Response of the Endophytic Microbial Composition in Amaranthus Roots to Different Fertilization Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Site, Test Material, and Fertilizer Descriptions
2.2. Experimental Design
2.3. Sample Collection
2.4. Analysis of the Amaranthin Content
2.5. Analysis of the Endophytic Microbial Compositions
2.6. Statistical Analyses
3. Results
3.1. Effect of Different Fertilization Treatments on the Amaranthin Content
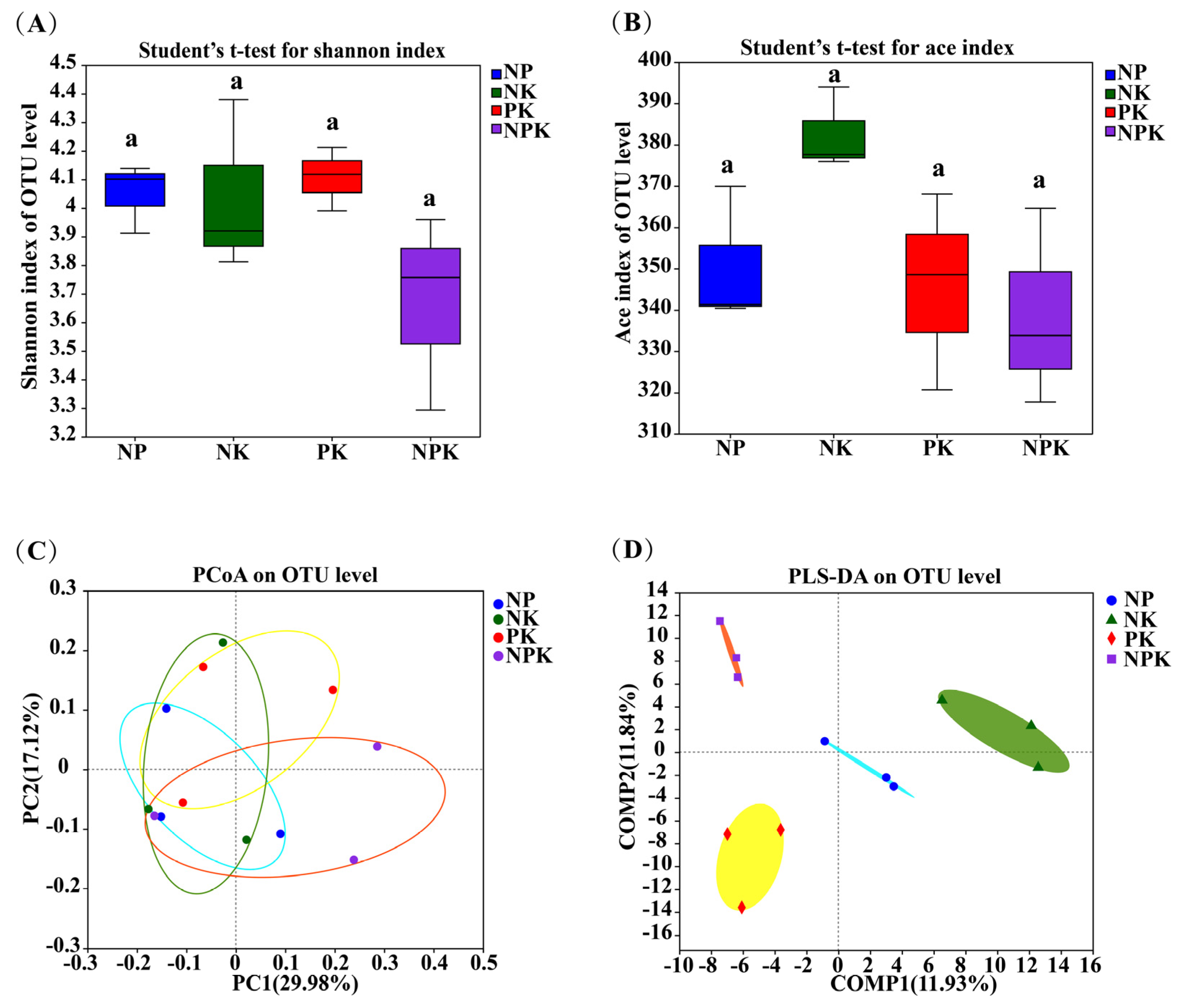
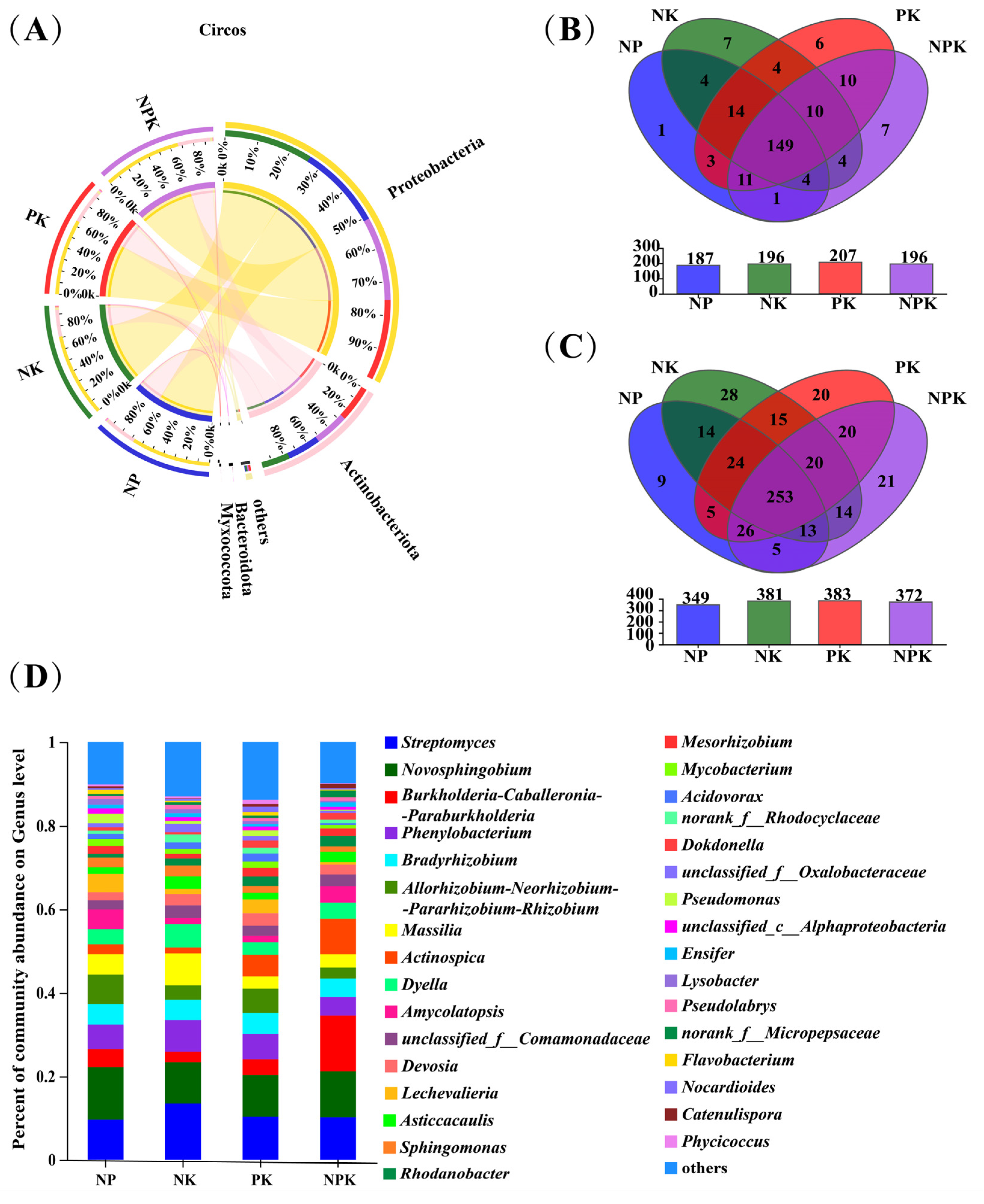
3.2. Effect of Different Fertilization Treatments on the Composition of Endophytic Bacteria in the Amaranth Root System
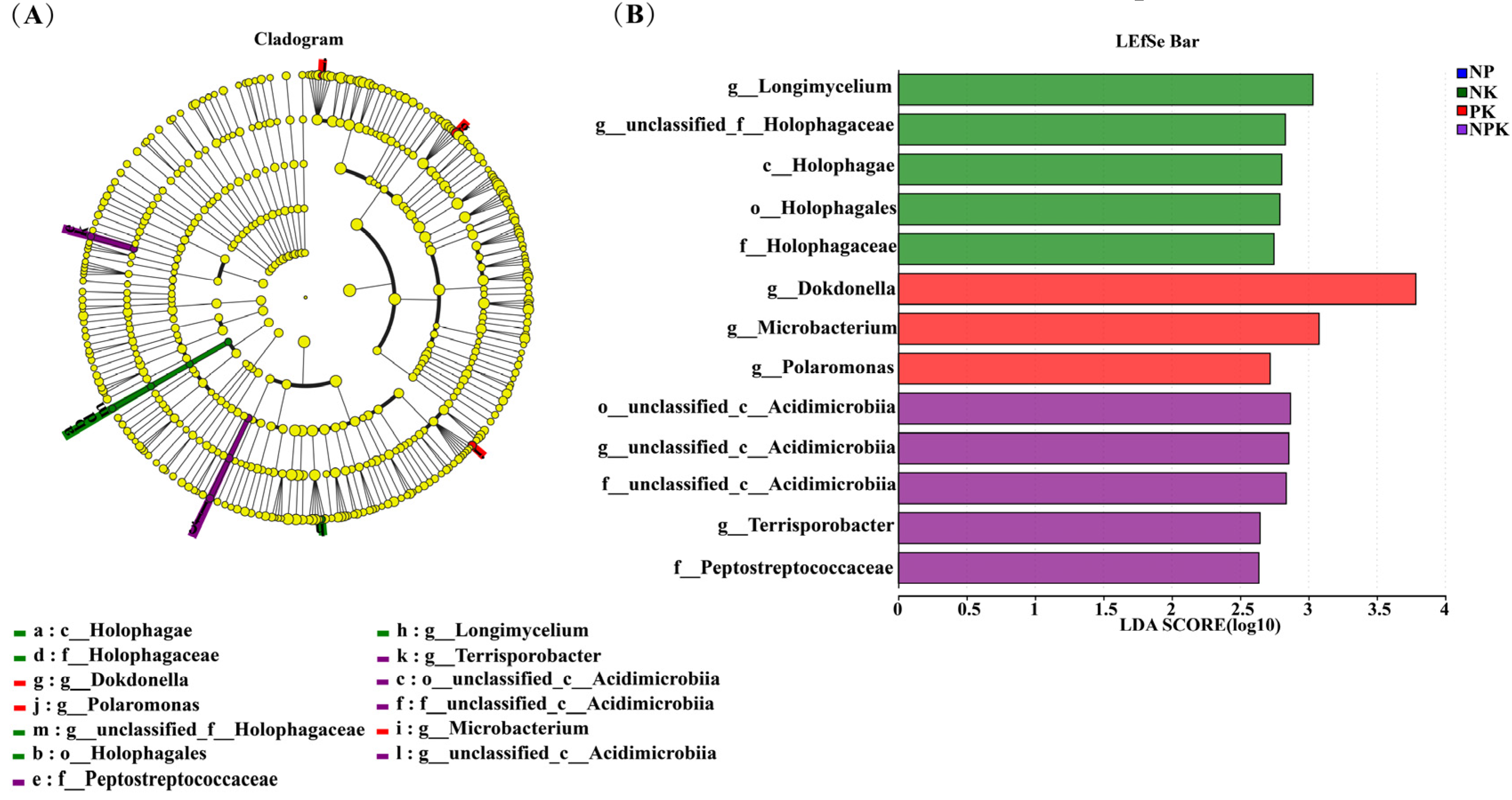
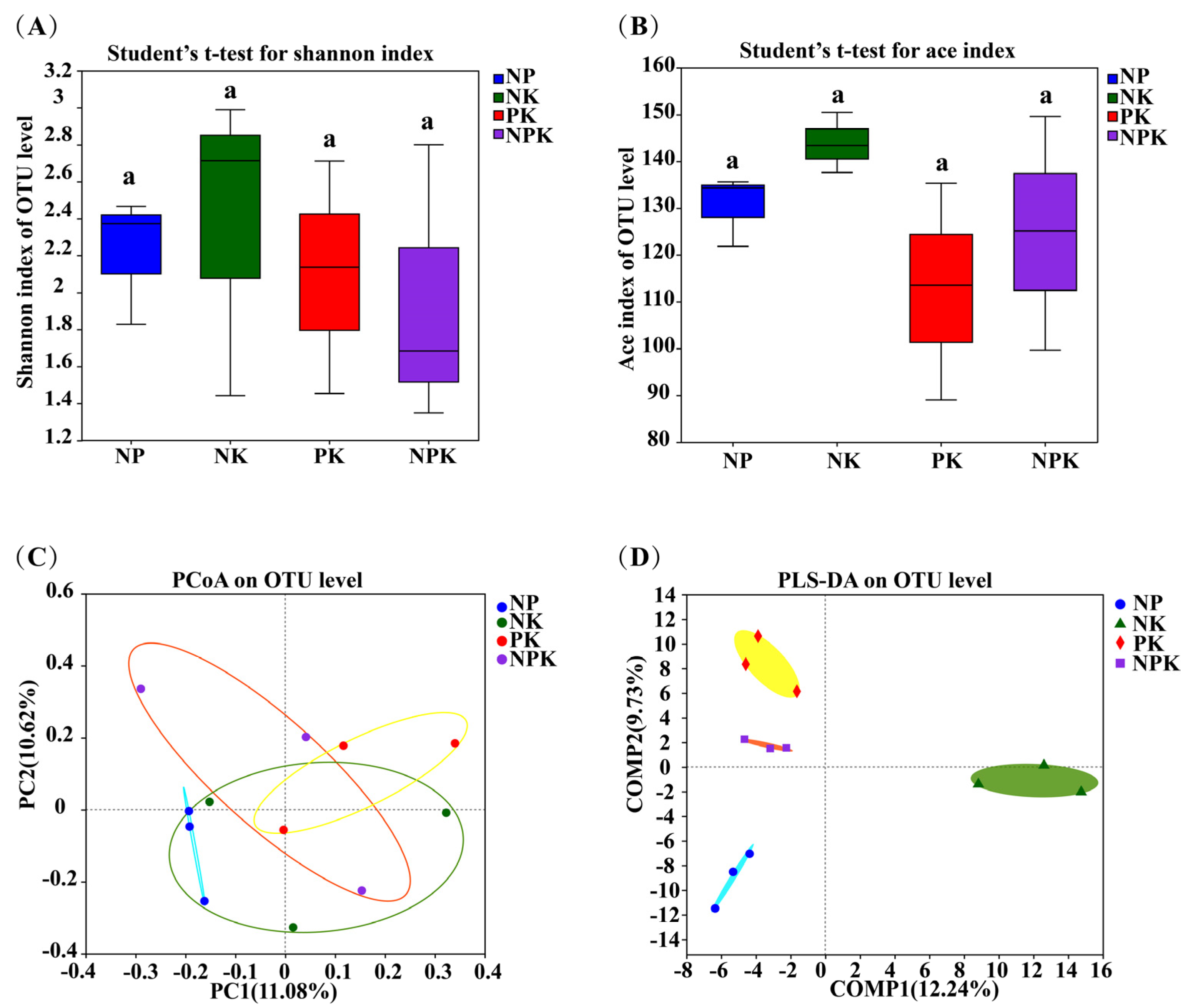
