Mitigating Effect of Exogenous Melatonin on Salt and Drought Stress in Cyperus esculentus L. during the Tillering Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Measurement Items and Methods
2.2.1. Determination of Plant Morphological Indicators and Biomass
2.2.2. Indicators of Osmoregulation in Blade
2.2.3. Antioxidant System Activity of Blade
2.3. Statistical Analysis
3. Results
3.1. Effects of Exogenous Melatonin on Morphological Indicators and Biomass in C. esculentus under Salt and Drought Stress
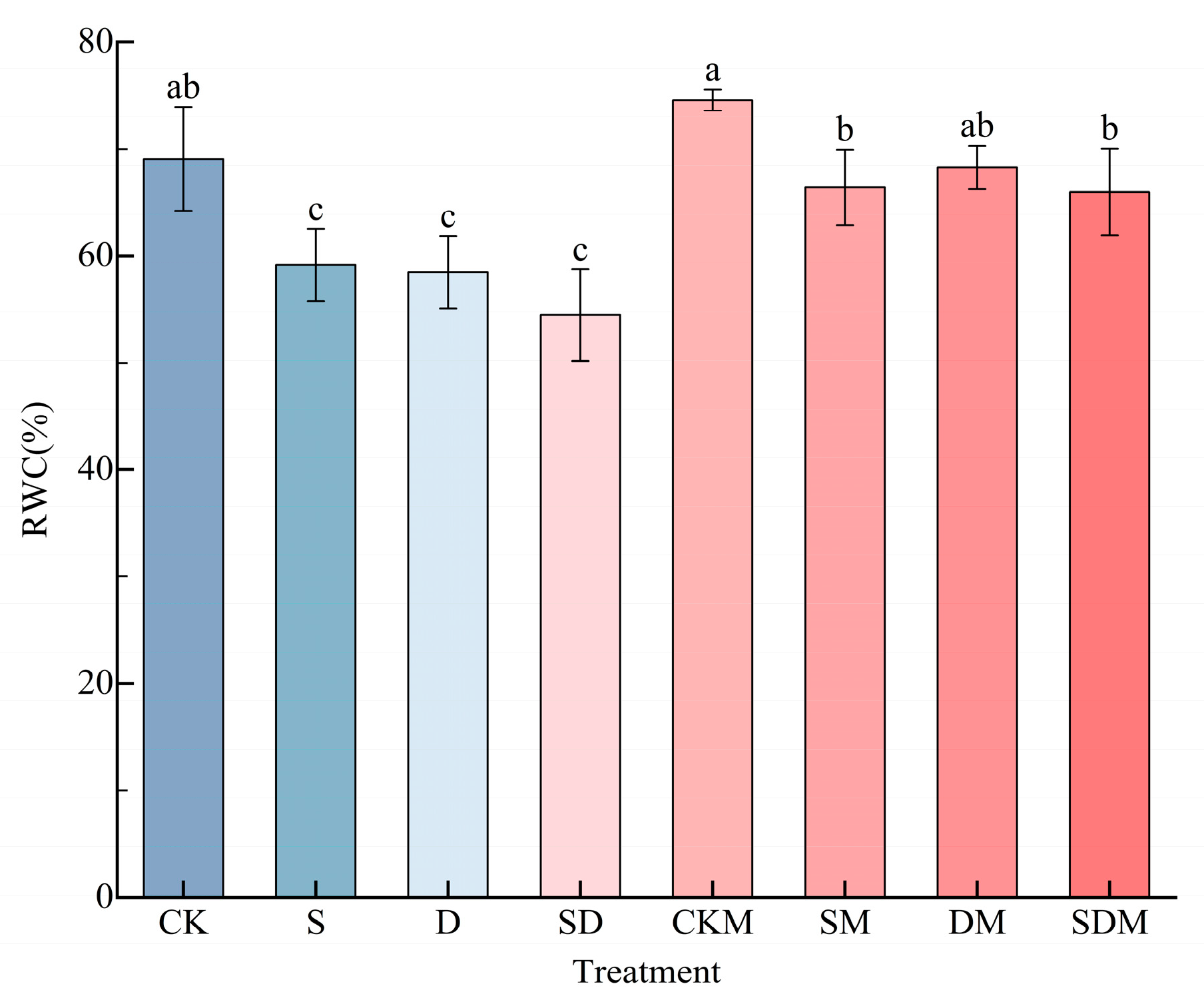
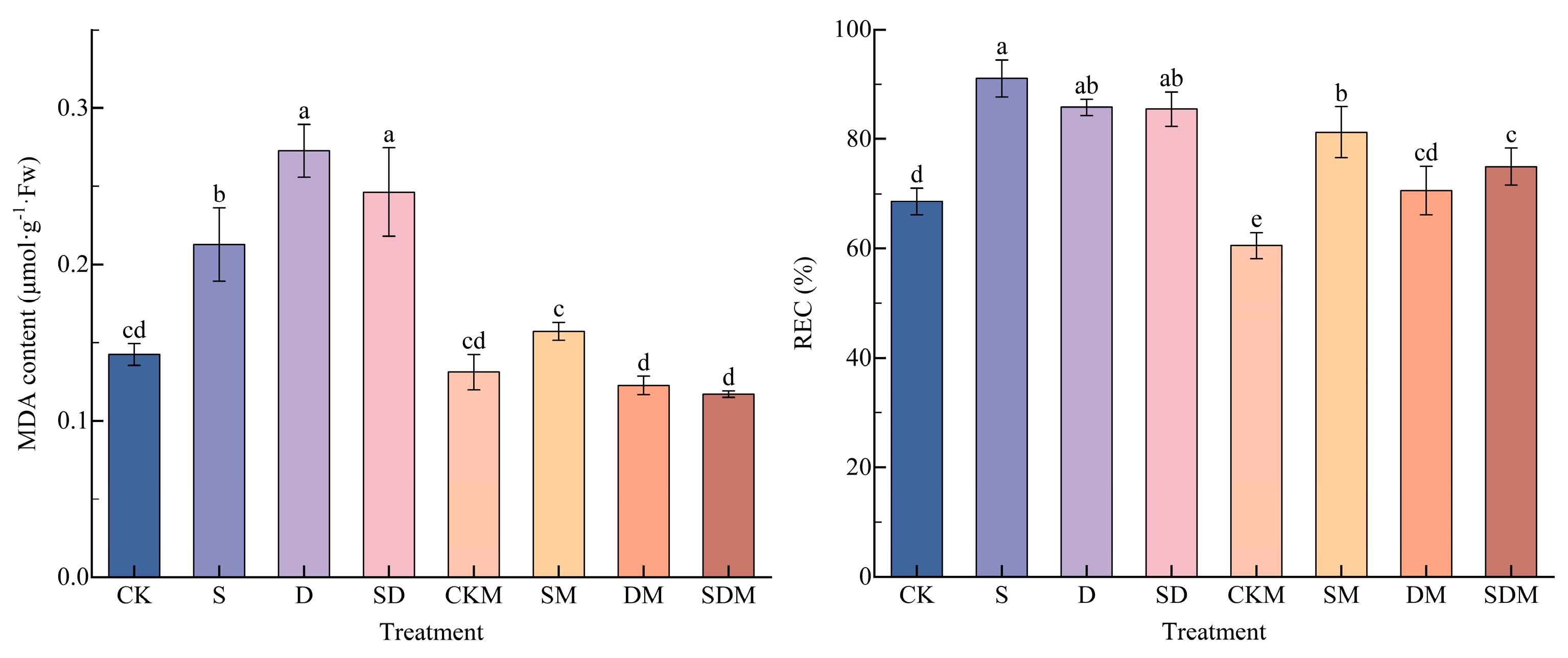
3.2. Effects of Exogenous Melatonin on the Osmotic Regulation Index of Blades in C. esculentus under Salt and Drought Stress
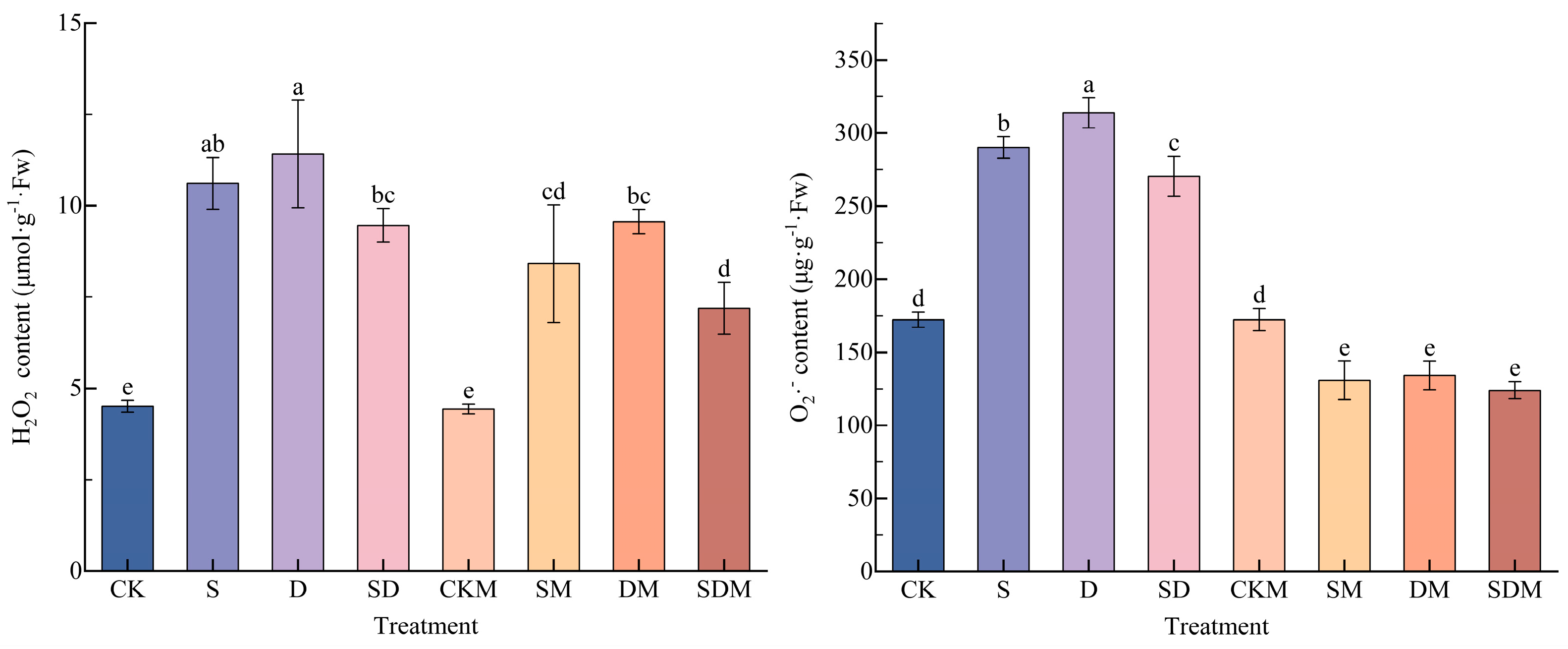
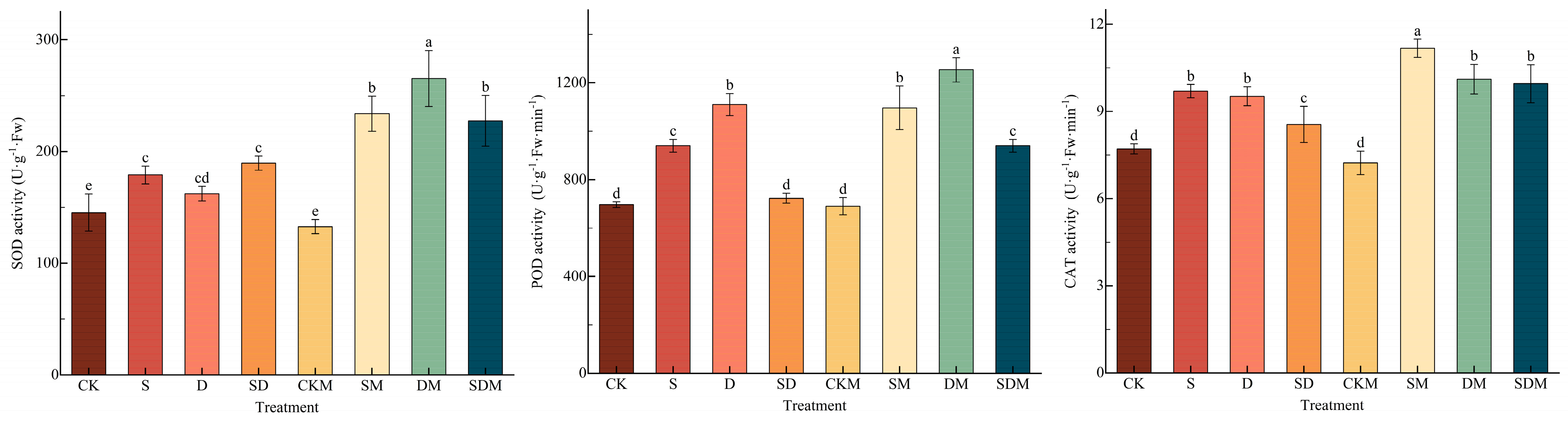
3.3. Effects of Exogenous Melatonin on the Antioxidant System Activity of Blades of C. esculentus under Salt and Drought Stress
3.4. Evaluation of Stress Resistance of Cyperus esculentus Seedlings
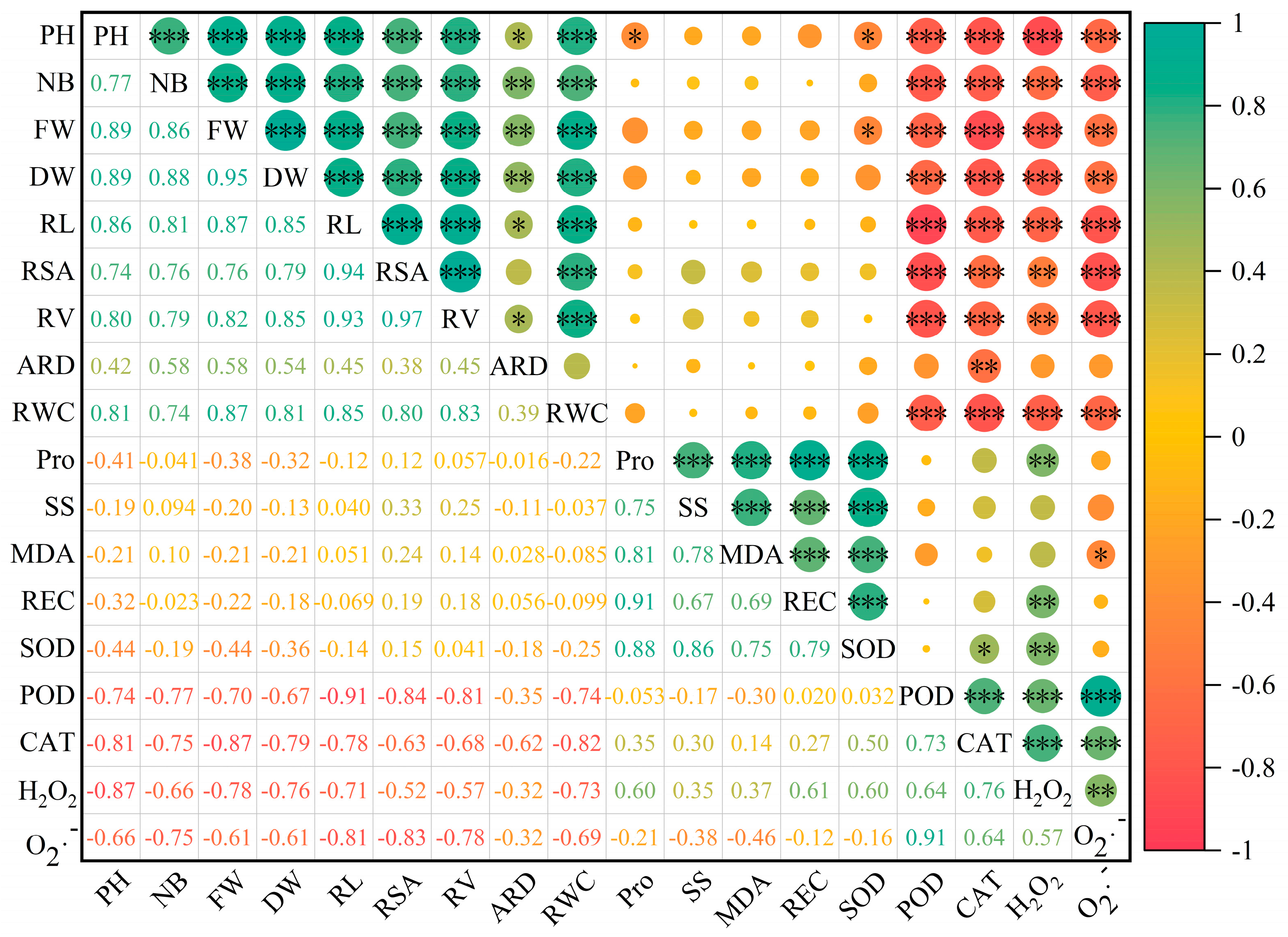
3.4.1. Correlation Analysis
3.4.2. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Zelm, E.V.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Wicke, B.; Smeets, E.; Dornburg, V.; Vashev, B.; Gaiser, T.; Turkenburg, W.; Faaij, A. Correction: The global technical and economic potential of bioenergy from salt-affected soils. Energy Environ. Sci. 2020, 13, 2585. [Google Scholar] [CrossRef]
- Neupane, D.; Adhikari, P.; Bhattarai, D.; Rana, B.; Ahmed, Z.; Sharma, U.; Adhikari, D. Does climate change affect the yield of the top three cereals and food security in the world? Earth 2022, 3, 45–71. [Google Scholar] [CrossRef]
- Paul, K.; Pauk, J.; Kondic-Spika, A.; Grausgruber, H.; Allahverdiyev, T.; Sass, L.; Vass, I. Co-occurrence of mild salinity and drought synergistically enhances biomass and grain retardation in wheat. Front. Plant Sci. 2019, 10, 10501. [Google Scholar] [CrossRef]
- Li, P.C.; Yang, X.Y.; Wang, H.M.; Pan, T.; Yang, J.Y.; Wang, Y.Y.; Xu, Y.; Yang, Z.F.; Xu, C.W. Metabolic responses to combined water deficit and salt stress in maize primary roots. J. Integr. Agric. 2021, 20, 109–119. [Google Scholar] [CrossRef]
