Exogenous Selenium Endows Salt-Tolerant and Salt-Sensitive Soybeans with Salt Tolerance through Plant-Microbial Coactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Preparation
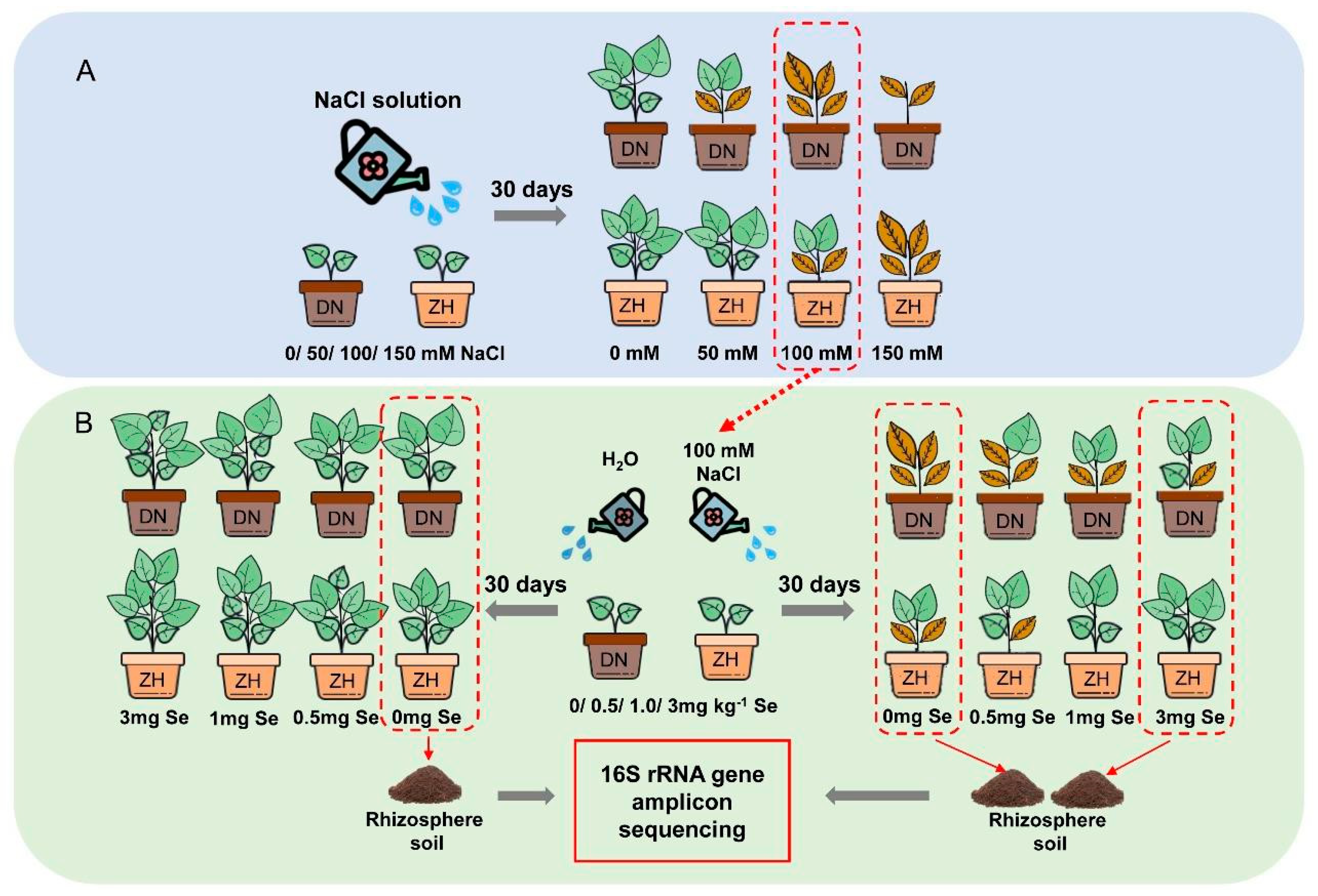
2.2. Experiment Designation
2.3. Sample Collection
2.4. Physical and Chemical Indexes Analysis
2.5. Determination of Soil Enzyme Activity
2.6. DNA Extraction and 16 S rRNA Sequencing
2.7. Microbiome Analysis
2.8. Statistical Analysis
3. Results
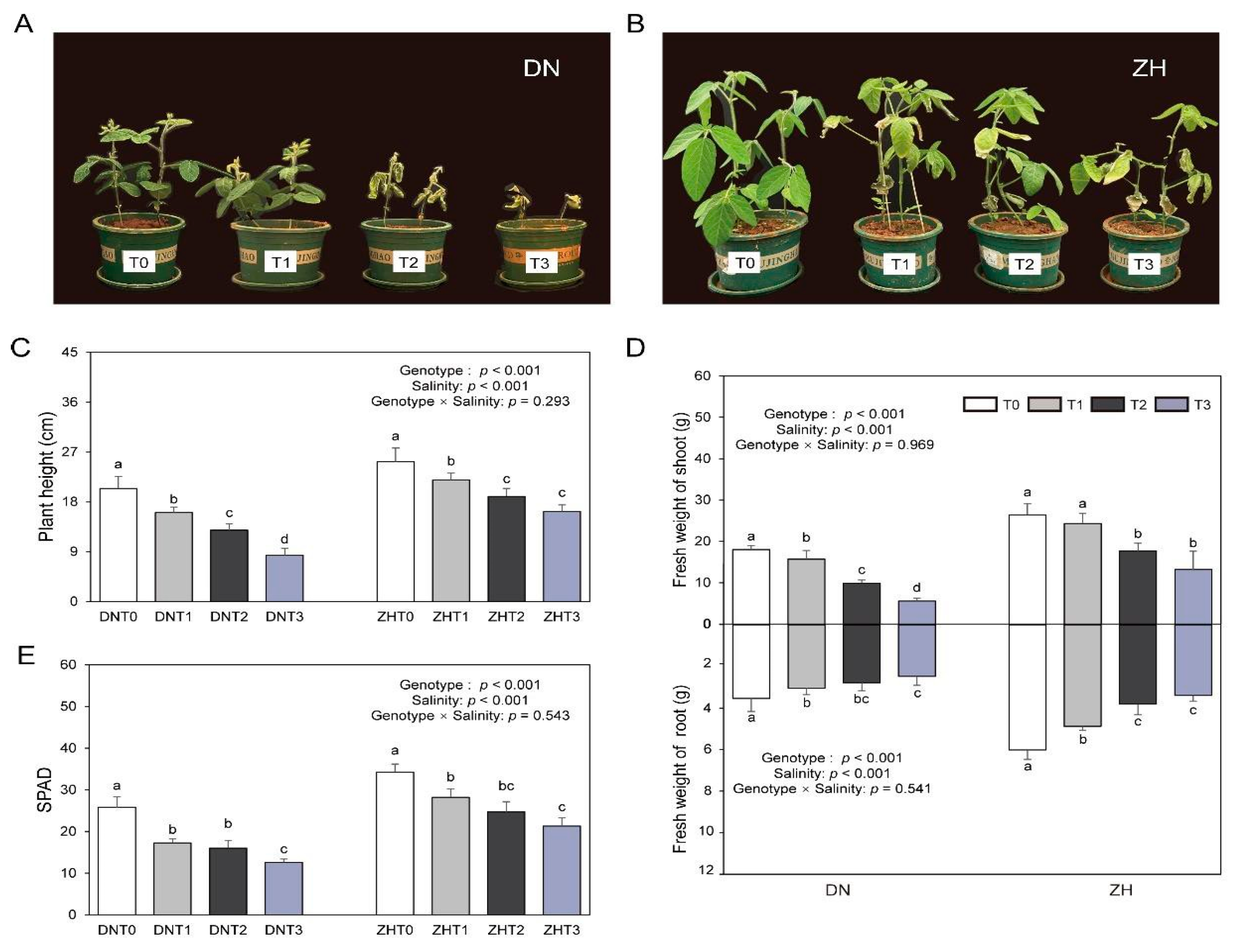
3.1. Effects of Salt Stress on Growth of Soybean
3.2. Effects of Salt Stress on Rhizosphere Soil Enzyme Activities of Soybean
3.3. Effects of Se on Growth of Soybean
3.4. Effects of Se on Soybean Chemical Indexes under Salt Stress
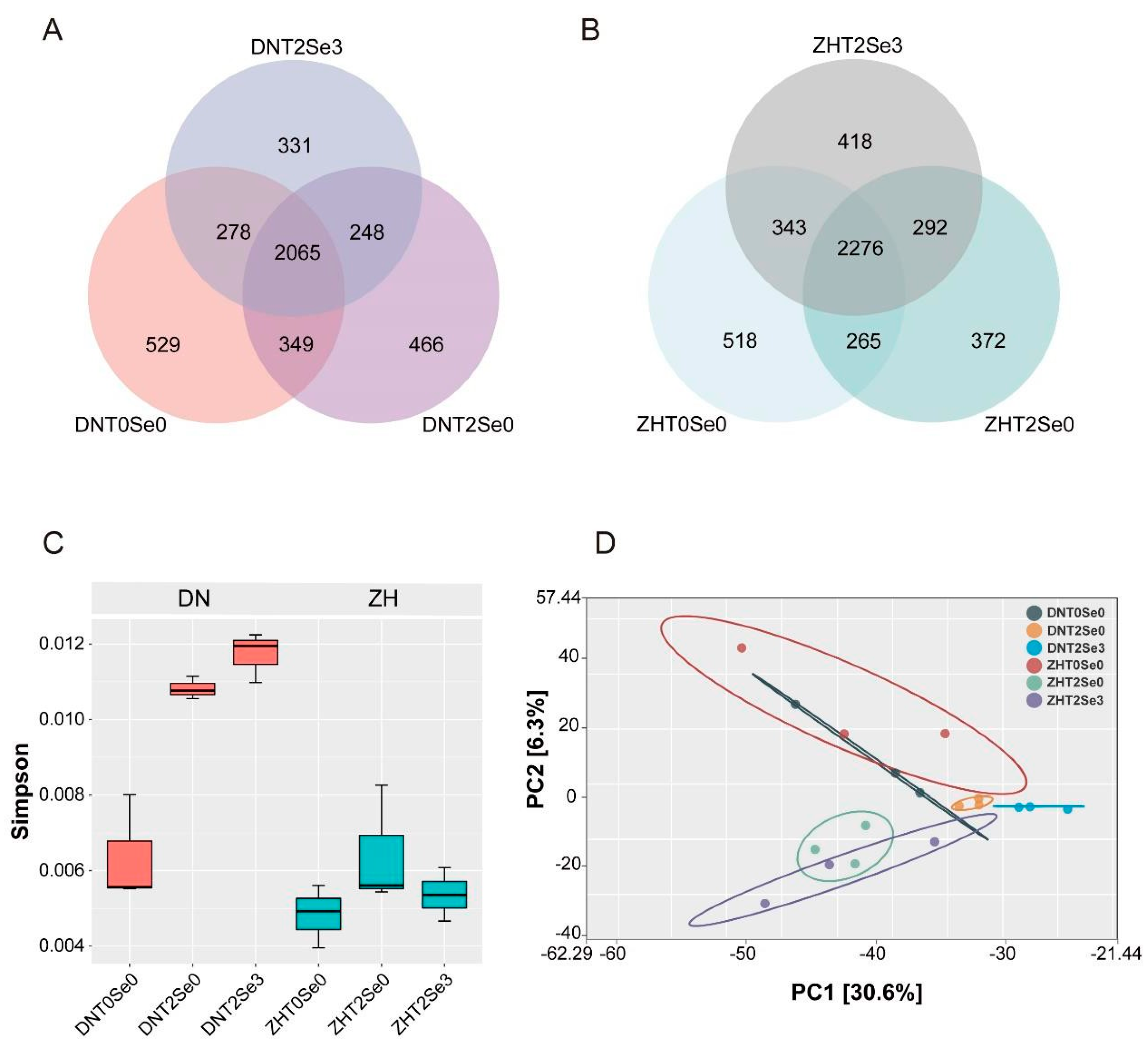
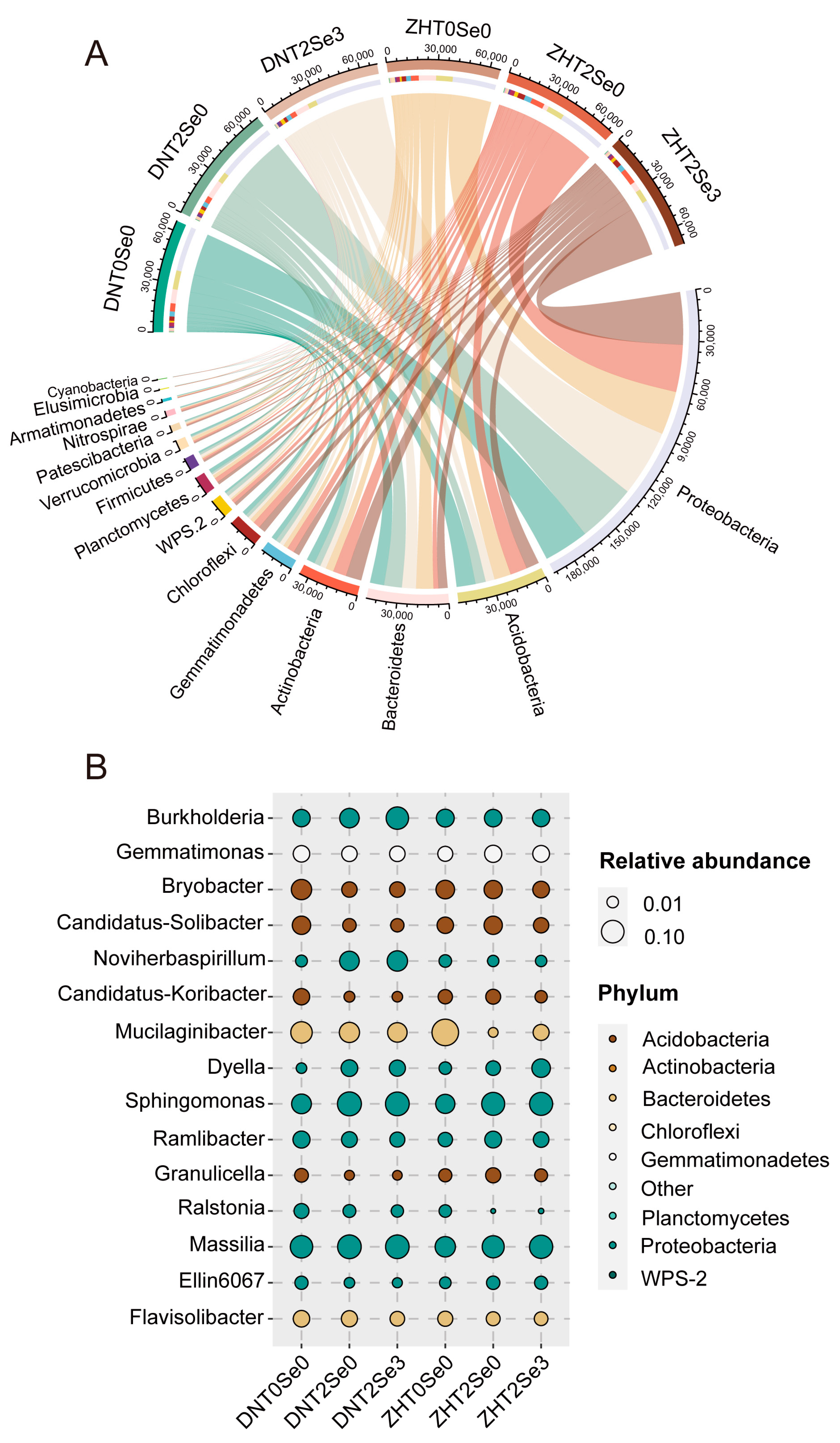
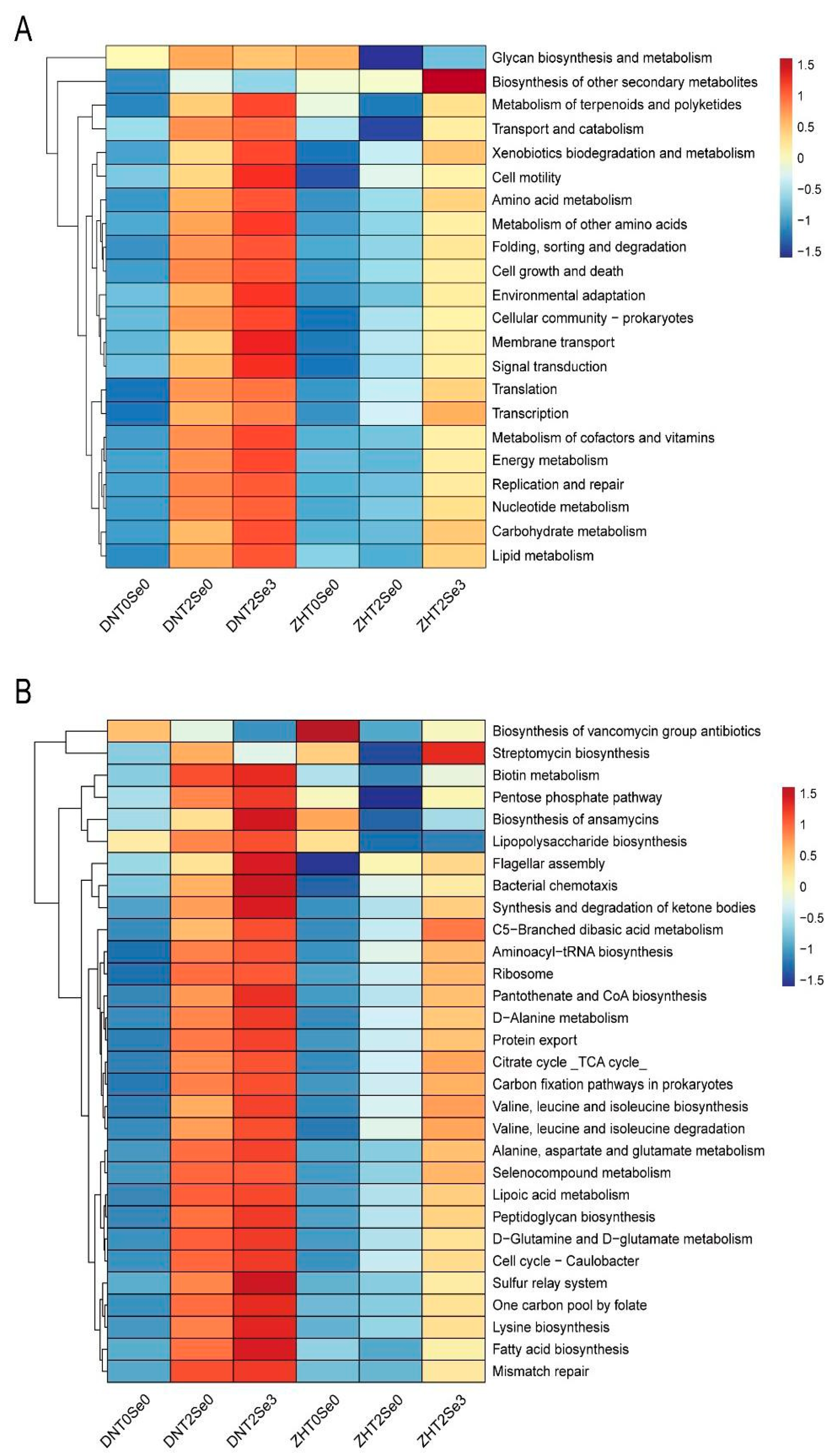
3.5. Effects of Se on Rhizosphere Microecology of Soybean under Salt Stress
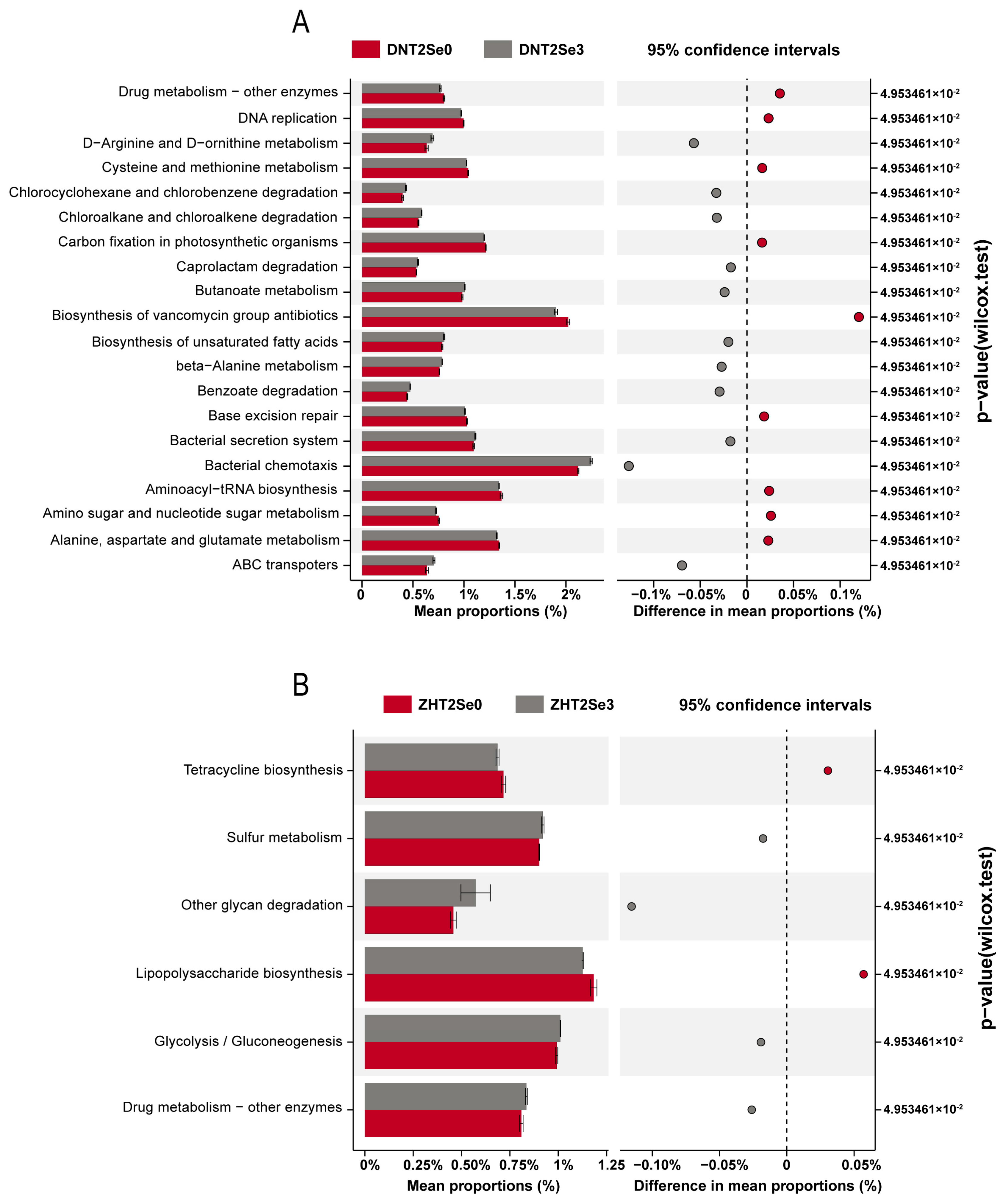
3.6. Effects of Se on Rhizosphere Microorganisms Functions of Soybean under Salt Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Litalien, A.; Zeeb, B. Curing the earth: A review of anthropogenic soil salinization and plant-based strategies for sustainable mitigation. Sci. Total Environ. 2020, 698, 134235. [Google Scholar] [CrossRef] [PubMed]
- Shelden, M.C.; Munns, R. Crop root system plasticity for improved yields in saline soils. Front. Plant Sci. 2023, 14, 1120583. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Peng, T.; Xue, S.W. Mechanisms of plant saline-alkaline tolerance. J. Plant Physiol. 2023, 281, 153916. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Nagpal, S.; Sharma, P. Potential of plant growth-promoting rhizobacteria-plant interactions in mitigating salt stress for sustainable agriculture: A review. Pedosphere 2022, 32, 223–245. