Exploring the Agronomic Performance and Molecular Characterization of Diverse Spring Durum Wheat Germplasm in Kazakhstan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Durum Wheat Material
2.2. Experimental Sites and Weather
2.3. Field Experimentation
2.4. Molecular Analysis
2.4.1. DNA Isolation
2.4.2. PCR Amplification
2.5. Statistical Analysis
2.5.1. Morphological Data Analysis
2.5.2. Molecular Markers Data Analysis
3. Results
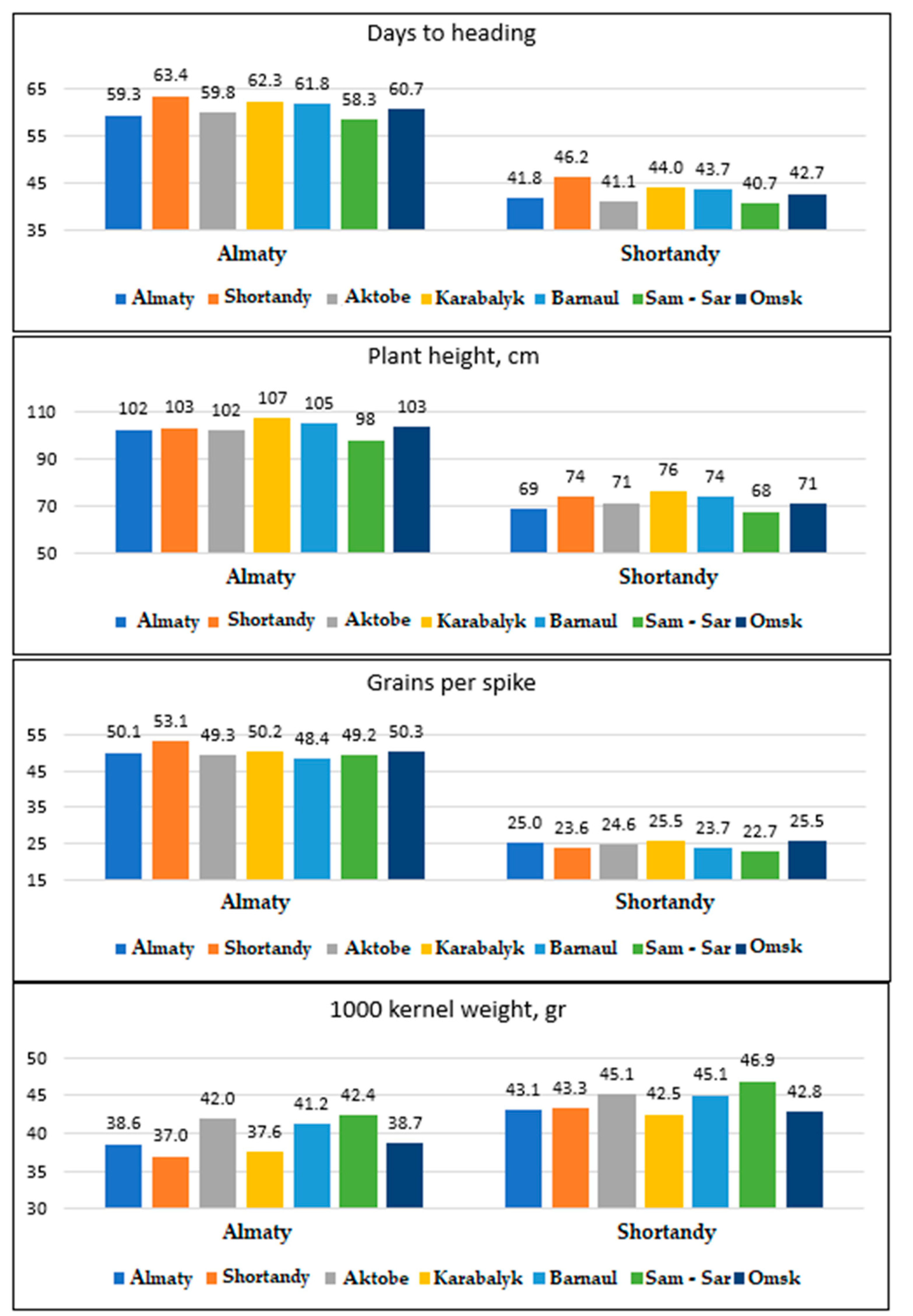
3.1. Comparison of Durum Wheat Agronomic Performance at Two Sites
3.2. Agronomic Performance of Durum Wheat Germplasm Originating from Different Breeding Programs
3.3. Superior Spring Durum Wheat Germplasm Identified in the Study
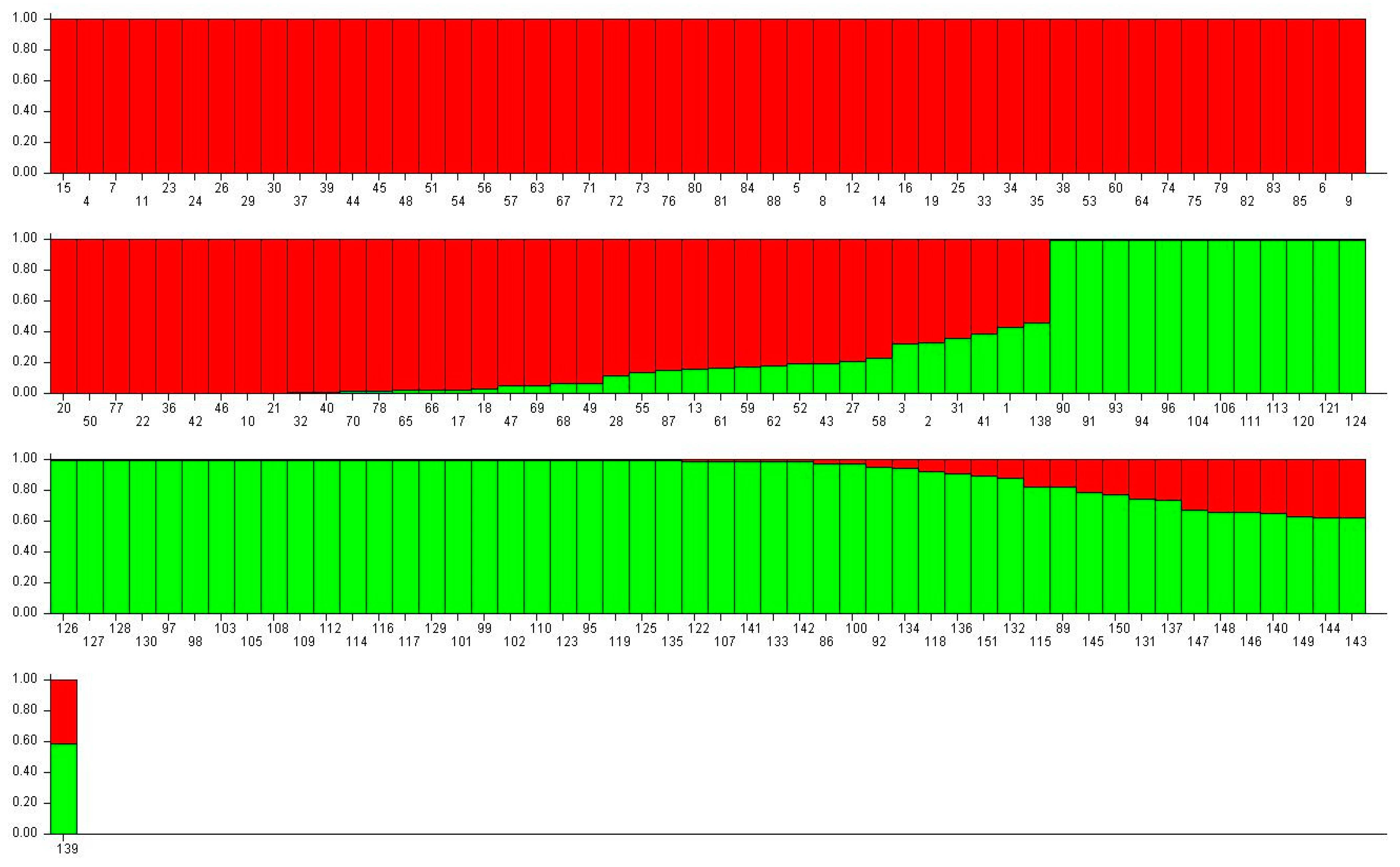
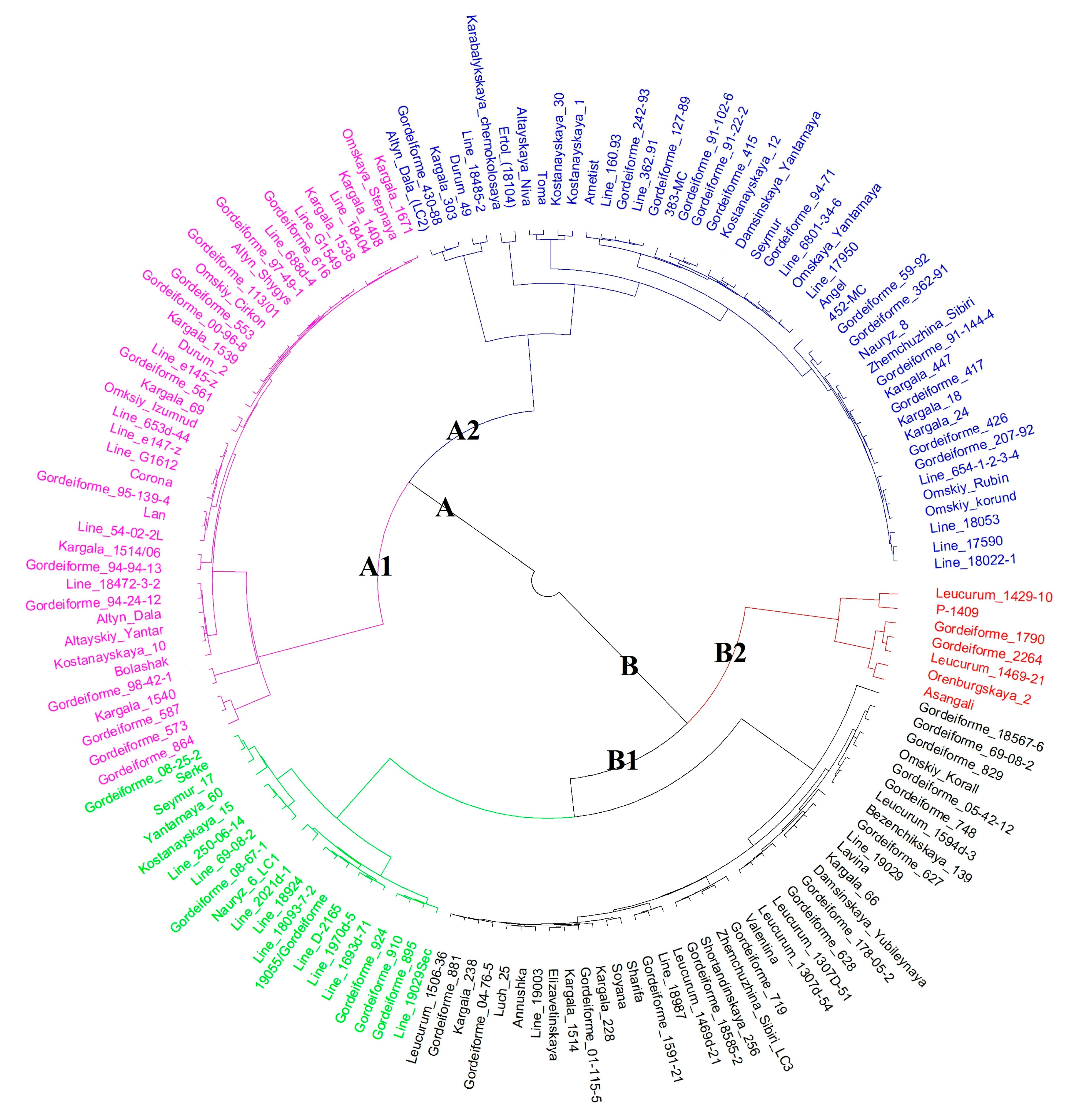
3.4. Molecular Characterization
4. Discussion
4.1. Phenotypic Characterization
4.2. Molecular Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Available online: https//www.fao.org/faostat/en/#home (accessed on 27 May 2023).
- Morgounov, A.; Zykin, V.; Sereda, G.; Urazaliev, R. Siberian and North Kazakhstan Wheat Pool. In The World Wheat Book, A History of Wheat Breeding; Bonjean, A., Angus, W., Eds.; Tec & Doc-Lavoisier: La Chaussée-Saint-Victor, France, 2001; Volume 3, pp. 755–772. [Google Scholar]
- Del Maro Polo, M.; Santos, N.; Syzdykov, Y. Adoption of Climate Technologies in the Agrifood System, Investment Opportunities in Kazakhstan; FAO: Rome, Italy, 2022; 47p. [Google Scholar]
- Babkenov, A.; Babkenova, S.; Abdullayev, K.; Kairzhanov, Y. Breeding Spring Soft Wheat for Productivity, Grain Quality, and Resistance to Adverse External Factors in Nothern Kazakhstan. J. Ecol. Eng. 2020, 21, 8–12. [Google Scholar] [CrossRef]