- Shamloo-Dashtpagerdi, R.; Aliakbari, M.; Lindlöf, A.; Tahmasebi, S. A systems biology study unveils the association between a melatonin biosynthesis gene, O-methyl transferase 1 (OMT1) and wheat (Triticum aestivum L.) combined drought and salinity stress tolerance. Planta 2022, 255, 99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cheng, K.; Ma, B.; Zhang, W.; Zheng, L.; Wang, Y. CaCl2 promotes the cross adaptation of Reaumuria trigyna to salt and drought by regulating Na+, ROS accumulation and programmed cell death. Plant Physiol. Biochem. 2023, 195, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Abdelraheem, A.; Esmaeili, N.; O’Connell, M.; Zhang, J. Progress and perspective on drought and salt stress tolerance in cotton. Ind. Crops Prod. 2019, 130, 118–129. [Google Scholar] [CrossRef]
- Duan, W.J.; Lu, B.; Liu, L.T.; Meng, Y.J.; Ma, X.Y.; Li, J.; Zhang, K.; Sun, H.C.; Zhang, Y.J.; Dong, H.Z.; et al. Effects of Exogenous Melatonin on Root Physiology, Transcriptome and Metabolome of Cotton Seedlings under Salt Stress. Int. J. Mol. Sci. 2022, 23, 9456. [Google Scholar] [CrossRef]
- de Queiroz, G.C.M.; de Medeiros, J.F.; da Silva, R.R.; da Silva Morais, F.M.; de Sousa, L.V.; de Souza, M.V.P.; da Nóbrega Santos, E.; Ferreira, F.N.; da Silva, J.M.C.; Clemente, M.I.; et al. Growth, Solute Accumulation, and Ion Distribution in Sweet Sorghum under Salt and Drought Stresses in a Brazilian Potiguar Semiarid Area. Agriculture 2023, 13, 803. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.L.; Liu, L.N.; Xie, Q.; Sui, N. Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front. Plant Sci. 2020, 10, 101722. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Jan, R.; Asif, S.; Farooq, M.; Jang, Y.H.; Kim, E.G.; Kim, N.; Kim, K.M. Exogenous melatonin induces salt and drought stress tolerance in rice by promoting plant growth and defense system. Sci. Rep. 2024, 14, 1214. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bano, A.; Rai, S.; Mishra, R.; Singh, M.; Sharma, S.; Pathak, N. Mechanistic insights of plant-microbe interaction towards drought and salinity stress in plants for enhancing the agriculture productivity. Plant Stress 2022, 4, 100073. [Google Scholar] [CrossRef]