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagon, D.; Gomez-Cadenas, A.; Mittler, R. Plant responses to climate change: Metabolic changes under combined abiotic stresses. J. Exp. Bot. 2022, 73, 3339–3354. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Gao, H.T.; Zhou, Y.G.; Jing, Y.; Li, S.Q.; Yan, Z.; Xu, K.H.; Zhou, F.X.; Zhang, W.P.; Yang, X.Q.; et al. Unfolding molecular switches for salt stress resilience in soybean: Recent advances and prospects for salt-tolerant smart plant production. Front. Plant Sci. 2023, 14, 1162014. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, J.; Daliakopoulos, I.N.; del Moral, F.; Hueso, J.J.; Tsanis, I.K. A Review of Soil-Improving Cropping Systems for Soil Salinization. Agronomy 2019, 9, 295. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rathod, S.; Manohara, K.K.; Gireesh, C.; Anantha, M.S.; Sakhare, A.S.; Parmar, B.; Yadav, B.K.; Bandumula, N.; Raihan, F.; et al. Salt Stress in Plants and Mitigation Approaches. Plants 2022, 11, 717. [Google Scholar] [CrossRef]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.T. An Overview of Hazardous Impacts of Soil Salinity in Crops, Tolerance Mechanisms, and Amelioration through Selenium Supplementation. Int. J. Mol. Sci. 2020, 21, 148. [Google Scholar] [CrossRef]
- Singhal, R.K.; Fahad, S.; Kumar, P.; Choyal, P.; Javed, T.; Jinger, D.; Singh, P.; Saha, D.; Md, P.; Bose, B.; et al. Beneficial elements: New Players in improving nutrient use efficiency and abiotic stress tolerance. Plant Growth Regul. 2022, 100, 237–265. [Google Scholar] [CrossRef]
- Cai, M.; Zhao, X.; Wang, X.; Shi, G.; Hu, C. Se changed the component of organic chemicals and Cr bioavailability in pak choi rhizosphere soil. Environ. Sci. Pollut. Res. Int. 2021, 28, 67331–67342. [Google Scholar] [CrossRef]
