Comparative Analysis of Biodegradable Mulches on Soil Bacterial Community and Pepper Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Location, Climate, and Soil
2.1.2. Experimental Design of Plastic Mulch and Plot Trials
2.1.3. Soil Sample Collection
2.2. Methods
2.2.1. Plastic Mulch Degradation and Soil Temperature
2.2.2. Measurement of Agronomic Traits of Chili Pepper
2.2.3. Measurement of Soil Enzyme Activity
2.2.4. Soil Chemical Property Testing
2.2.5. Soil Total DNA Extraction and High-Throughput Sequencing and Analysis
2.2.6. Statistical Analysis
3. Results
3.1. Degradation Characteristics of Plastic Mulch
3.2. The Influence of Plastic Mulch on the Agronomic Traits and Yield of Pepper
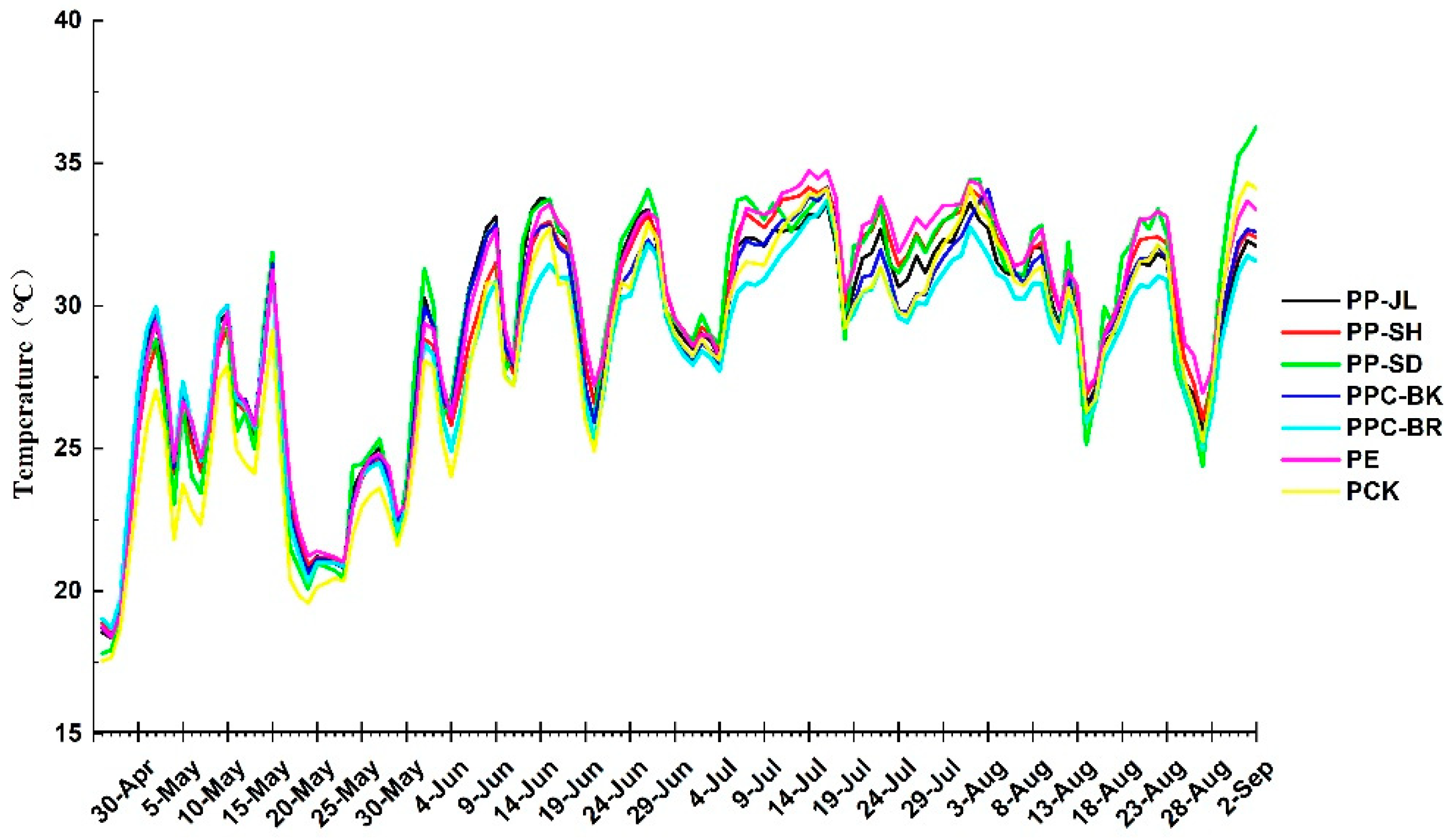
3.3. The Influence of Plastic Mulch on Soil Temperature
3.4. The Impact of Plastic Mulch on Soil Enzyme Activity
3.5. The Impact of Plastic Mulch Covering on Soil Bacterial Community Diversity
3.6. Analysis of the Correlation between Environmental Factors and Microbial Community
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, H.; Yan, C.; Liu, Q.; Ding, W.; Chen, B.; Li, Z. Effects of plastic mulching and plastic residue on agricultural production: A meta-analysis. Sci. Total Environ. 2019, 651, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Hinojosa, A.; Karlsen-Ayala, E.; Boyd, N.S.; Strauss, S.L. Impact of repeated fumigant applications on soil properties, crop yield, and microbial communities in a plastic-mulched tomato production system. Sci. Total Environ. 2024, 919, 170659. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, C.; Zhang, P.; Jia, Z. Long-term effects of plastic mulching on soil structure, organic carbon and yield of rainfed maize. Agr. Water Manag. 2023, 287, 108447. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A.; Fatima Rizvi, Z. Impact of plastic mulching as a major source of microplastics in agroecosystems. J. Hazard. Mater. 2023, 445, 130455. [Google Scholar] [CrossRef] [PubMed]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug. Deliver. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tian, Y.; Wang, J.; Zhang, R.; Zhu, J. Enhanced degradation and gas barrier of PBAT through composition design of aliphatic units. Polym. Degrad. Stab. 2022, 195, 109795. [Google Scholar] [CrossRef]
- Chen, G.Q.; Albertsson, A.-C. Polyhydroxyalkanoates and Other Biopolymers. Biomacromolecules 2019, 20, 3211–3212. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Ruiz, H.; Martin-Closas, L.; Pelacho, A.M. Biodegradable plastic mulches: Impact on the agricultural biotic environment. Sci. Total Environ. 2021, 750, 141228. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Galafassi, S.; Di Pippo, F.; Pojar, I.; Massarelli, C.; Uricchio, V.F. A critical review of biodegradable plastic mulch films in agriculture: Definitions, scientific background and potential impacts. TrAC Trend Anal. Chem. 2024, 170, 117391. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Liu, J.; Liu, X.; Dong, Y.; Huang, X.; Zhen, Z.; Lv, J.; He, W. Degradability and properties of PBAT-based biodegradable mulch films in field and their effects on cotton planting. Polymers 2022, 14, 3157. [Google Scholar] [CrossRef]