- Yolcu, S.; Alavilli, H.; Ganesh, P.; Panigrahy, M.; Song, K. Salt and Drought Stress Responses in Cultivated Beets (Beta vulgaris L.) and Wild Beet (Beta maritima L.). Plants 2021, 10, 1843. [Google Scholar] [CrossRef] [PubMed]
- Kaura, V.; Malhotra, P.K.; Mittal, A.; Sanghera, G.S.; Kaur, N.; Bhardwaj, R.D.; Cheema, R.S.; Kaur, G. Physiological, biochemical, and gene expression responses of sugarcane under cold, drought and salt stresses. J. Plant Growth Regul. 2023, 42, 6367–6376. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, S.; Ding, F. Melatonin mitigates chilling-induced oxidative stress and photosynthesis inhibition in tomato plants. Antioxidants 2020, 9, 218. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015, 66, 647–656. [Google Scholar] [CrossRef]
- Gao, W.; Feng, Z.; Bai, Q.; He, J.; Wang, Y. Melatonin-mediated regulation of growth and antioxidant capacity in salt-tolerant naked oat under salt stress. Int. J. Mol. Sci. 2019, 20, 1176. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.; Ruiz, J.H. Melatonin and reactive oxygen and nitrogen species: A model for the plant redox network. Melatonin Res. 2019, 2, 152–168. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Mora-Poblete, F.; Arnao, M.B.; Naz, S.; Anwar, M.; Altaf, M.M.; Shahid, S.; Shakoor, A.; et al. Phytomelatonin: An overview of the importance and mediating functions of melatonin against environmental stresses. Physiol. Plant. 2021, 172, 820–846. [Google Scholar] [CrossRef] [PubMed]
- Moustafa-Farag, M.; Elkelish, A.; Dafe, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; El-Ezz, A.A.; Almoneafy, A.; Mahmoud, A.; Awad, M.; et al. Role of melatonin in plant tolerance to soil stressors: Salinity, pH and heavy metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef]
- Muhammad, I.; Yang, L.; Ahmad, S.; Farooq, S.; Khan, A.; Muhammad, N.; Ullah, S.; Adnan, M.; Ali, S.; Zhou, X.B.; et al. Melatonin-priming enhances maize seedling drought tolerance by regulating the antioxidant defense system. Plant Physiol. 2023, 191, 2301–2315. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, R.; Ge, J.; Liu, J.; Wang, W.; Xu, M.; Zhang, R.; Hussain, S.; Wei, H.; Dai, Q. Exogenous melatonin confers enhanced salinity tolerance in rice by blocking the ROS burst and improving Na+/K+ homeostasis. Environ. Exp. Bot. 2021, 189, 104530. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, X.; Yu, N.; Chen, J.; Wang, H.; Shen, Q.; Chen, G.; Wang, Y. Melatonin Promotes Rice Seed Germination under Drought Stress by Regulating Antioxidant Capacity. Phyton 2023, 92, 1571–1587. [Google Scholar] [CrossRef]
- Guo, T.; Wan, C.; Huang, F.; Wei, C. Evaluation of quality properties and antioxidant activities of tiger nut (Cyperus esculentus L.) oil produced by mechanical expression or/with critical fluid extraction. LWT-Food Sci. Technol. 2021, 141, 110915. [Google Scholar] [CrossRef]
- Adebayo, S.F.; Arinola, S.O. Effect of germination on the nutrient and antioxidant properties of tigernut (Cyperus esculentus). J. Biol. Agric. Healthc. 2017, 7, 88–94. [Google Scholar] [CrossRef]
- Sánchez-Zapata, E.; Fernández-López, J.; Angel Pérez-Alvarez, J. Tiger nut (Cyperus esculentus) commercialization: Health aspects, composition, properties, and food applications. Compr. Rev. Food Sci. Food Saf. 2012, 11, 366–377. [Google Scholar] [CrossRef]
- Follak, S.; Belz, R.; Bohren, C.; De Castro, O.; Del Guacchio, E.; Pascual-Seva, N.; Schwarz, M.; Verloove, F.; Essl, F. Biological flora of central Europe: Cyperus esculentus L. Perspect. Plant Ecol. Evol. Syst. 2016, 23, 33–51. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, X.; Zhang, T.; Zhao, C.; Guan, S.; Pu, Y.; Gao, F. Tiger nut (Cyperus esculentus L.): Nutrition, processing, function and applications. Foods 2022, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Cheng, Z.; Long, C.; Su, M.; Yang, D. Comprehensive development of chufa (Cyperus esculentus L. var. sativus). China Oils Fats 2007, 09, 61–63. [Google Scholar] [CrossRef]
- Ying, Y.Q.; Song, L.L.; Jacobs, D.F.; Mei, L.; Liu, P.; Jin, S.H.; Wu, J.S. Physiological response to drought stress in Camptotheca acuminata seedlings from two provenances. Front. Plant Sci. 2015, 6, 124077. [Google Scholar] [CrossRef] [PubMed]