- Lei, Z.; Li, Q.Q.; Tang, Y.N.; Zhang, H.; Han, C.; Wang, X.; Zhao, X.H.; Shi, G.Y. Selenium enhanced nitrogen accumulation in legumes in soil with rhizobia bacteria. J. Clean. Prod. 2022, 380, 134960. [Google Scholar] [CrossRef]
- Rahman, M.; Rahman, K.; Sathi, K.S.; Alam, M.M.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Supplemental Selenium and Boron Mitigate Salt-Induced Oxidative Damages in Glycine max L. Plants 2021, 10, 2224. [Google Scholar] [CrossRef]
- Jiang, C.Q.; Zu, C.L.; Lu, D.J.; Zheng, Q.S.; Shen, J.; Wang, H.Y.; Li, D.C. Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci. Rep. 2017, 7, 42039. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, M.; Feng, F.; Tian, Z. Toward a “Green Revolution” for Soybean. Mol. Plant 2020, 13, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.J.; Niu, L.; Cai, Q.A.; Wei, J.; Shang, L.X.; Yang, X.D.; Ma, R. Improved salt-tolerance of transgenic soybean by stable over-expression of AhBADH gene from Atriplexhortensis. Plant Cell Rep. 2023, 42, 1291–1310. [Google Scholar] [CrossRef] [PubMed]
- Noor, J.; Ullah, A.; Saleem, M.H.; Tariq, A.; Ullah, S.; Waheed, A.; Okla, M.K.; Al-Hashimi, A.; Chen, Y.L.; Ahmed, Z.; et al. Effect of Jasmonic Acid Foliar Spray on the Morpho-Physiological Mechanism of Salt Stress Tolerance in Two Soybean Varieties (Glycine max L.). Plants 2022, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.P.; Wang, X.S.; Zhang, Z.Y.; Li, C.X.; Xing, Y.M.; Luo, Y.Q.; Li, D.H.; Ma, Z.Y.; Cai, H. Symbiotic System Establishment between Piriformospora indica and Glycine max and Its Effects on the Antioxidant Activity and Ion-Transporter-Related Gene Expression in Soybean under Salt Stress. Int. J. Mol. Sci. 2022, 23, 14961. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, R.; Jiang, Q.Y.; Sun, X.J.; Zhang, H.; Hu, Z. GmNAC06, a NAC domain transcription factor enhances salt stress tolerance in soybean. Plant Mol. Biol. 2021, 105, 333–345. [Google Scholar] [CrossRef]