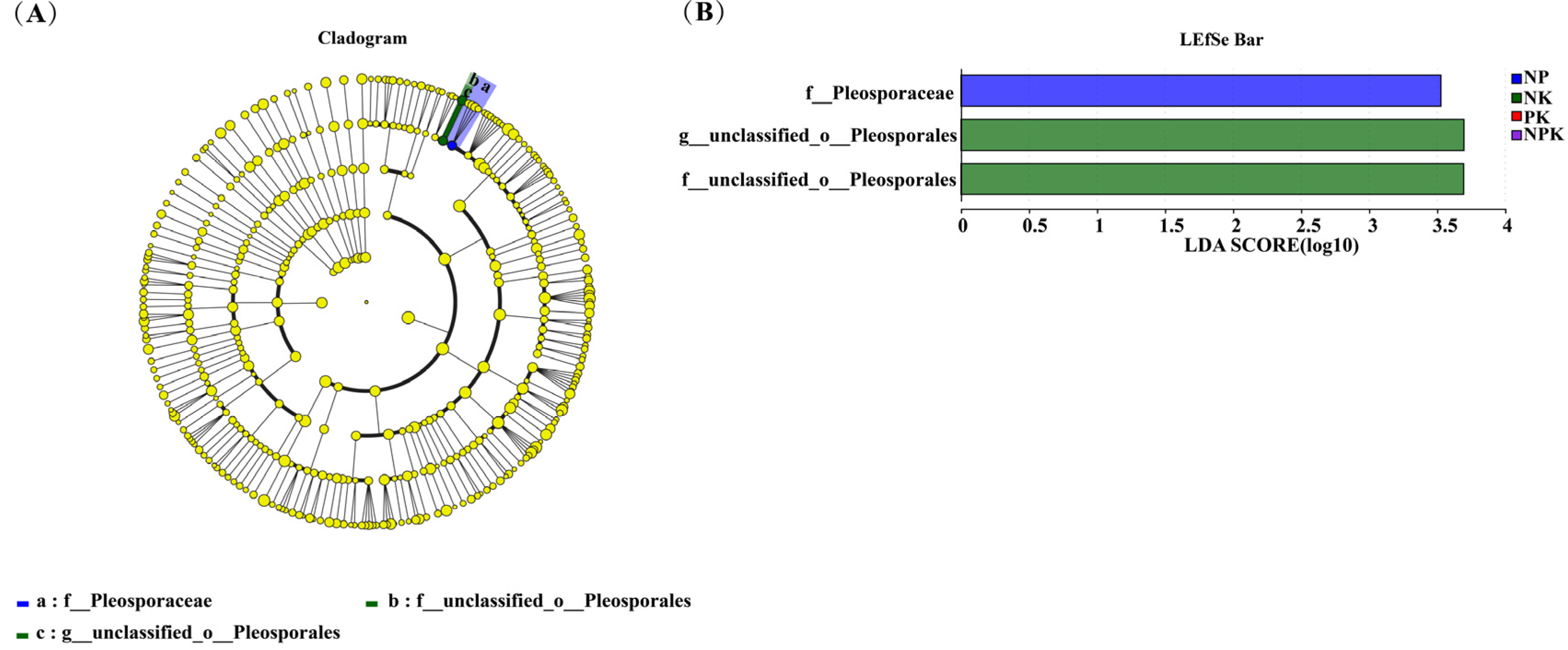
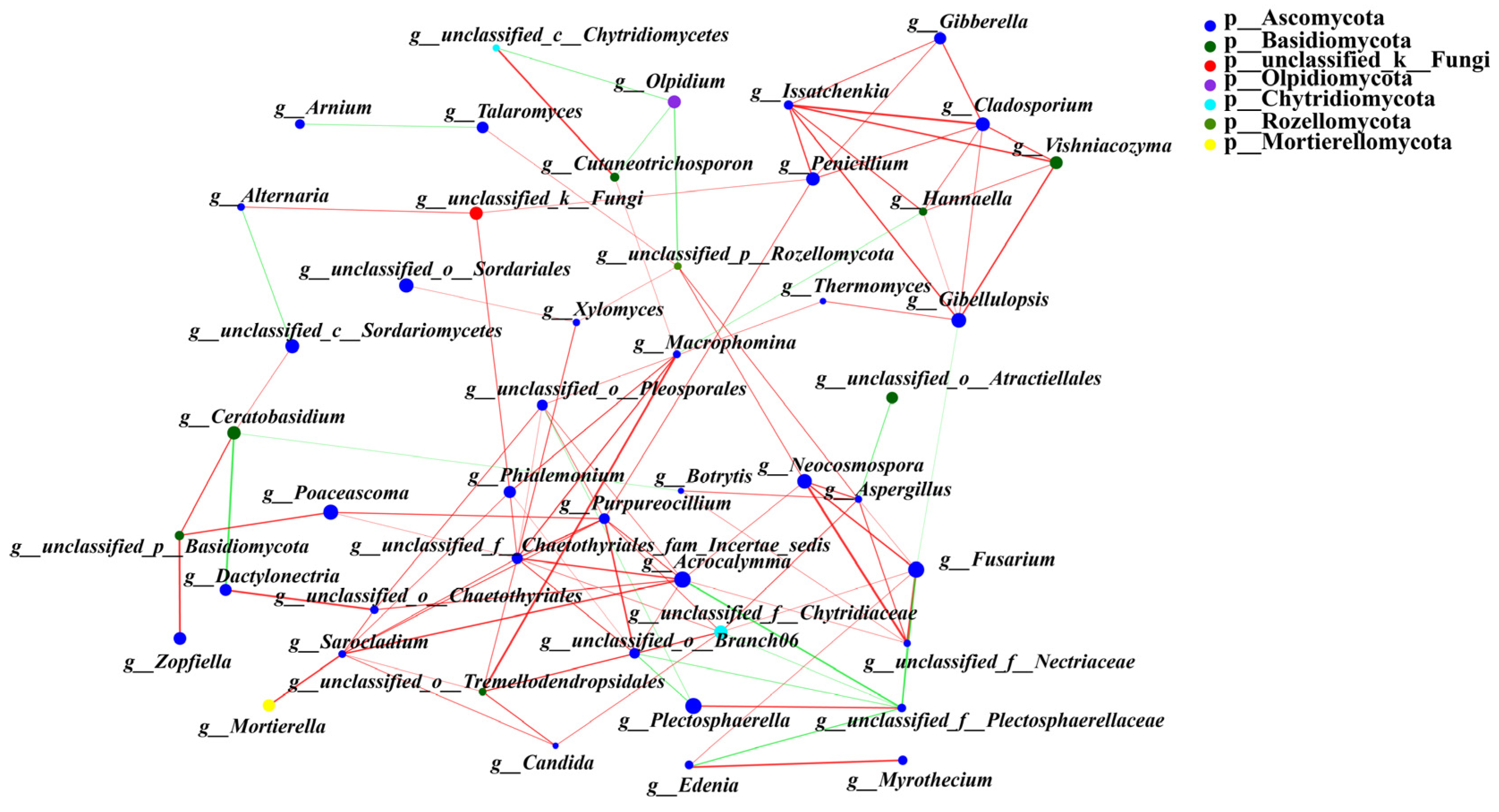
3.3. Effects of Different Fertilization Treatments on Endophytic Fungi in Amaranth Roots
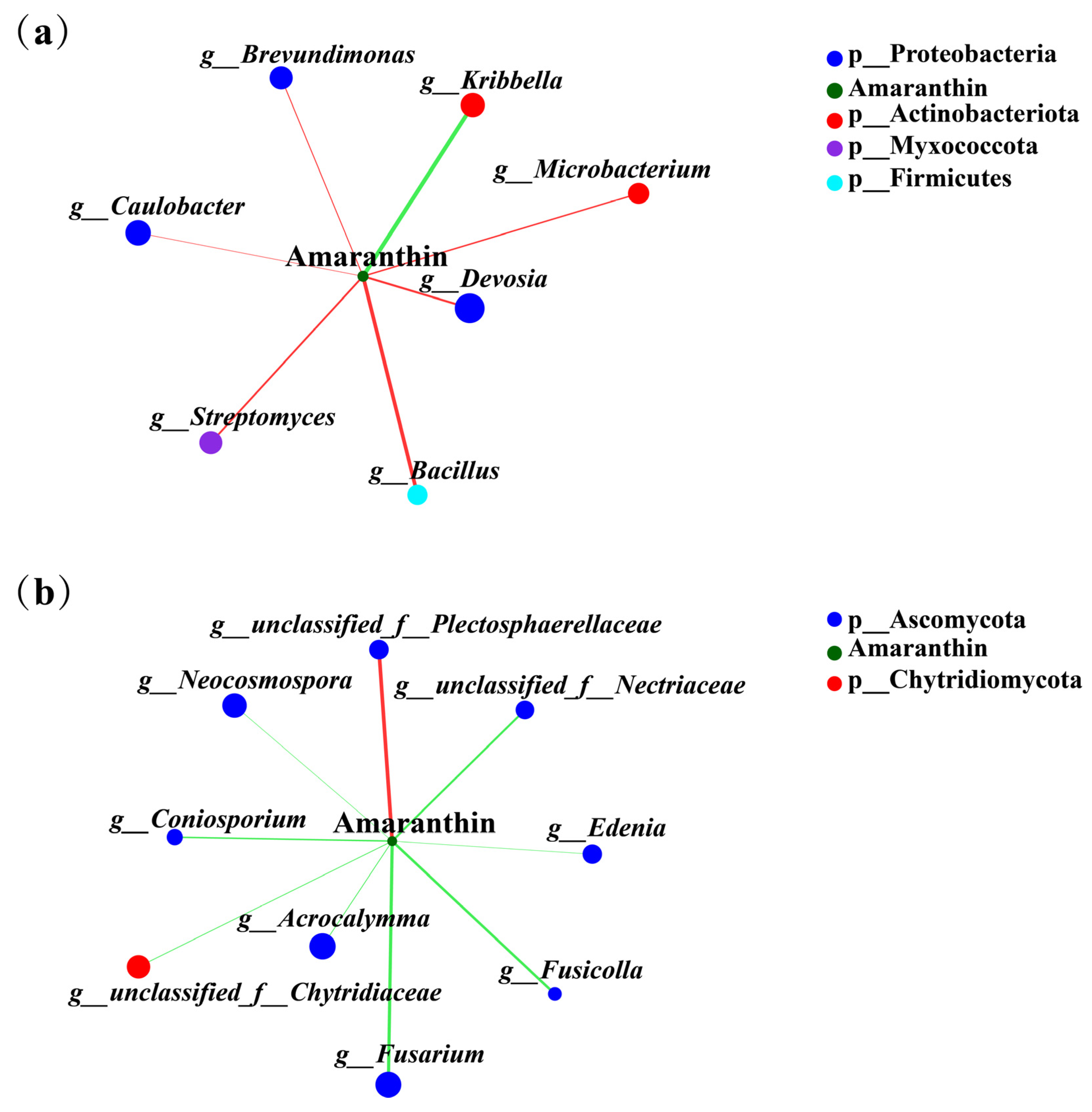
3.4. Correlation Analysis of Amaranthin with Endophytic Bacteria and Fungi
4. Discussion
4.1. Effects of Different Fertilization Treatments on the Amaranthin Content
4.2. Effects of Different Fertilization Treatments on the Endophytic Bacterial Community Structures in Amaranth Roots
4.3. Effects of Different Fertilization Treatments on the Endophytic Fungal Community Structures in Amaranth Roots
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarker, U.; Islam, M.T.; Rabbani, M.G.; Oba, S.Y. Genotypic variability for nutrient, antioxidant, yield and yield contributing traits in vegetable amaranth. J. Food Agric. Environ. 