Significant Effects of Long-Term Application of Straw and Manure Combined with NPK Fertilizers on Olsen P and PAC in Red Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Used Material
| Latitude | Longitude | Altitude (m) | MAT (°C) | MAP (mm) | MAE (mm) |
|---|---|---|---|---|---|
| 26°45′ | 111°52′ | 120 | 18.0 | 1426 | 1435 |
| SOC (g kg−1) | TN (g kg−1) | AN (mg kg−1) | TP (g kg−1) | Olsen-P0 (mg kg−1) | TK (g kg−1) |
| 7.9 | 1.07 | 79 | 0.45 | 13.9 | 13.7 |
| AK (mg kg−1) | pH | Sand (%) | Silt (%) | Clay (%) | BD (g cm−3) |
| 104 | 5.7 | 24.3 | 31.8 | 43.9 | 1.2 |
| Treatment | Inorganic Fertilizer (N-P-K kg ha−1 yr−1) | Manure (t ha−1 yr−1) | Types of Manure | Straw | Crop Rotation | |
|---|---|---|---|---|---|---|
| NPK (1) | C (2)-210-36.7-69.7 | W-90-15.7-30 | 0 | - | 0 | Wheat-Corn |
| NPKS | C-210-36.7-69.7 | W-90-15.7-30 | 0 | - | 1/2 straw returning to field | |
| M | C-0-0-0 | W-0-0-0 | C-42/W-18 | Pig manure | 0 | |
| MNPK | C-63-36.7-69.7 | W-27-15.7-30 | C-29.4/W-12.6 | Pig manure | 0 | |
2.2. Experimental Design and Field Management
2.3. Soil Sampling and Analysis
2.4. Data Analysis
2.5. Statistical Analyses
3. Results
3.1. Increase of Olsen P under P Surplus
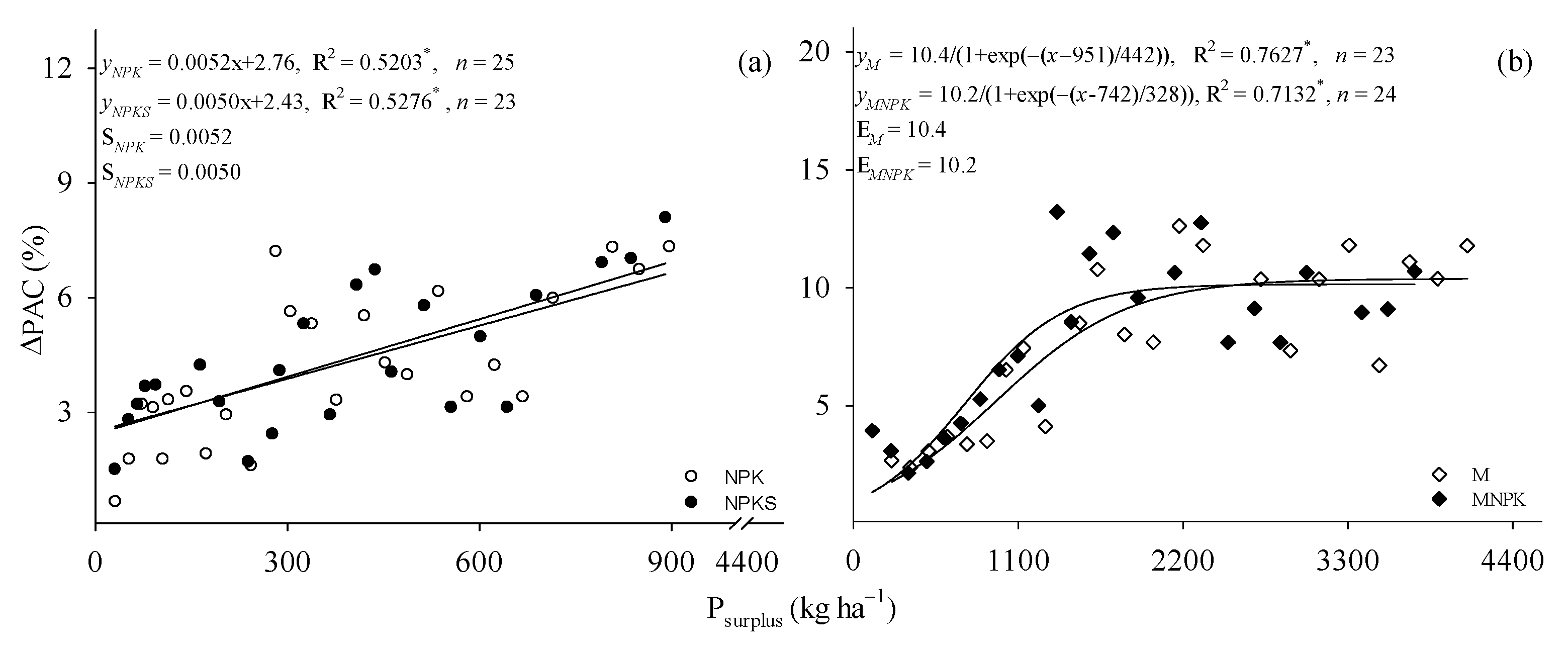
3.2. Increase of PAC under P Surplus
3.3. Comparison of P Fraction Changes for Jiang-Gu Method
3.4. Comparison of P Fraction Changes for Hedley Method
| Treatment | 2000 (year) | 2013 (year) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔCa2-P (1) (mg kg−1) | ΔCa8-P (mg kg−1) | ΔAl-P (mg kg−1) | ΔFe-P (mg kg−1) | ΔO-P (mg kg−1) | ΔCa10-P (mg kg−1) | ΔCa2-P (mg kg−1) | ΔCa8-P (mg kg−1) | ΔAl-P (mg kg−1) | ΔFe-P (mg kg−1) | ΔO-P (mg kg−1) | ΔCa10-P (mg kg−1) | |
| NPK (2) | 2.3 ± 1.0 bB | 6.1 ± 4.2 cB | 47.5 ± 10.7 cB | 344.4 ± 94.1 bA | 8.4 ± 2.9 aA | 6.6 ± 3.6 aB | 88.7 ± 26.3 cA | 73.9 ± 11.1 cA | 96.0 ± 13.2 aA | 90.5 ± 33.6 bB | 65.1 ± 13.6 bA | 31.9 ± 5.6 bA |