- Jin, T.; Shan, Z.; Zhou, S.; Yang, Q.Q.; Gai, J.Y.; Li, Y. GmDNAJC7 from Soybean Is Involved in Plant Tolerance to Alkaline-Salt, Salt, and Drought Stresses. Agronomy 2022, 12, 1419. [Google Scholar] [CrossRef]
- Cao, D.; Li, Y.Y.; Liu, B.H.; Kong, F.J.; Tran, L.S.P. Adaptive Mechanisms of Soybean Grown on Salt-Affected Soils. Land Degrad. Dev. 2018, 29, 1054–1064. [Google Scholar] [CrossRef]
- Ardie, S.W.; Xie, L.N.; Takahashi, R.; Liu, S.K.; Takano, T. Cloning of a high-affinity K+ transporter gene PutHKT2;1 from Puccinellia tenuiflora and its functional comparison with OsHKT2;1 from rice in yeast and Arabidopsis. J. Exp. Bot. 2009, 60, 3491–3502. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, J.C.; Bremner, J.M. A Rapid and Precise Method for Routine Determination of Organic-Carbon in Soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Iqbal, A.; He, L.; Khan, A.; Wei, S.Q.; Akhtar, K.; Ali, I.; Ullah, S.; Munsif, F.; Zhao, Q.; Jiang, L.G. Organic Manure Coupled with Inorganic Fertilizer: An Approach for the Sustainable Production of Rice by Improving Soil Properties and Nitrogen Use Efficiency. Agronomy 2019, 9, 651. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts I. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Kotroczo, Z.; Veres, Z.; Fekete, I.; Krakomperger, Z.; Toth, J.A.; Lajtha, K.; Tothmeresz, B. Soil enzyme activity in response to long-term organic matter manipulation. Soil Biol. Biochem. 2014, 70, 237–243. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef]
- Liu, L.L.; Wu, Y.M.; Yin, M.Q.; Ma, X.Y.; Yu, X.A.; Guo, X.; Du, N.; Eller, F.; Guo, W.H. Soil salinity, not plant genotype or geographical distance, shapes soil microbial community of a reed wetland at a fine scale in the Yellow River Delta. Sci. Total Environ. 2023, 856, 159136. [Google Scholar] [CrossRef]