- Morgounov, A.; Sonder, K.; Abugalieva, A.; Bhadauria, V.; Cuthbert, R.D.; Shamanin, V.; Zelenskiy, Y.; DePauw, R.M. Effect of climate change on spring wheat yields in North America and Eurasia in 1981–2015 and implications for breeding. PLoS ONE 2018, 13, e0204932. [Google Scholar] [CrossRef] [PubMed]
- De Vita, P.; Taranto, F. Durum wheat (Triticum turgidum ssp. durum) breeding to meet the challenge of climate change. Adv. Plant Breed. Strateg. 2019, 5, 471–524. [Google Scholar]
- Tajibayev, D.; Yusov, V.S.; Chudinov, V.A.; Mal’chikov, P.N.; Rozova, M.A.; Shamanin, V.P.; Shepelev, S.S.; Sharma, R.; Tsygankov, V.I.; Morgounov, A.I. Genotype by environment interactions for spring durum wheat in Kazakhstan and Russia. Ecol. Genet. Genom. 2021, 21, 100099. [Google Scholar] [CrossRef]
- Evdokimov, M.G.; Yusov, V.S.; Morgounov, A.I.; Zelensky, Y.I. Drought tolerance gene pool in developing adaptive varieties of durum wheat identified in study nurseries under the Kazakhstan-Siberian program. Vavilovskii Zhurnal Genetiki i Selektsii 2017, 21, 515–522. [Google Scholar] [CrossRef] [Green Version]
- Baloch, F.S.; Kurt, C.; Arıoğlu, H.; Özkan, H. Assaying of diversity among Soybean (Glycin max L.) and peanut (Arachis hypogaea L.) genotypes at DNA level. Turk. J. Agric. For. 2010, 34, 285–301. [Google Scholar] [CrossRef]
- Yaman, M. Evaluation of genetic diversity by morphological, biochemical and molecular markers in sour cherry genotypes. Mol. Biol. Rep. 2022, 49, 5293–5301. [Google Scholar] [CrossRef]
- Pinar, H.; Yahya, H.N.; Erċışlı, S.; Coskun, O.F.; Yaman, M.; Turgunbaev, K.; Uzun, A. Molecular Characterization of Barberry Genotypes from Turkey and Kyrgyzstan. Erwerbs-Obstbau 2021, 63, 403–407. [Google Scholar] [CrossRef]