2014, 12, 168–174. Available online: https://www.researchgate.net/publication/267509472 (accessed on 10 November 2023).
- Sarker, U.; Oba, S. Nutraceuticals, antioxidant pigments, and phytochemicals in the leaves of Amaranthus spinosus and Amaranthus viridis weedy species. Sci. Rep. 2019, 9, 20413. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Islam, M.T.; Rabbani, M.G.; Oba, S. Variability, heritability and genetic association in vegetable amaranth (Amaranthus tricolor L.). Span. J. Agric. Res. 2015, 13, e0702. [Google Scholar] [CrossRef]
- Chakrabarty, T.; Sarker, U.; Hasan, M. Variability in mineral compositions, yield and yield contributing traits of stem amaranth (Amaranthus lividus). Genetika 2018, 50, 995–1010. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 2014, 15, 19–38. [Google Scholar] [CrossRef]
- Anton, G.; Živa, P.; Klemen, E.; Mihelič, R.; Suhadolc, M. Combined effects of long-term tillage and fertilization regimes on soil organic carbon, microbial biomass, and abundance of the total microbial communities and N-functional guilds. Appl. Soil. Ecol. 2023, 188, 104876. [Google Scholar] [CrossRef]
- Sebastiana, M.; Rafael JL, B.; Bellido, L.L.; Romero, V.M.; Moreno, F.; Murillo, J.M. Long-term effect of tillage, rotation and nitrogen fertilizer on soil quality in a Mediterranean Vertisol. Soil. Tillage Res. 2011, 114, 97–107. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Tang, Z.H.; Wang, J.D.; Zhang, Y.C. Long-term organic fertilization reshapes the communities of bacteria and fungi and enhances the activities of C- and P-cycling enzymes in calcareous alluvial soil. Appl. Soil. Ecol. 2024, 194, 105204. [Google Scholar] [CrossRef]
- Zhang, M.K.; Zhang, H.M. Co-transport of dissolved organic matter and heavy metals in soils induced by excessive phosphorus applications. J. Environ. Sci. 2010, 22, 598–606. [Google Scholar] [CrossRef]
- Tsavkelova, E.A.; Cherdyntseva, T.A.; Lobakova, E.S.; Kolomeĭtseva, G.L.; Netrusov, A.I. Microbiota of the orchidrhizoplane. Mikrobiologiia 2001, 70, 567–573. Available online: https://pubmed.ncbi.nlm.nih.gov/11558285/ (accessed on 10 November 2023).