- Ozden, M.; Demirel, U.; Kahraman, A. Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci. Hortic. 2009, 119, 163–168. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Javeed, H.M.R.; Ali, M.; Skalicky, M.; Nawaz, F.; Qamar, R.; Rehman, A.U.; Faheem, M.; Mubeen, M.; Iqbal, M.M.; ur Ruhman, M.H.; et al. Lipoic acid combined with melatonin mitigates oxidative stress and promotes root formation and growth in salt-stressed canola seedlings (Brassica napus L.). Molecules 2021, 26, 3147. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.E.; Zhao, Y.Q.; Ding, C.B.; Liao, J.Q.; Hu, C.; Zhou, L.J.; Zhang, Z.W.; Yuan, S.; Yuan, M. Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, A.; Sun, H.; Li, P.; Liu, X.; Guo, C.; Zhang, Y.; Zhang, K.; Bai, Z.; Dong, H. The effect of exogenous melatonin on root growth and lifespan and seed cotton yield under drought stress. Ind. Crops Prod. 2023, 204, 117344. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Zhang, J.; Guo, X.; Zhang, Z.; Sun, J.; Chen, X.; Wang, Y. Exogenous melatonin improves physiological characteristics and promotes growth of strawberry seedlings under cadmium stress. Hortic. Plant J. 2021, 7, 13–22. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Gao, G.; Ali, I.; Wu, X.; Tang, M.; Chen, L.; Jiang, L.; Liang, T. Effects of various seed priming on morphological, physiological, and biochemical traits of rice under chilling stress. Front. Plant Sci. 2023, 14, 1146285. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Sami, A.; Xu, Q.Q.; Wu, L.L.; Zheng, W.Y.; Chen, Z.P.; Jin, X.Z.; Zhang, H.; Li, Y.; Yu, Y.; et al. Effects of seed priming treatments on the germination and development of two rapeseed (Brassica napus L.) varieties under the co-influence of low temperature and drought. PLoS ONE 2012, 16, e0257236. [Google Scholar] [CrossRef] [PubMed]
- Smirnof, N. The role of active oxygen in the response of plants towaterd eficit and desiccation. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef]
- Hanif, S.; Saleem, M.F.; Sarwar, M.; Irshad, M.; Shakoor, A.; Wahid, M.A.; Khan, H.Z. Biochemically triggered heat and drought stress tolerance in rice by proline application. J. Plant Growth Regul. 2021, 40, 305–312. [Google Scholar] [CrossRef]
