Huayu 963 (Shandong Luhua Agricultural Science Co., Ltd., Laiyang, China) | 20 cm | 20 cm | 250,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; He, L.; Wang, T.; Xiao, T.; Zou, Z.; Wang, M.; Cai, X.; Yao, B.; Yang, Y.; Wu, K. The Effectiveness of Mixed Food Attractant for Managing Helicoverpa armigera (Hübner) and Agrotis ipsilon (Hufnagel) in Peanut Fields. Agronomy 2024, 14, 986. https://doi.org/10.3390/agronomy14050986
Wang L, He L, Wang T, Xiao T, Zou Z, Wang M, Cai X, Yao B, Yang Y, Wu K. The Effectiveness of Mixed Food Attractant for Managing Helicoverpa armigera (Hübner) and Agrotis ipsilon (Hufnagel) in Peanut Fields. Agronomy. 2024; 14(5):986. https://doi.org/10.3390/agronomy14050986
Chicago/Turabian StyleWang, Liying, Limei He, Tongwei Wang, Tao Xiao, Zongfeng Zou, Meng Wang, Xiaoling Cai, Bingtao Yao, Yu Yang, and Kongming Wu. 2024. "The Effectiveness of Mixed Food Attractant for Managing Helicoverpa armigera (Hübner) and Agrotis ipsilon (Hufnagel) in Peanut Fields" Agronomy 14, no. 5: 986. https://doi.org/10.3390/agronomy14050986