- Lacava, P.T.; Bogas, A.C.; Cruz, F.P.N. Plant Growth Promotion and Biocontrol by Endophytic and Rhizospheric Microorganisms from the Tropics: A Review and Perspectives. Front. Sustain. Food Syst. 2022, 6, 796113. [Google Scholar] [CrossRef]
- Singh, C.B. Role of endophytic microorganisms in sustainable agriculture. NeBIO 2012, 3, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Segaran, G.; Sathiavelu, M. Chapter 4—Antimicrobial metabolites from endophytic microorganisms and its mode of action. Biocontrol Mech. Endophytic Microorg. 2022, 75–88, 9780323884785. [Google Scholar] [CrossRef]
- Pacifico, D.; Squartini, A.; Crucitti, D.; Barizza, E.; Lo Schiavo, F.; Muresu, R.; Carimi, F.; Zottini, M. The role of the endophytic microbiome in the grapevine response to environmental triggers. Front. Plant Sci. 2019, 10, 1256. [Google Scholar] [CrossRef] [PubMed]
- Schlaeppi, K.; Dombrowski, N.; Oter, R.G.; Themaat, E.V.L.V.; Lefert, P.S. Quantitative divergence of the bacterial root microbiota in Arabidopsis thaliana relatives. Proc. Natl. Acad. Ences 2013, 111, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Garrido-Oter, R.; Münch, P.C.; Weiman, A.; Dröge, J.; Pan, Y.; McHardy, A.C.; Schulze-Lefert, P. Structure and Function of the Bacterial Root Microbiota in Wild and Domesticated Barley. Cell Host Microbe 2015, 17, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Guo, G.; Li, M.; Liang, X.-Y.; Gu, Y.-Y. Diversity of endophytic bacteria of mulberry (Morus L.) under cold conditions. Front. Microbiol. 2022, 13, 923162. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Chen, S.; Liang, T.; Yang, S.; Tan, H. Response of endophytic bacteria in sugarcane roots to different slow-release fertilizers with dicyandiamide (DCD) and humic acid (HA) applications. Environ. Technol. Innov. 2023, 32, 103244. [Google Scholar] [CrossRef]
- Toledo, S.; Gargaglione, V.; Peri, P.L. Mineral fertilization impacts microbial activity and endophytic fungi but not microbial biomass in semiarid grasslands. Pedobiologia 2024, 102, 150929. [Google Scholar] [CrossRef]
- Sindhu, L.; Song, Y.; Li, L.; Dong, Z.; Yang, Q.; Mangi, S.S.; Guo, W.; Yang, L.; Cui, H.; Lv, S.; et al. Meta-omics revealed that nitrogen fertilization alters the endophytic fungi divergence in maize field ecosystem. Ecol. Indic. 2024, 160, 111852. [Google Scholar] [CrossRef]
- Ma, Y.; Weisenhorn, P.; Guo, X.; Wang, D.; Yang, T.; Shi, Y.; Zhang, H.; Chu, H. Effect of long-term fertilization on bacterial communities in wheat endosphere. Pedosphere 2021, 31, 538–548. [Google Scholar] [CrossRef]
- Sun, A.; Jiao, X.-Y.; Chen, Q.; Wu, A.-L.; Zheng, Y.; Lin, Y.-X.; He, J.-Z.; Hu, H.-W. Microbial communities in crop phyllosphere and root endosphere are more resistant than soil microbiota to fertilization. Soil. Biol. Biochem. 2021, 153, 108113. [Google Scholar] [CrossRef]
- Pedraza, R.O.; Bellone, C.H.; de Bellone, S.C.; Boa Sorte, P.M.F.; dos Santos Teixeira, K.R. Azospirillum inoculation and nitrogen fertilization effect on grain yield and on the diversity of endophytic bacteria in the phyllosphere of rice rainfed crop. Eur. J. Soil. Biol. 2009, 45, 36–43. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, G.; Stirling, E.; Zhang, H.; Chen, S.; Xu, C.; Zhang, X.; Ge, T.; Wang, D. Nitrogen fertilization modulates rice seed endophytic microbiomes and grain quality. Sci. Total Environ. 2023, 857 Pt 2, 159181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Cao, C.; Han, S.; Zhao, W.; Li, Q.; Liu, X.; Kong, L. Effects of fertilizer reduction coupled with straw returning on soil fertility, wheat root endophytic bacteria, and the occurrence of wheat crown rot. Front. Microbiol. 2023, 14, 1143480. [Google Scholar] [CrossRef] [PubMed]
- Cooper-Driver, G.A. Contributions of Jeffrey Harborne and coworkers to the study of anthocyanins. Phytochemistry 2001, 56, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Hazli, U.H.A.M.; Abdul-Aziz, A.; Mat-Junit, S.; Chee, C.F.; Kong, K.W. Solid-liquid extraction of bioactive compounds with antioxidant potential from Alternanthera sesillis (red) and identification of the polyphenols using UHPLC-QqQ-MS/MS. Food Res. Int. 2019, 115, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Yang, D.; Lin, X.; Zhou, X.; Li, Z.; Kurokawa, H.; Matsui, H.; Fujita, T.; Yang, S.-D. Differences in endophytic bacterial and fungal compositions in roots between red and green Amaranthus sp. S. Afr. J. Bot. 2023, 163, 275–284. [Google Scholar] [CrossRef]
- Chen, S.; Sun, Y.; Wei, Y.; Li, H.; Yang, S. Different rhizosphere soil microbes are recruited by tomatoes with different fruit color phenotypes. BMC Microbiol. 2022, 22, 210. [Google Scholar] [CrossRef] [PubMed]
- Timoneda, A.; Feng, T.; Sheehan, H.; Walker-Hale, N.; Pucker, B.; Lopez-Nieves, S.; Guo, R.; Brockington, S. The evolution of betalain biosynthesis in Caryophyllales. New Phytol. 2019, 224, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.; Wang, Z.Q.; Gong, W.H.; Yang, L.F. Optimum dosage test of nitrogen, phosphorus and potassium fertilizers in amaranth. Vegetables 2015, 2, 29–31. Available online: https://kns.cnki.net/kcms2/article/abstract?v=8WLnD7pOpNFi_PphG5xeLIX0HoKITDlbLr0AomwERBpbRyxl0XUeL5alpeP0ZXjKgk3w7DhWs_cRxEu_RQp1kIiNdqqRBO_CcSfnZHG4FpMtQ_4y0MUgBgZYKbOXggCQ5UCCNiUvae4FL7VkYSe9sA==&uniplatform=NZKPT&language=CHS (accessed on 10 November 2023).