- Piper, F.I.; Paula, S. The role of nonstructural carbohydrates storage in forest resilience under climate change. Curr. For. Rep. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Cao, X.; Shen, Q.; Ma, S.; Liu, L.; Cheng, J. Physiological andpiptranscriptional responses to progressive soil water deficit in three mulberry cultivars. Front. Plant Sci. 2020, 11, 1310. [Google Scholar] [CrossRef] [PubMed]
- Dugasa, M.T.; Cao, F.; Ibrahim, W.; Wu, F. Differences in physiological and biochemical characteristics in response to single and combined drought and salinity stresses between wheat genotypes differing in salt tolerance. Physiol. Plant. 2019, 165, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Alagoz, S.M.; Hadi, H.; Toorchi, M.; Pawowski, T.A.; Lajayer, B.A.; Price, G.W.; Farooq, M.; Astatkie, T. Morpho-physiological responses and growth indices of triticale to drought and salt stresses. Sci. Rep. 2023, 13, 8896. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, M.; Wu, X.; Wang, Y.; Zhang, R. Physiological and transcriptomic analyses of the effects of exogenous melatonin on drought tolerance in maize (Zea mays L.). Plant Physiol. Biochem. 2021, 168, 128–142. [Google Scholar] [CrossRef]
- Gökçe, A.F.; Gökçe ZN, Ö.; Junaid, M.D.; Chaudhry, U.K. Evaluation of biochemical and molecular response of onion breeding lines to drought and salt stresses. Sci. Hortic. 2023, 311, 111802. [Google Scholar] [CrossRef]
- Zhang, W.; Bao, G.; Tang, W.; Dai, G.; Xiao, J.; Liu, J.; Wang, Z.; Xi, J. Physiological response of barley seedlings to salinity and artemisinin combined stresses under freeze-thaw environment. Environ. Sci. Pollut. Res. Int. 2022, 29, 70552–70563. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.Z.; Jia, Q.; Ibrahim, A.K.; Niyitanga, S.; Zhang, L. Mechanisms and signaling pathways of salt tolerance in crops: Understanding from the transgenic plants. Trop. Plant Biol. 2020, 13, 297–320. [Google Scholar] [CrossRef]
- Zhan, H.; Nie, X.; Zhang, T.; Li, S.; Wang, X.; Du, X.; Tong, W.; Song, W. Melatonin: A small molecule but important for salt stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 709. [Google Scholar] [CrossRef] [PubMed]
- EL-Bauome, H.A.; Abdeldaym, E.A.; Abd El-Hady, M.A.; Darwish, D.B.E.; Alsubeie, M.S.; El-Mogy, M.M.; Basahi, M.A.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Alzuaibr, F.M.; et al. Exogenous proline, methionine, and melatonin stimulate growth, quality, and drought tolerance in cauliflower plants. Agriculture 2022, 12, 1301. [Google Scholar] [CrossRef]