- Yaman, M.; Uzun, A. Morphological and molecular identification of hybrid individuals obtained by interspecies hybridization (Prunus armeniaca × Prunus salicina). Int. J. Agric. Nat. Sci. 2021, 14, 7–15. [Google Scholar]
- Nadeem, M.A.; Nawaz, M.A.; Shahid, M.Q.; Doğan, Y.; Comertpay, G.; Yıldız, M.; Hatipoğlu, R.; Ahmad, F.; Alsaleh, A.; Labhane, N.; et al. DNA molecular markers in plant breeding, current status and recent advancements in genomic selection and genome editing. Biotechnol. Biotechnol. Equip. 2018, 32, 261–285. [Google Scholar] [CrossRef] [Green Version]
- Kalendar, R.; Antonius, K.; Smýkal, P.; Shulman, A.H. iPBS, a universal method for DNA fingerprinting and retrotransposon isolation. Theor. Appl. Genet. 2010, 121, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Baloch, F.S.; Guizado, S.J.V.; Altaf, M.T.; Yüce, I.; Çilesiz, Y.; Bedir, M.; Nadeem, M.A.; Hatipoglu, R.; Gómez, J.C.C. Applicability of inter-primer binding site iPBS-retrotransposon marker system for the assessment of genetic diversity and population structure of Peruvian rosewood (Aniba rosaeodora Ducke) germplasm. Mol. Biol. Rep. 2022, 49, 2553–2564. [Google Scholar] [CrossRef]
- Andeden, E.E.; Baloch, F.S.; Derya, M.; Kilian, B.; Özkan, H. iPBS Retrotransposons based genetic diversity and relationship among wild annual Cicer species. J. Plant Biochem. Biotechnol. 2013, 22, 453–466. [Google Scholar] [CrossRef]
- Yaman, M. Determination of genetic diversity in european cranberrybush (Viburnum opulus L.) genotypes based on morphological, phytochemical and ISSR markers. Genet. Resour. Crop Evol. 2022, 69, 1889–1899. [Google Scholar] [CrossRef]
- Yildiz, E.; Pinar, H.; Uzun, A.; Yaman, M.; Sumbul, A.; Ercisli, S. Identification of genetic diversity among Juglans regia L. genotypes using molecular, morphological, and fatty acid data. Genet. Resour. Crop Evol. 2021, 68, 1425–1437. [Google Scholar] [CrossRef]