| NPKS | 3.3 ± 0.4 bB | 14.3 ± 1.1 bB | 64.2 ± 6.0 bB | 348.4 ± 70.4 bA | −5.3 ± 0.8 cB | −4.5 ± 0.9 cB | 98.4 ± 23.3 cA | 84.5 ± 20.0 cA | 102.4 ± 20.8 aA | 100.7 ± 8.5 bB | 67.5 ± 10.1 bA | 30.7 ± 8.3 bA |
| M | 58.8 ± 21.0 aB | 63.6 ± 27.8 aB | 114.6 ± 36.1 aA | 552.0 ± 119.5 aA | 0.2 ± 0.5 bB | 8.5 ± 2.4 bB | 145.1 ± 21.2 bA | 130.6 ± 23.5 bA | 127.4 ± 34.4 aA | 154.7 ± 24.8 aB | 102.8 ± 6.9 aA | 69.9 ± 5.8 aA |
| MNPK | 69.5 ± 20.1 aB | 68.3 ± 21.7 aB | 151.5 ± 45.2 aA | 707.8 ± 161.2 aA | −5.9 ± 0.8 cB | 16.9 ± 4.3 aB | 205.6 ± 16.1 aA | 250.9 ± 33.3 aA | 136.1 ± 23.0 aA | 160.9 ± 24.2 aB | 104.4 ± 11.9 aA | 76.1 ± 10.7 dA |
| Treatment | ΔCa2-P (%) | ΔCa8-P (%) | ΔAl-P (%) | ΔFe-P (%) | ΔO-P (%) | ΔCa10-P (%) | ΔCa2-P (%) | ΔCa8-P (%) | ΔAl-P (%) | ΔFe-P (%) | ΔO-P (%) | ΔCa10-P (%) |
| NPK | −2.0 ± 1.5 bB | −0.3 ± 0.4 bB | 0.9 ± 0.3 bA | 32.3 ± 12.3 aA | −27.8 ± 9.5 aB | −3.1 ± 1.1 aB | 18.0 ± 8.1 aA | 16.6 ± 6.0 aA | 13.0 ± 33 aA | 6.2 ± 2.2 aB | −46.2 ± 9.3 aA | −3.2 ± 0.4 abA |
| NPKS | −2.3 ± 0.9 bB | −0.5 ± 0.7 bB | 1.0 ± 0.3 bA | 32.2 ± 7.7 aA | −26.3 ± 4.8 aB | −4.1 ± 0.9 aB | 18.4 ± 7.2 aA | 14.3 ± 4.4 aA | 15.7 ± 9 aA | 5.1 ± 1.1 aB | −45.9 ± 11.8 aA | −4.1 ± 1.0 bA |
| M | 1.8 ± 0.5 aA | 4.8 ± 2.0 aA | 4.8 ± 1.2 aA | 23.9 ± 7.7 aA | −31.0 ± 10.7 aB | −4.2 ± 2.1 aB | 16.5 ± 4.9 aB | 15.1 ± 7.4 aB | 11.3 ± 5.6 aB | 5.5 ± 0.9 aB | −38.2 ± 5.7 aA | −8.2 ± 0.9 cA |
| MNPK | 1.8 ± 0.5 aA | 3.6 ± 1.2 aA | 5.4 ± 2.2 aA | 26.4 ± 10.8 aA | −33.0 ± 8.8 aB | −4.1 ± 2.8 aB | 18.2 ± 4.3 aB | 24.3 ± 8.8 aB | 7.4 ± 2.2 aB | 1.8 ± 0.6 bB | −49.7 ± 9.0 aA | −2.0 ± 0.5 aA |
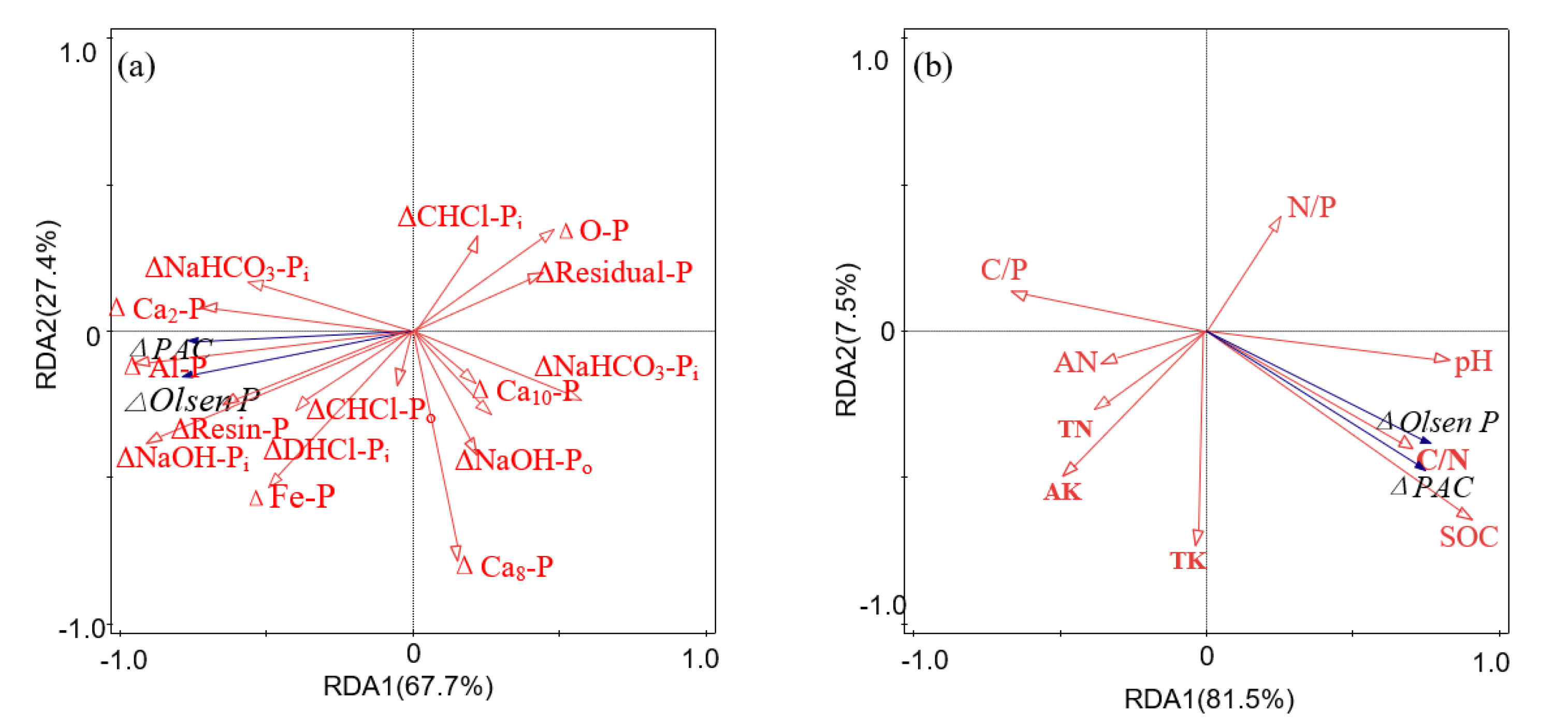
3.5. Influence Factors of ΔOlsen P and ΔPAC
4. Discussion
4.1. ΔOlsen P, ΔPAC, and P surplus