- Khan, W.U.D.; Ramzani, P.M.A.; Anjum, S.; Abbas, F.; Iqbal, M.; Yasar, A.; Ihsan, M.Z.; Anwar, M.N.; Baqar, M.; Tauqeer, H.M.; et al. Potential of miscanthus biochar to improve sandy soil health, in situ nickel immobilization in soil and nutritional quality of spinach. Chemosphere 2017, 185, 1144–1156. [Google Scholar] [CrossRef]
- Henneron, L.; Kardol, P.; Wardle, D.A.; Cros, C.; Fontaine, S. Rhizosphere control of soil nitrogen cycling: A key component of plant economic strategies. New Phytol. 2020, 228, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- York, L.M.; Carminati, A.; Mooney, S.J.; Ritz, K.; Bennett, M.J. The holistic rhizosphere: Integrating zones, processes, and semantics in the soil influenced by roots. J. Exp. Bot. 2016, 67, 3629–3643. [Google Scholar] [CrossRef]
- Barquero, M.; Poveda, J.; Laureano-Marin, A.M.; Ortiz-Liebana, N.; Branas, J.; Gonzalez-Andres, F. Mechanisms involved in drought stress tolerance triggered by rhizobia strains in wheat. Front. Plant Sci. 2022, 13, 1036973. [Google Scholar] [CrossRef] [PubMed]
- Abalos, D.; Jeffery, S.; Sanz-Cobena, A.; Guardia, G.; Vallejo, A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric. Ecosyst. Environ. 2014, 189, 136–144. [Google Scholar] [CrossRef]
- Jian, S.Y.; Li, J.W.; Chen, J.; Wang, G.S.; Mayes, M.A.; Dzantor, K.E.; Hui, D.F.; Luo, Y.Q. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Udvardi, M.; Poole, P.S. Transport and Metabolism in Legume-Rhizobia Symbioses. Ann. Rev. Plant Biol. 2013, 64, 781–805. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wang, M.; Bi, D. Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the growth of sorrel seedlings under salt stress. Plant Growth Regul. 2005, 45, 155–163. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Lanza, M.; dos Reis, A.R. Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef]