- Nadeem, M.A. Deciphering the genetic diversity and population structure of Turkish bread wheat germplasm using iPBS-retrotransposons markers. Mol. Biol. Rep. 2021, 48, 6739–6748. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Nadeem, M.A.; Barut, M.; Habyarimana, E.; Chaudhary, H.J.; Khalil, I.H.; Alsaleh, A.; Hatipoğlu, R.; Karaköy, T.; Kurt, C.; et al. Genetic diversity, population structure and marker-trait association for 100-seed weight in international safflower panel using silicoDArT marker information. Plants 2020, 9, 652. [Google Scholar] [CrossRef]
- Baloch, F.S.; Alsaleh, A.; de Miera, L.E.S.; Hatipoğlu, R.; Çiftçi, V.; Karaköy, T.; Yildiz, M.; Özkan, H. DNA based iPBS-retrotransposon markers for investigating the population structure of pea (Pisum sativum) germplasm from Turkey. Biochem. Syst. Ecol. 2015, 61, 244–252. [Google Scholar] [CrossRef]
- Baloch, F.S.; Derya, M.; Andeden, E.E.; Alsaleh, A.; Cömertpay, G.; Kilian, B.; Özkan, H. Inter-primer binding site retrotransposon and inter-simple sequence repeat diversity among wild Lens species. Biochem. Syst. Ecol. 2015, 58, 162–168. [Google Scholar] [CrossRef]
- Arystanbekkyzy, M.; Nadeem, M.A.; Aktas, H.; Yeken, M.Z.; Zencirci, N.; Nawaz, M.A.; Ali, F.; Haider, M.S.; Tunc, K.; Chung, G.; et al. Phylogenetic and taxonomic relationship of turkish wild and cultivated emmer (Triticum turgidum ssp. dicoccoides) revealed by iPBS retrotransposons markers. Int. J. Agric. Biol. 2019, 21, 155–163. [Google Scholar]
- KazGidroMet. National Hydrometeorological Service of the Republic of Kazakhstan. Available online: https://www.kazhydromet.kz/ru/about/o-nacionalnoy-gidrometeorologicheskoy-sluzhbe-kazahstana (accessed on 15 March 2023).