4.2. Change of P Fractions
4.3. Influencing Factors of ΔOlsen P and ΔPAC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Demay, J.; Ringeval, B.; Pellerin, S.; Nesme, T. Half of global agricultural soil phosphorus fertility derived from anthropogenic sources. Nat. Geosci. 2023, 16, 69–74. [Google Scholar] [CrossRef]
- Wu, Q.H.; Zhang, S.X.; Zhu, P.; Huang, S.M.; Wang, B.R.; Zhao, L.P.; Xu, M.G. Characterizing differences in the phosphorus activation coefficient of three typical cropland soils and the influencing factors under long-term fertilization. PLoS ONE 2017, 12, e0176437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Zhao, T.; Tarin, M.W.K.; Han, Y.Z.; Hu, W.F.; Rong, J.D.; He, T.Y.; Zheng, Y.S. Effect of various mulch materials on chemical properties of soil, leaves and shoot characteristics in Dendrocalamus Latiflorus Munro forests. Plants 2021, 10, 2302. [Google Scholar] [CrossRef] [PubMed]
- Abdala, D.B.; Ghosh, A.K.; Silva, I.; Novais, R.; Venegas, V. Phosphorus saturation of a tropical soil and related P leaching caused by poultry litter addition. Agric. Ecosyst. Environ. 2012, 162, 15–23. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, S.; Hu, R.; Li, Y. Aggregate stability and size distribution of red soils under different land uses integrally regulated by soil organic matter, and iron and aluminum oxides. Soil Tillage Res. 2017, 167, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.H.; Yan, X.J.; Wang, M.K.; Cai, Y.Y.; Weng, X.F.; Su, D.; Guo, G.X.; Wang, W.Q.; Hou, Y.; Ye, D.L.; et al. Long-term excessive phosphorus fertilization alters soil phosphorus fractions in the acidic soil of pomelo orchards. Soil Tillage Res. 2022, 215, 105214. [Google Scholar] [CrossRef]