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. Melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Gao, L.; Xu, L.; Zheng, Q.; Sun, S.; Liu, X.; Zhang, Z.; Tian, Z.; Dai, T.; Sun, J. Melatonin alleviates chromium toxicity by altering chromium subcellular distribution and enhancing antioxidant metabolism in wheat seedlings. Environ. Sci. Pollut. Res. Int. 2023, 30, 50743–50758. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Zhang, H.H.; Xu, N.; Li, X.; Han, Y.; Ren, J.W.; Li, M.B. Special issue in honour of Prof. Reto J. Strasser–Effects of exogenous abscisic acid on the photosynthetic function and reactive oxygen species metabolism of tobacco leaves under drought stress. Photosynthetica 2020, 58, 585–594. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, B.; Gao, S.; Xu, K. Grafting improves tomato drought tolerance through enhancing photosynthetic capacity and reducing ROS accumulation. Protoplasma 2019, 256, 1013–1024. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Zhu, T.; Zhao, C.; Li, L.; Chen, M. The role of melatonin in salt stress responses. Int. J. Mol. Sci. 2019, 20, 1735. [Google Scholar] [CrossRef]
- Khan MS, S.; Ahmed, S.; ul Ikram, A.; Hannan, F.; Yasin, M.U.; Wang, J.; Zhao, B.; Islam, F.; Chen, J. Phytomelatonin: A key regulator of redox and phytohormones signaling against biotic/abiotic stresses. Redox Biol. 2023, 102805. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Melatonin Content (μmol·L−1) | NaCl Content (mmol·L−1) | Field Capacity (%) |
|---|---|---|---|
| CK | 0 | 0 | 70 |
| S | 0 | 200 | 70 |
| D | 0 | 0 | 50 |
| SD | 0 | 200 | 50 |
| CKM | 150 | 0 | 70 |
| SM | 150 | 200 | 70 |
| DM | 150 | 0 | 50 |
| SDM | 150 | 200 | 50 |
| Treatment | Plant Height (cm) | Number of Blade | Fresh Weight (g) | Dry Weight (g) |
|---|---|---|---|---|
| CK | 32.07 ± 0.43 a | 27.00 ± 0.82 ab | 11.90 ± 0.54 b | 2.38 ± 0.15 a |
| S | 24.94 ± 0.46 c | 17.67 ± 2.05 d | 7.76 ± 0.15 f | 1.22 ± 0.07 d |
| D | 24.30 ± 0.93 c | 18.00 ± 0.82 d | 7.53 ± 0.40 f | 1.42 ± 0.03 cd |