- Pask, A.J.D.; Pietragalla, J.; Mullan, D.M.; Reynolds, M.P. Physiological Breeding II, A Field Guide to Wheat Phenotyping; CIMMYT: México-Veracruz, Mexico, 2012; pp. 72–113. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh leaf tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Yeh, F.C.; Yang, R.; Boyle, T.J.; Ye, Z.; Xiyan, J.M. PopGene32, Microsoft Windows-Based Freeware for Population Genetic Analysis, version 1.32; Molecular Biology and Biotechnology Centre, University of Alberta: Edmonton, AB, Canada, 2000. [Google Scholar]
- Roldán-Ruiz, I.; Dendauw, J.; Van Bockstaele, E.; Depicker, A.; De Loose, M. AFLP markers reveal high polymorphic rates in ryegrasses (Lolium spp.). Mol. Breed. 2000, 6, 125–134. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5, genetic analysis in Excel. Population genetic software for teaching and research an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE, a simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [Green Version]
- Zatybekov, A.; Anuarbek, S.; Abugalieva, S.; Turuspekov, Y. Phenotypic and genetic variability of a tetraploid wheat collection grown in Kazakhstan. Vavilovskii Zhurnal Genet Selektsii 2020, 24, 605–612. [Google Scholar] [CrossRef]
- Anuarbek, S.; Abugalieva, S.; Pecchioni, N.; Laidò, G.; Maccaferri, M.; Tuberosa, R.; Turuspekov, Y. Quantitative trait loci for ag-ronomic traits in tetraploid wheat for enhancing grain yield in Kazakhstan environments. PLoS ONE 2020, 15, e0234863. [Google Scholar] [CrossRef]
- Trethowan, R.M.; Morgunov, A.; He, Z.; De Pauw, R.; Crossa, J.; Warburton, M.; Baytasov, A.; Zhang, C.; Mergoum, M.; Alvarado, G. The global adaptation of bread wheat at high latitudes. Euphytica 2006, 152, 303–316. [Google Scholar] [CrossRef]
- Yusov, V.S.; Evdokimov, M.G.; Kiriakova, M.N.; Glushakov, D.A. Using the gene pool of CIMMYT cultivars and lines in spring durum wheat breeding in Western Siberia. Proc. Appl. Bot. Genet. Breed. 2022, 183, 95–103. (In Russian) [Google Scholar] [CrossRef]
- Azhgaliev, T.B. Cereals. In State List of Breeding Achievements Proposed for Use in the Republic of Kazakhstan, 2nd ed.; Sutula, Y.V., Nurgaziev, R.E., Sharipova, G.A., Zhubatganov, A.A., Yeskakov, D.G., Turgaraeva, A.K., Seitpenbetova, G.M., Gabdola, A.J., Eds.; Ministry of Agriculture of the Republic of Kazakhstan: Astana, Kazakhstan, 2022; pp. 12–13. [Google Scholar]
- Mhlaba, Z.B.; Mashilo, J.; Shimelis, H.; Assefa, A.B.; Modi, A.T. Progress in genetic analysis and breeding of tepary bean (Phaseolus acutifolius A. Gray), A review. Sci. Hortic. 2018, 237, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.K.; Chand, P. Assessment of genetic diversity among twenty Indian wheat (Triticum aestivum L.) cultivars using simple sequence repeat (SSR) markers. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 1708–1717. [Google Scholar]
- Domb, K.; Keidar, D.; Yaakov, B.; Khasdan, V.; Kashkush, K. Transposable elements generate population-specific insertional patterns and allelic variation in genes of wild emmer wheat (Triticum turgidum ssp. dicoccoides). BMC Plant Biol. 2017, 17, 175. [Google Scholar] [CrossRef] [Green Version]
- Vuorinen, A.L.; Kalendar, R.; Fahima, T.; Korpelainen, H.; Nevo, E.; Schulman, A.H. Retrotransposon-based genetic diversity assessment in wild emmer wheat (Triticum turgidum ssp. dicoccoides). Agronomy 2018, 8, 107. [Google Scholar] [CrossRef] [Green Version]
- Demirel, F. Genetic diversity of Emmer wheats using iPBS markers. Avrupa Bilim ve Teknoloji Dergisi 2020, 20, 640–646. [Google Scholar]