- Schiavon, M.; Lima, L.W.; Jiang, Y.; Hawkesford, M.J. Effects of Selenium on Plant Metabolism and Implications for Crops and Consumers. In Selenium in Plants: Molecular, Physiological, Ecological and Evolutionary Aspects; Pilon Smits, E.A.H., Winkel, L.H.E., Lin, Z.Q., Eds.; Plant Ecophysiology; Springer: Berlin/Heidelberg, Germany, 2017; Volume 11, pp. 257–275. [Google Scholar]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Hosseinifard, M.; Stefaniak, S.; Javid, M.G.; Soltani, E.; Wojtyla, L.; Garnczarska, M. Contribution of Exogenous Proline to Abiotic Stresses Tolerance in Plants: A Review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Ahmed, S.; Akram, W.; Li, G.H.; Yasin, N.A. Selenium seed priming enhanced the growth of salt-stressed Brassica rapa L. through improving plant nutrition and the antioxidant system. Front. Plant Sci. 2023, 13, 359. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Chu, J.; Liang, L.; Geng, W.; Li, J.; Hou, G. Selenium improves recovery of wheat seedlings at rewatering after drought stress. Russ. J. Plant Physiol. 2012, 59, 701–707. [Google Scholar] [CrossRef]
- Shams, M.; Khadivi, A. Mechanisms of salinity tolerance and their possible application in the breeding of vegetables. BMC Plant Biol. 2023, 23, 139. [Google Scholar] [CrossRef]
- Mann, A.; Lata, C.; Kumar, N.; Kumar, A.; Kumar, A.; Sheoran, P. Halophytes as new model plant species for salt tolerance strategies. Front. Plant Sci. 2023, 14, 1137211. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fan, S.Y.; Gong, H.J.; Guo, J. Roles of salicylic acid in selenium-enhanced salt tolerance in tomato plants. Plant Soil 2023, 484, 569–588. [Google Scholar] [CrossRef]