| SD | 24.00 ± 1.06 c | 19.00 ± 0.82 cd | 6.67 ± 0.50 g | 1.02 ± 0.06 e |
| CKM | 31.87 ± 1.58 a | 27.33 ± 1.25 ab | 13.42 ± 0.28 a | 2.50 ± 0.16 a |
| SM | 28.66 ± 1.81 b | 24.67 ± 1.70 b | 9.64 ± 0.26 d | 1.96 ± 0.09 b |
| DM | 27.99 ± 0.61 b | 28.00 ± 1.63 a | 10.85 ± 0.21 c | 1.93 ± 0.03 b |
| SDM | 27.52 ± 0.75 b | 21.67 ± 0.47 c | 8.73 ± 0.34 e | 1.50 ± 0.06 c |
| Treatment | RL (cm) | RSA (cm2) | RV (cm3) | ARD (mm) |
|---|---|---|---|---|
| CK | 1312.11 ± 47.72 a | 307.64 ± 15.06 b | 6.52 ± 0.48 a | 0.81 ± 0.08 a |
| S | 997.71 ± 47.02 c | 235.28 ± 8.83 c | 4.61 ± 0.59 c | 0.76 ± 0.04 a |
| D | 812.93 ± 46.90 d | 203.72 ± 10.67 d | 4.27 ± 0.69 c | 0.78 ± 0.08 a |
| SD | 716.84 ± 69.56 e | 147.16 ± 11.13 e | 2.49 ± 0.32 d | 0.77 ± 0.07 a |
| CKM | 1338.24 ± 52.85 a | 307.61 ± 12.24 b | 6.95 ± 0.47 a | 0.81 ± 0.06 a |
| SM | 1180.98 ± 52.16 b | 334.39 ± 21.48 a | 7.03 ± 0.55 a | 0.78 ± 0.04 a |
| DM | 1232.57 ± 56.32 b | 313.05 ± 28.15 ab | 6.61 ± 0.47 a | 0.82 ± 0.07 a |
| SDM | 1200.82 ± 65.05 b | 288.99 ± 27.19 b | 5.69 ± 0.67 b | 0.78 ± 0.04 a |
| Treatment | Principal Component Score | Comprehensive Score (F) | Comprehensive Score Ranking | |
|---|---|---|---|---|
| PC1 (F1) | PC2 (F2) | |||
| CK | 0.32 | −0.43 | 0.08 | 5 |
| S | −0.29 | −0.05 | −0.19 | 6 |
| D | −0.33 | −0.11 | −0.23 | 7 |
| SD | −0.38 | −0.37 | −0.34 | 8 |
| CKM | 0.41 | −0.5 | 0.11 | 3 |
| SM | 0.07 | 0.6 | 0.21 | 2 |
| DM | 0.16 | 0.61 | 0.27 | 1 |
| SDM | 0.03 | 0.26 | 0.09 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Luo, X.; Wang, Z.; Liu, J. Mitigating Effect of Exogenous Melatonin on Salt and Drought Stress in Cyperus esculentus L. during the Tillering Stage. Agronomy 2024, 14, 1009. https://doi.org/10.3390/agronomy14051009
Wang N, Luo X, Wang Z, Liu J. Mitigating Effect of Exogenous Melatonin on Salt and Drought Stress in Cyperus esculentus L. during the Tillering Stage. Agronomy. 2024; 14(5):1009. https://doi.org/10.3390/agronomy14051009
Chicago/Turabian StyleWang, Ningning, Xuemei Luo, Zhen Wang, and Jianguo Liu. 2024. "Mitigating Effect of Exogenous Melatonin on Salt and Drought Stress in Cyperus esculentus L. during the Tillering Stage" Agronomy 14, no. 5: 1009. https://doi.org/10.3390/agronomy14051009