- Kizilgeci, F.; Bayhan, B.; Türkoğlu, A.; Haliloglu, K.; Yildirim, M. Exploring genetic diversity and Population structure of five Aegilops species with inter-primer binding site (iPBS) markers. Mol. Biol. Rep. 2022, 49, 8567–8574. [Google Scholar] [CrossRef] [PubMed]
- Haliloğlu, K.; Türkoğlu, A.; Öztürk, A.; Niedbała, G.; Niazian, M.; Wojciechowski, T.; Piekutowska, M. Genetic Diversity and Population Structure in Bread Wheat Germplasm from Türkiye Using iPBS-Retrotransposons-Based Markers. Agronomy 2023, 13, 255. [Google Scholar] [CrossRef]
- Marzang, N.; Abdollahi Mandoulakani, B.; Shaaf, S.; Ghadimzadeh, M.; Bernousi, I.; Abbasi Holasou, H.; Sadeghzadeh, B. IRAP and REMAP-based genetic diversity among Iranian, Turkish, and International Durum wheat (Triticum turgidum L.) cultivars. J. Agric. Sci. Technol. 2020, 22, 271–285. [Google Scholar]
- Carvalho, A.; Guedes-Pinto, H.; Martins-Lopes, P.; Lima-Brito, J. Genetic variability of Old Portuguese bread wheat cultivars as-sayed by IRAP and REMAP markers. Ann. Appl. Biol. 2010, 156, 337–345. [Google Scholar] [CrossRef]
- Alemu, A.; Feyissa, T.; Letta, T.; Abeyo, B. Genetic diversity and population structure analysis based on the high density SNP markers in Ethiopian durum wheat (Triticum turgidum ssp. durum). BMC Genet. 2020, 21, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, F.; Yılmaz, A.; Nadeem, M.A.; Habyarimana, E.; Subaşı, I.; Nawaz, M.A.; Chaudhary, H.J.; Shahid, M.Q.; Ercişli, S.; Zia, M.A.; et al. Mobile genomic element diversity in world collection of safflower (Carthamus tinctorius L.) panel using iPBS retrotransposon markers. PLoS ONE 2019, 14, e0211985. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.K.; Pandey, A.; Thomas, G.; Akkaya, M.S.; Kayis, S.A.; Ozsensoy, Y.; Hamurcu, M.; Gezgin, S.; Topal, A.; Hakki, E.E. Genetic diversity and population structure of wheat in India and Turkey. AoB Plants 2015, 7, plv083. [Google Scholar] [CrossRef]
- Pour, A.H.; Karahan, F.; Ilhan, E.; Ilçim, A.; Haliloglu, K. Genetic structure and diversity of Adonis, L. (Ranunculaceae) populations collected from Turkey by inter-primer binding site (iPBS) retrotransposon markers. Turk. J. Bot. 2019, 43, 585–596. [Google Scholar] [CrossRef]
- Solouki, M.; Mehdikhani, H.; Zeinali, H.; Emamjomeh, A.A. Study of genetic diversity in Chamomile (Matricaria chamomilla) based on morphological traits and molecular markers. Sci. Hortic. 2008, 117, 281–287. [Google Scholar] [CrossRef]
- Newell, M.A.; Cook, D.; Hofmann, H.; Jannink, J.L. An algorithm for deciding the number of clusters and validation using simulated data with application to exploring crop population structure. Ann. App. Stat. 2013, 1, 1898–1916. [Google Scholar] [CrossRef] [Green Version]
- Bouchet, S.; Pot, D.; Deu, M.; Rami, J.F.; Billot, C.; Perrier, X.; Rivallan, R.; Gardes, L.; Xia, L.; Wenzl, P.; et al. Genetic structure, linkage disequilibrium and signature of selection in sorghum, lessons from physically anchored DArT markers. Pone 2012, 7, e33470. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Breeding Program | Geographical Site 1 | Coordinates | Precipitation May–August, mm | Number of Entries in the Study |
|---|---|---|---|---|
| Aktobe Agric. Research Station (KZ) | Aktobe, Aktobe region | 50.3519° N, 57.3928° E | 131 | 20 |
| Karabalyk Agric. Research Station (KZ) | Karabalyk, Kostanay region | 53.8540° N, 62.1015° E | 218 | 25 |
| Scientific Production Center of Grain Farming named after A.I. Barayev (KZ) | Shortandy, Akmola region | 51.4024° N, 71.0049° E | 225 | 14 |
| Kazakh Scientific Research Institute of Agriculture and Plant Growing (KZ) | Almaty, Almaty region | 43.2475° N, 76.6959° E | 509 | 25 |
| Altay Agric. Research Institute (RU) | Barnaul, Altay region | 53.4125° N, 83.5190° E | 230 | 20 |