- Xiao, J.; Sun, Y.; He, Y.; Tang, X.F.; Yang, S.D.; Huang, J.Y. Comparison of Rhizospheric and Endophytic Bacterial Compositions between Netted and Oriental Melons. Microbiol. Spectr. 2023, 11, e0402722. [Google Scholar] [CrossRef] [PubMed]
- Piattelli, M.; Nicola, M.; Castrogiovanni, V. The effect of kinetin on amaranthin synthesis in Amaranthus tricolor in the dark. Phytochemistry 1971, 10, 289–293. [Google Scholar] [CrossRef]
- Zhou, H.; Jia, S.; Gao, Y.; Li, X.; Lin, Y.; Yang, F.; Ni, K. Characterization of phyllosphere endophytic lactic acid bacteria reveals a potential novel route to enhance silage fermentation quality. Commun. Biol. 2024, 7, 117. [Google Scholar] [CrossRef]
- Meng, J.X.; Gao, Y.; Han, M.L. In vitro Anthocyanin Induction and Metabolite Analysis in Malus spectabilis Leaves Under Low Nitrogen Conditions. Hortic. Plant J. 2020, 6, 284–292. [Google Scholar] [CrossRef]
- Hassan, A. Effect of Nitrogen Fertilizer Levels in the Form of Organic, Inorganic and Bio fertilizer Applications on Growth, Yield and Quality of Strawberry. Middle E. J. Appl. Sci. 2015, 5, 604–617. [Google Scholar]
- Neshev, N.; Manolov, I. Influence of mineral fertilization on the leaf plastid pigment content and tuber quality of potatoes. Agrar. Nauk. 2015, 7, 35–40. [Google Scholar]
- Stobart, A.K.; Hendry GA, F.; Ei-Hussein, S.; Kinsman, L.T. The Effect of Potassium on Amaranthin Synthesis in Seedlings of Amaranthus caudatus L. Z. Für Pflanzenphysiol. 1980, 96, 217–225. [Google Scholar] [CrossRef]
- Romero, E.M.; Marina, M.; Pieckenstain, F.L. The communities of tomato (Solanum lycopersicum L.) leaf endophytic bacteria, analysed by 16S-ribosomal RNA gene pyrosequencing. Fems Microbiol. Lett. 2014, 351, 187–194. [Google Scholar] [CrossRef]
- Natacha, B.; Horton, M.W.; Joy, B. Bacterial Communities Associated with the Leaves and the Roots of Arabidopsis thaliana. PLoS ONE 2013, 8, e56329. [Google Scholar] [CrossRef]
- Bakker, M.G.; Manter, D.K.; Sheflin, A.M.; Weir, T.L.; Vivanco, J.M. Harnessing the rhizosphere microbiome through plant breeding and agricultural management. Plant Soil. 2012, 360, 1–13. [Google Scholar] [CrossRef]
- Qin, S.; Xing, K.; Jiang, J.H.; Xu, L.H.; Li, W.J. Biodiversity, bioactive natural products and biotechnological potential of plant-associated endophytic actinobacteria. Appl. Microbiol. Biotechnol. 2011, 89, 457–473. [Google Scholar] [CrossRef]
- Bérdy, J. Erratum: Thoughts and facts about antibiotics: Where we are now and where we are heading. J. Antibiot. 2012, 65, 441. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Ning, P.; Zheng, L.; Huang, J.B.; Li, G.Q.; Hsiang, T. Fumigant activity of volatiles of Strep-T. globisporus JK-1 against Penicillium italicum on Citrus microcarpa. Postharvest Biol. Technol. 2010, 58, 157–165. [Google Scholar] [CrossRef]
- Polturak, A.A. “La Vie en Rose”: Biosynthesis, sources, and applications of betalain pigments. Mol. Plant 2018, 11, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zheng, X.; Pan, J.; Peng, L.; Cheng, C.; Wang, X.; Zhao, C.; Zhang, Z.; Lin, Y.; Xu, X.; et al. RNA-sequencing analysis reveals betalain metabolism in the leaf of Amaranthus tricolor L. PLoS ONE 2019, 14, e0216001. [Google Scholar] [CrossRef]
- Kim, D. Streptomyces cytochrome P450 enzymes and their roles in the biosynthesis of macrolide therapeutic agents. Biomol. Ther. 2019, 27, 127–133. [Google Scholar] [CrossRef]
- Nzuza, N.; Padayachee, T.; Syed, P.R.; Kryś, J.D.; Chen, W.; Gront, D.; Nelson, D.R.; Syed, K. Ancient bacterial class Alphaproteobacteria cytochrome P450 monooxygenases can be found in other bacterial species. Int. J. Mol. Sci. 2021, 22, 5542. [Google Scholar] [CrossRef]
- Wu, Y.C.; Yuan, J.; Yao, Y.E. Effects of volatile organic compounds from Streptomyces albulus NJZJSA2 on growth of two fungal pathogens. J. Basic. Microbiol. 2015, 55, 1104–1117. [Google Scholar] [CrossRef]
- Boukaew, S.; Cheirsilp, B.; Prasertsan, P.; Yossan, S. Antifungal effect of volatile organic compounds produced by Streptomyces salmonis PSRDC-09 against the anthracnose pathogen Colle totric hum gloeos porioides PSU-03 in postharvest chili fruit. J. Appl. Microbiol. 2021, 131, 1452–1463. [Google Scholar] [CrossRef]
- Sohn, J.H.; Kwon, K.K.; Kang, J.H.; Jung, H.B.; Kim, S.J. Novosphingobium pentaromativorans sp. nov., a high-molecular-mass polycyclic aromatic hydrocarbon-degrading bacterium isolated from estuarine sediment. Int. J. Syst. Evol. Microbiol. 2004, 54 Pt 5, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Pang, S.; Zhou, Z.; Wu, X.; Li, J.; Huang, Y.; Zhang, W.; Lei, Q.; Bhatt, P.; Mishra, S.; et al. Novel pathway of acephate degradation by the microbial consortium ZQ01 and its potential for environmental bioremediation. J. Hazard. Mater. 2022, 426, 127841. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Hu, L.; Shen, W. The involvement of α-proteobacteria Phenylobacterium in maintaining the dominance of toxic Microcystis blooms in Lake Taihu, China. Environ. Microbiol. 2020, 23, 1066–1078. [Google Scholar] [CrossRef]