- Mushtaq, N.U.; Alghamdi, K.M.; Saleem, S.; Shajar, F.; Tahir, I.; Bahieldin, A.; Rehman, R.U.; Hakeem, K.R. Selenate and selenite transporters in proso millet: Genome extensive detection and expression studies under salt stress and selenium. Front. Plant Sci. 2022, 13, 1060154. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.C.; Wang, Z.W.; Zhu, K.F.; Wu, W.M. Comparative metagenomic analysis reveals rhizosphere microbial community composition and functions help protect grapevines against salt stress. Front. Microbiol. 2023, 14, 1102547. [Google Scholar] [CrossRef]
- Winkel, L.H.E.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Banuelos, G.S. Selenium Cycling Across Soil-Plant-Atmosphere Interfaces: A Critical Review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Vemuri, H.; Garai, S.; Mondal, M.; Bhatt, R.; et al. Selenium Biofortification: Roles, Mechanisms, Responses and Prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef]
- Hagh-Doust, N.; Mikryukov, V.; Anslan, S.; Bahram, M.; Puusepp, R.; Dulya, O.; Tedersoo, L. Effects of nitrogen deposition on carbon and nutrient cycling along a natural soil acidity gradient as revealed by metagenomics. New Phytol. 2023, 238, 2607–2620. [Google Scholar] [CrossRef]
- Li, Y.Y.; Ma, K.; Song, W.; Zhou, J.Y.; Liu, X.; Wang, M.Q.; Tu, Q.C. Environmental heterogeneity and dispersal limitation simultaneously determine the spatial scaling of different microbial functional groups. Sci. Total Environ. 2023, 885, 163854. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.H.; Yang, J.; Zheng, H.X.; Zhang, F.N.; Li, S.M.; Chen, Z.T.; Sui, N. Interactions between the soil bacterial community assembly and gene regulation in salt-sensitive and salt-tolerant sweet sorghum cultivars. Land Degrad. Dev. 2022, 33, 2985–2997. [Google Scholar] [CrossRef]
- Fan, W.Q.; Tang, F.; Wang, J.N.; Dong, J.Q.; Xing, J.; Shi, F.L. Drought-induced recruitment of specific root-associated bacteria enhances adaptation of alfalfa to drought stress. Front. Microbiol. 2023, 14, 1114400. [Google Scholar] [CrossRef]