| Omsk Agricultural Research Center (RU) | Omsk, Omsk region | 55.0404° N, 73.3604° E | 238 | 25 |
| Samara Agric. Research Institute (RU) | Bezenchuk, Samara region 2 | 52.9644° N, 49.4187° E | 180 | 14 |
| Southeast Agricultural Research Institute (RU) | Saratov, Saratov region 2 | 51.3420° N, 45.5952° E | 175 | 5 |
| Primer Name | Sequence | Annealing Temperature (°C) |
|---|---|---|
| 2228 | CATTGGCTCTTGATACCA | 53 |
| 2074 | GCTCTGATACCA | 50 |
| 2226 | CGGTGACCTTTGATACCA | 53 |
| 2239 | ACCTAGGCTCGGATGCCA | 55 |
| 2245 | GAGGTGGCTCTTATACCA | 50 |
| 2252 | TCATGGCTCATGATACCA | 52 |
| 2256 | GACCTAGCTCTAATACCA | 51 |
| 2270 | ACCTGGCGTGCCA | 55 |
| 2271 | GGCTCGGATGCCA | 55 |
| 2389 | ACATCCTTCCCA | 50 |
| Trait | Almaty | Shortandy | LSD 0.05 | Correlation Almaty-Shortandy 2 |
|---|---|---|---|---|
| Days to heading | 60.7 | 42.7 | 0.6 | 0.698 *** |
| Plant height, cm | 103.2 | 71.9 | 1.3 | 0.534 *** |
| Spikes/plant | 1.17 1 | 1.50 | - | −0.067 |
| Spike length, cm | 8.8 | 6.2 | 0.2 | 0.453 *** |
| Awn length, cm | 11.5 1 | 8.9 | - | 0.633 *** |
| Spikelets/spike | 18.8 | 12.1 | 0.2 | 0.450 *** |
| Grains/spike | 49.9 | 24.6 | 0.7 | 0.277 *** |
| Grains/spikelet | 2.66 | 2.03 | 0.05 | 0.279 *** |
| Grain weight/spike, g | 2.07 | 1.15 | 0.04 | 0.164 * |
| 1000 KW | 39.8 | 44.1 | 0.7 | 0.699 *** |
| Yield, g/m2 | 243 | 170 | 9 | −0.006 |
| Trait | Coefficients of Correlation with Grain Yield: | |||
|---|---|---|---|---|
| Almaty | Shortandy | |||
| 2020 | 2022 | 2021 | 2022 | |
| Days to heading | −0.140 | −0.412 *** | 0.209 ** | 0.290 *** |
| Plant height, cm | 0.241 ** | 0.202 * | 0.570 *** | 0.219 ** |
| Spikes/plant | 0.025 | - | 0.197 * | 0.082 |
| Spike length, cm | 0.173 * | −0.078 | 0.429 *** | 0.221 ** |
| Awn length, cm | 0.101 | - | 0.215 ** | 0.013 |
| Spikelets/spike | 0.151 | −0.084 | 0.423 *** | 0.216 ** |
| Grains/spike | 0.229 ** | 0.193 * | 0.422 *** | 0.213 ** |
| Grains/spikelet | 0.164 * | 0.275 *** | 0.252 ** | 0.092 |
| Grain weight/spike, g | 0.330 *** | 0.509 *** | 0.422 *** | 0.265 *** |
| 1000 KW | 0.100 | 0.377 *** | 0.194 * | 0.063 |
| Entry | Germplasm Name | Originator | Days to Heading | Plant Height, cm | Grains/Main Spike | 1000 KW, g | Yield, g/m2-Rank | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Almaty | Shortandy | Almaty | Shortandy | Almaty | Shortandy | Almaty | Shortandy | Almaty | Shortandy | |||||
| 172 | Nauryz-6 (LC-1) | Almaty | 60.2 | 41.8 | 108 | 67 | 43.9 | 27.2 | 36.2 | 40.6 | 203 | 200 | ||
| 47 | Altyn-Dala (LC-2) | Karabalyk | 61.5 | 43.5 | 107 | 75 | 46.7 | 23.3 | 44.8 | 48.3 | 286 | 190 | ||
| 121 | Zhemch. Sibiri (LC-3) | Omsk | 61.0 | 37.5 | 110 | 70 | 49.7 | 29.8 | 41.2 | 43.1 | 274 | 204 | ||
| 149 | Line-250-06-14 | Shortandy | 66.5 | 49.8 | 96 | 71 | 54.4 | 24.4 | 38.6 | 44.7 | 436 | 1 | 208 | 16 |
| 27 | Gordeiforme-91-22-2 | Omsk | 53.5 | 41.5 | 106 | 72 | 49.8 | 23.1 | 44.6 | 42.3 | 411 | 2 | 147 | 117 |
| 68 | Ertol | Almaty | 60.5 | 43.0 | 105 | 71 | 47.8 | 20.4 | 49.4 | 44.9 | 399 | 3 | 156 | 97 |
| 26 | Gordeiforme-91-102-6 | Omsk | 52.5 | 38.8 | 107 | 70 | 45.3 | 30.0 | 45.4 | 42.2 | 357 | 4 | 151 | 110 |
| 160 | Line-1970d-5 | Samara | 60.5 | 42.0 | 101 | 70 | 55.5 | 24.4 | 41.7 | 46.7 | 341 | 5 | 146 | 119 |
| 69 | Altayskiy Yantar | Barnaul | 61.0 | 42.8 | 98 | 73 | 50.1 | 23.4 | 40.8 | 44.1 | 330 | 6 | 142 | 127 |
| 25 | Gordeiforme-94-71 | Omsk | 60.0 | 41.8 | 108 | 74 | 48.4 | 26.2 | 46.0 | 46.5 | 322 | 7 | 189 | 34 |
| 147 | Serke | Almaty | 58.5 | 39.0 | 105 | 63 | 50.6 | 21.4 | 40.2 | 38.5 | 321 | 8 | 167 | 67 |
| 157 | Gordeiforme-08-67-1 | Omsk | 64.0 | 45.8 | 108 | 73 | 64.2 | 28.7 | 36.4 | 41.5 | 315 | 9 | 169 | 64 |