- Serrano-Ruiz, H.; Martin-Closas, L.; Pelacho, A.M. Impact of buried debris from agricultural biodegradable plastic mulches on two horticultural crop plants: Tomato and lettuce. Sci. Total Environ. 2023, 856, 159167. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, H.; Jin, T.; Liu, H.; Men, J.; Cai, G.; Cernava, T.; Duan, G.; Jin, D. Biodegradable mulch films significantly affected rhizosphere microbial communities and increased peanut yield. Sci. Total Environ. 2023, 871, 162034. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Flury, M.; Schaeffer, S.M.; Chang, Y.; Tao, Z.; Jia, Z.; Li, S.; Ding, F.; Wang, J. Agronomic performance of polyethylene and biodegradable plastic film mulches in a maize cropping system in a humid continental climate. Sci. Total Environ. 2021, 786, 147460. [Google Scholar] [CrossRef]
- Somanathan, H.; Sathasivam, R.; Sivaram, S.; Mariappan Kumaresan, S.; Muthuraman, M.S.; Park, S.U. An update on polyethylene and biodegradable plastic mulch films and their impact on the environment. Chemosphere 2022, 307, 135839. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, S.; Martín-Closas, L.; Pelacho, A.M.; DeBruyn, J.M.J.F.i.M. Biodegradable plastic mulch films: Impacts on soil microbial communities and ecosystem functions. Front. Microbiol. 2018, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, S.; Sintim, H.; DeBruyn, J. Effects of biodegradable plastic film mulching on soil microbial communities in two agroecosystems. PeerJ 2020, 8, e9015. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, S.; Liquet y Gonzalez, J.; Henderson, K.; Anunciado, M.; Hayes, D.; DeBruyn, J. Soil microbial communities associated with biodegradable plastic mulch films. Front. Microbiol. 2020, 11, 2840. [Google Scholar] [CrossRef]
- Abbate, C.; Scavo, A.; Pesce, G.R.; Fontanazza, S.; Restuccia, A.; Mauromicale, G. Soil bioplastic mulches for agroecosystem sustainability: A comprehensive review. Agriculture 2023, 13, 197. [Google Scholar] [CrossRef]
- Chen, N.; Li, X.; Shi, H.; Yuehong, Z.; Hu, Q.; Sun, Y.n. Modeling effects of biodegradable film mulching on evapotranspiration and crop yields in Inner Mongolia. Agr. Water Manag. 2023, 275, 107996. [Google Scholar] [CrossRef]
- Men, J.; Liu, H.; Jin, T.; Cai, G.; Cao, H.; Cernava, T.; Jin, D. The color of biodegradable mulch films is associated with differences in peanut yield and bacterial communities. Environ. Res. 2024, 248, 118342. [Google Scholar] [CrossRef]
- Quezada, M.; Maria, R.; Munguia, L.; Juan, P.; Linares, C. Plastic mulching and availability of soil nutrients in cucumber crop. Terra 1995, 13, 136–147. [Google Scholar]
- Bergstrom, D.; Monreal, C.; King, D. Sensitivity of soil enzyme activities to conservation practices. Soil Sci. Soc. Am. J. 1998, 62, 1286–1295. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Maspons, J.; Molowny-Horas, R.; Fernández-Martínez, M.; Janssens, I.; Richter, A.; Ciais, P.; Obersteiner, M.; Penuelas, J. The effect of global change on soil phosphatase activity. Glob. Chang. Biol. 2021, 27, 5989–6003. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Lauber, C.; Ramirez, K.; Zaneveld, J.; Bradford, M.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities. ISME J. 2011, 6, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Kielak, A.; Pijl, A.S.; van Veen, J.A.; Kowalchuk, G.A. Phylogenetic diversity of Acidobacteria in a former agricultural soil. ISME J. 2009, 3, 378–382. [Google Scholar] [CrossRef]