- Sharma, I.; Kashyap, S.; Agarwala, N. Biotic stress-induced changes in root exudation confer plant stress tolerance by altering rhizospheric microbial community. Front. Plant Sci. 2023, 14, 1132824. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.Y.; Cao, X.S.; Wang, C.X.; Chen, F.R.; Zou, H.; Yue, L.; Wang, Z.Y. Crosstalk between in situ root exudates and rhizobacteria to promote rice growth by selenium nanomaterials. Sci. Total Environ. 2023, 878, 163175. [Google Scholar] [CrossRef]
- Rudrappa, T.; Czymmek, K.J.; Pare, P.W.; Bais, H.P. Root-Secreted Malic Acid Recruits Beneficial Soil Bacteria. Plant Physiol. 2008, 148, 1547–1556. [Google Scholar] [CrossRef]



| Physicochemical Properties | Value |
|---|---|
| pH | 6.03 |
| electrical conductivity [ms·cm−1] | 0.47 |
| Organic matter [g·kg−1] | 14.52 |
| Alkaline hydrolyzed nitrogen [mg·kg−1] | 34.18 |
| Available P [mg·kg−1] | 50.50 |
| Available K [mg·kg−1] | 201.41 |
| Total Se [mg·kg−1] | 0.090 |
| Available Se [mg·kg−1] | 0.073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, C.; Wuriyanghan, H.; Lei, Z.; Tang, Y.; Zhang, H.; Zhao, X. Exogenous Selenium Endows Salt-Tolerant and Salt-Sensitive Soybeans with Salt Tolerance through Plant-Microbial Coactions. Agronomy 2023, 13, 2271. https://doi.org/10.3390/agronomy13092271
Wang Y, Xu C, Wuriyanghan H, Lei Z, Tang Y, Zhang H, Zhao X. Exogenous Selenium Endows Salt-Tolerant and Salt-Sensitive Soybeans with Salt Tolerance through Plant-Microbial Coactions. Agronomy. 2023; 13(9):2271. https://doi.org/10.3390/agronomy13092271
Chicago/Turabian StyleWang, Yin, Chao Xu, Hada Wuriyanghan, Zheng Lei, Yanni Tang, Huan Zhang, and Xiaohu Zhao. 2023. "Exogenous Selenium Endows Salt-Tolerant and Salt-Sensitive Soybeans with Salt Tolerance through Plant-Microbial Coactions" Agronomy 13, no. 9: 2271. https://doi.org/10.3390/agronomy13092271