| 89 | Kargala-1408 | Aktobe | 56.0 | 41.5 | 103 | 68 | 47.4 | 23.6 | 44.8 | 48.2 | 315 | 10 | 155 | 100 |
| Average for top ten Almaty performers | 59.3 | 42.6 | 104 | 70 | 51.3 | 24.6 | 42.8 | 44.0 | 355 | - | 163 | - | ||
| 86 | Gordeiforme-00-96-8 | Omsk | 68.5 | 46.2 | 103 | 74 | 59.4 | 31.6 | 28.2 | 40.7 | 214 | 99 | 286 | 1 |
| 155 | Gordeiforme-924 | Barnaul | 65.0 | 46.5 | 102 | 81 | 57.5 | 23.7 | 43.9 | 49.1 | 310 | 15 | 268 | 2 |
| 154 | Gordeiforme-910 | Barnaul | 63.0 | 45.0 | 107 | 74 | 53.1 | 29.1 | 42.0 | 48.4 | 174 | 132 | 246 | 3 |
| 59 | Gordeiforme-95-139-4 | Omsk | 61.0 | 43.8 | 101 | 77 | 53.1 | 25.8 | 44.3 | 50.6 | 258 | 62 | 242 | 4 |
| 23 | Gordeiforme-242-93 | Karabalyk | 61.5 | 44.7 | 103 | 77 | 43.8 | 24.1 | 37.2 | 46.1 | 224 | 94 | 241 | 5 |
| 118 | Leucurum-1469d-21 | Samara | 58.0 | 40.8 | 107 | 78 | 47.5 | 28.8 | 49.0 | 51.1 | 266 | 47 | 240 | 6 |
| 123 | Kargala-238 | Aktobe | 60.5 | 48.3 | 106 | 77 | 51.7 | 22.3 | 40.6 | 45.7 | 194 | 117 | 239 | 7 |
| 45 | Seymur | Almaty | 65.0 | 48.8 | 96 | 71 | 60.8 | 32.2 | 29.2 | 37.9 | 144 | 144 | 235 | 8 |
| 151 | Gordeiforme-1790 | Karabalyk | 64.0 | 47.0 | 110 | 80 | 56.9 | 27.8 | 37.4 | 43.0 | 292 | 29 | 225 | 9 |
| 150 | Kostanayskaya-15 | Karabalyk | 63.5 | 47.8 | 108 | 81 | 41.9 | 25.9 | 40.0 | 44.4 | 263 | 53 | 222 | 10 |
| Average for top ten Shrotandy performers | 63.0 | 45.9 | 104 | 77 | 52.6 | 27.1 | 39.2 | 45.7 | 234 | - | 244 | - | ||
| Primer | TNB 1 | PB | Polymorphism (%) | Ne | h | I | ht | PIC |
|---|---|---|---|---|---|---|---|---|
| 2228 | 23 | 20 | 86.96 | 1.248 | 0.165 | 0.277 | 0.165 | 0.165 |
| 2074 | 36 | 35 | 97.22 | 1.435 | 0.265 | 0.412 | 0.246 | 0.262 |
| 2226 | 44 | 44 | 100.00 | 1.514 | 0.309 | 0.472 | 0.293 | 0.308 |
| 2239 | 36 | 33 | 91.67 | 1.524 | 0.306 | 0.462 | 0.270 | 0.307 |
| 2245 | 32 | 32 | 100.00 | 1.498 | 0.293 | 0.446 | 0.293 | 0.294 |
| 2252 | 33 | 32 | 96.97 | 1.403 | 0.251 | 0.395 | 0.233 | 0.251 |
| 2256 | 31 | 29 | 93.55 | 1.407 | 0.260 | 0.406 | 0.260 | 0.260 |
| 2270 | 36 | 27 | 75.00 | 1.487 | 0.266 | 0.385 | 0.257 | 0.267 |
| 2271 | 38 | 33 | 86.84 | 1.480 | 0.280 | 0.420 | 0.261 | 0.281 |
| 2389 | 36 | 32 | 88.88 | 1.186 | 0.118 | 0.201 | 0.056 | 0.118 |
| Mean | 345 | 317 | 91.88 | 1.418 | 0.251 | 0.388 | 0.233 | 0.251 |
| Source | Df 1 | SS | MS | Est. Var. | % |
|---|---|---|---|---|---|
| Among Population | 2 | 2,467,708 | 822,569 | 22,257 | 41% |
| Within Populations | 147 | 4,765,921 | 32,421 | 32,421 | 59% |
| Total | 150 | 7,233,629 | 54,678 | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajibayev, D.; Mukin, K.; Babkenov, A.; Chudinov, V.; Dababat, A.A.; Jiyenbayeva, K.; Kenenbayev, S.; Savin, T.; Shamanin, V.; Tagayev, K.; et al. Exploring the Agronomic Performance and Molecular Characterization of Diverse Spring Durum Wheat Germplasm in Kazakhstan. Agronomy 2023, 13, 1955. https://doi.org/10.3390/agronomy13071955
Tajibayev D, Mukin K, Babkenov A, Chudinov V, Dababat AA, Jiyenbayeva K, Kenenbayev S, Savin T, Shamanin V, Tagayev K, et al. Exploring the Agronomic Performance and Molecular Characterization of Diverse Spring Durum Wheat Germplasm in Kazakhstan. Agronomy. 2023; 13(7):1955. https://doi.org/10.3390/agronomy13071955
Chicago/Turabian StyleTajibayev, Daniyar, Kadyrzhan Mukin, Adylkhan Babkenov, Vladimir Chudinov, Abdelfattah A. Dababat, Karlyga Jiyenbayeva, Serik Kenenbayev, Timur Savin, Vladimir Shamanin, Kuttymurat Tagayev, and et al. 2023. "Exploring the Agronomic Performance and Molecular Characterization of Diverse Spring Durum Wheat Germplasm in Kazakhstan" Agronomy 13, no. 7: 1955. https://doi.org/10.3390/agronomy13071955