- Bi, X.Y.; Li, X.D.; Yu, H.B. Development of a multiplex RT‒PCR assay for simultaneous detection of Cucumber green mottle mosaic virus and Acidovorax citrulli in watermelon. PeerJ 2019, 22, e7539. [Google Scholar] [CrossRef] [PubMed]
- Lay, C.Y.; Hamel, C.; St-Arnaud, M. Taxonomy and pathogenicity of Olpidium brassicae and its allied species. Fungal Biol. 2018, 122, 837–846. [Google Scholar] [CrossRef] [PubMed]
- James, T.Y.; Letcher, P.M.; Longcore, J.E.; Mozley-Standridge, S.E.; Porter, D.; Powell, M.J. A molecular phylogeny of the flagellated fungus (Chytridiomycota) and description of a new phylum (Blastocladiomycota). Mycologia 2006, 98, 860–871. [Google Scholar] [CrossRef]
- Ngoh, D.J.; Patrice, D.B.; Djongnang, G. Brown Spot and Stalk Rot Diseases of Maize (Zea Mays) and Susceptibility of Two Varieties to Physoderma maydis in Far North Cameroon. Int. J. Phytopathol. 2021, 10, 19–28. [Google Scholar] [CrossRef]
- Nikandrow, A. Acrocalymma medicaginis and Phomopsis sp. as Causal Agents of Crown Rot of Lucerne in Australia. J. Phytopathol. 2010, 130, 24–36. [Google Scholar] [CrossRef]
- Pan, R.; Deng, Q.; Deng, M.; Guan, M.; Xu, D.; Gai, Y.; Chen, W.; Yang, Y. This paper provides the first report of peanut foot rot caused by Neocosmospora vasinfecta in mainland China. Plant Pathol. 2010, 59, 1172. [Google Scholar] [CrossRef]
- Luo, H.; Hallen-Adams, H.E.; Walton, J.D. Processing of the phalloidin proprotein by prolyloligopeptidase from the mushroom conocybe albipes. J. Biol. Chem. 2009, 284, 18070–18077. [Google Scholar] [CrossRef] [PubMed]
- Nancy, J.A.; Robert, H.P.; Susan, P.M. Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. Toxin Rev. 2009, 28, 198–215. [Google Scholar] [CrossRef]
- Hassan, H.A.H. Gibberellin and auxin production plant root fungi and their biosynthesis under salinity-calcium interaction. Rostl. Vyrob. 2002, 48, 101–106. [Google Scholar] [CrossRef]
- Kinsman, L.T.; Pinfield, N.J.; Stobart, A.K. The hormonal control of amaranthin synthesis in Amaranthus caudatus seedlings. Planta 1975, 127, 207–212. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Z.; Guo, Q. This is the first report of wilt of sugar beet caused by G. nigrescens in the Xinjiang Region of China. Plant Dis. 2017, 101, 1318. [Google Scholar] [CrossRef]
- Avasthi, S.; Gautam, A.K.; Bhadauria, R. This article provides the first report of Cladosporium sphaerospermum causing leaf spot disease in Aloe vera in India. Crop Prot. 2016, 5, 649–654. [Google Scholar] [CrossRef]
- Manici, L.M.; Caboni, E.; Caputo, F.; Frattarelli, A.; Lucioli, S. Phytotoxins from Dactylonectria torresensis involved in replant disease of fruit trees. Rhizosphere 2021, 17, 100300. [Google Scholar] [CrossRef]
- Erper, I.; Ozer, G.; Alkan, M.; Zholdoshbekova, S.; Turkkan, M. First report of Dactylonectria torresensis causing black root rot in strawberry plants in Kyrgyzstan. J. Plant Pathol. 2021, 103, 379–380. [Google Scholar] [CrossRef]
- Hsuan, H.M.; Salleh, B.; Zakaria, L. Molecular Identification of Fusarium Species in Gibberella fujikuroi Species Complex from Rice, Sugarcane and Maize from Peninsular Malaysia. Int. J. Mol. Sci. 2011, 12, 6722–6732. [Google Scholar] [CrossRef]


| Treatment | N Fertilizer (mg/kg) | P Fertilizer (mg/kg) | K Fertilizer (mg/kg) |
|---|---|---|---|
| NP | 83.6 | 23.6 | - |
| NK | 83.6 | - | 22.2 |
| PK | - | 23.6 | 22.2 |
| NPK | 83.6 | 23.6 | 22.2 |
| Treatment | Amaranthin Content (mg/g FW) |
|---|---|
| NP | 0.48 ± 0.11 b |
| NK | 0.70 ± 0.28 ab |
| PK | 0.81 ± 0.05 ab |
| NPK | 0.84 ± 0.12 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Yang, D.; Zhou, X.; Wei, X.; Xie, Y.; Yang, S. Response of the Endophytic Microbial Composition in Amaranthus Roots to Different Fertilization Treatments. Agronomy 2024, 14, 965. https://doi.org/10.3390/agronomy14050965
Lin X, Yang D, Zhou X, Wei X, Xie Y, Yang S. Response of the Endophytic Microbial Composition in Amaranthus Roots to Different Fertilization Treatments. Agronomy. 2024; 14(5):965. https://doi.org/10.3390/agronomy14050965
Chicago/Turabian StyleLin, Xinru, Da Yang, Xinyan Zhou, Xun Wei, Yuanyuan Xie, and Shangdong Yang. 2024. "Response of the Endophytic Microbial Composition in Amaranthus Roots to Different Fertilization Treatments" Agronomy 14, no. 5: 965. https://doi.org/10.3390/agronomy14050965