- Macchi, M.; Martinez, M.; Tauil, R.; Valacco, P.; Morelli, I.; Coppotelli, B. Insights into the genome and proteome of Sphingomonas paucimobilis strain 20006FA involved in the regulation of polycyclic aromatic hydrocarbon degradation. World J. Microb. Biot. 2017, 34, 7. [Google Scholar] [CrossRef]
- Pathak, V.M. Review on the current status of polymer degradation: A microbial approach. Bioresour. Bioprocess. 2017, 4, 15. [Google Scholar] [CrossRef]
- Wufuer, R.; Li, W.; Wang, S.; Duo, J. Isolation and degradation characteristics of PBAT film degrading bacteria. Int. J. Environ. Res. Public Health 2022, 19, 17087. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.; Lauber, C.; Lozupone, C.; Caporaso, J.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an Arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Feng, Y.; Wang, L.; Xiao, X.; Xi, Y.; Luo, X.; Sun, R.; Ye, X.; Huang, Y.; et al. Consistent responses of the microbial community structure to organic farming along the middle and lower reaches of the Yangtze River. Sci. Rep. 2016, 6, 35046. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, Z.; Gharavi, S.; Soudi, M.; Moosavi-Nejad, Z. A survey of intact low-density polyethylene film biodegradation by terrestrial Actinobacterial species. Int. Microbiol. 2021, 24, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Huerta Lwanga, E.; Thapa, B.; Yang, X.; Gertsen, H.; Salánki, T.; Geissen, V.; Garbeva, P. Decay of low-density polyethylene by bacteria extracted from earthworm’s guts: A potential for soil restoration. Sci. Total Environ. 2018, 624, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Hiessl, S.; Steinbüchel, A. Functional diversity of Nocardia in metabolism. Environ. Microbiol. 2013, 16, 29–48. [Google Scholar] [CrossRef] [PubMed]




| May | June | July | |
|---|---|---|---|
| Monthly rainfall | 173.8 mm | 13 mm | 57.9 mm |
| Minimum air temperature | 15 °C | 16 °C | 25 °C |
| Maximum air temperature | 34 °C | 37 °C | 37 °C |
| Average air temperature | 23 °C | 28 °C | 30 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, T.; Li, L.; Peng, K.; Li, W.; Jin, D.; Chen, W.; Peng, J. Comparative Analysis of Biodegradable Mulches on Soil Bacterial Community and Pepper Cultivation. Agronomy 2024, 14, 905. https://doi.org/10.3390/agronomy14050905
Jin T, Li L, Peng K, Li W, Jin D, Chen W, Peng J. Comparative Analysis of Biodegradable Mulches on Soil Bacterial Community and Pepper Cultivation. Agronomy. 2024; 14(5):905. https://doi.org/10.3390/agronomy14050905
Chicago/Turabian StyleJin, Tuo, Lin Li, Kewei Peng, Wei Li, Decai Jin, Wu Chen, and Jianwei Peng. 2024. "Comparative Analysis of Biodegradable Mulches on Soil Bacterial Community and Pepper Cultivation" Agronomy 14, no. 5: 905. https://doi.org/10.3390/agronomy14050905