- Sun, F.X.; Sun, N.; Ma, X.Z.; Zhou, B.K.; Zhu, P.; Gao, H.J.; Xu, M.G. The application of fertilizer phosphorus affected Olsen P and the phosphorus fractions of Hedley method in black soil. Agronomy 2022, 12, 3146. [Google Scholar] [CrossRef]
- Cai, A.; Zhang, W.; Xu, M.; Wang, B.; Wen, S.; Shah, S.A.A. Soil fertility and crop yield after manure addition to acidic soils in South China. Nutr. Cycl. Agroecosyst. 2018, 111, 61–72. [Google Scholar] [CrossRef]
- Ahmed, W.; Huang, J.; Liu, K.; Ali, S.; Chen, D.Y. Impacts of long-term inorganic and organic fertilization on phosphorus adsorption and desorption characteristics in red paddies in southern china. PLoS ONE 2021, 16, e0246428. [Google Scholar] [CrossRef]
- Suriyagoda, L.; De Costa, W.A.J.M.; Lambers, H. Growth and phosphorus nutrition of rice when inorganic fertiliser application is partly replaced by straw under varying moisture availability in sandy and clay soils. Plant Soil 2014, 384, 53–68. [Google Scholar] [CrossRef]
- Amanullah; Khan, S.T.; Iqbal, A.; Fahad, S. Growth and productivity response of hybrid rice to application of animal manures, plant residues and phosphorus. Front. Plant Sci. 2016, 7, 1440. [Google Scholar]
- Zhuang, M.H.; Zhang, J.; Kong, Z.Y.; Fleming, R.M.; Zhang, C.Y.; Zhang, Z.Y. Potential environmental benefits of substituting nitrogen and phosphorus fertilizer with usable crop straw in China during 2000–2017. J. Clean. Prod. 2020, 267, 122125. [Google Scholar] [CrossRef]
- Whalen, J.K.; Chi, C. Phosphorus accumulation in cultivated soils from long-term annual applications of cattle feedlot manure. J. Environ. Qual. 2001, 30, 229. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.K.; Zhang, W.J.; Guo, Z.B.; Wang, D.Z.; Oenema, O. Evaluating crop response and environmental impact of theaccumulation of phosphorus due to long-term manuring of vertisol soilin northern China. Agric. Ecosyst. Environ. 2016, 219, 101–110. [Google Scholar] [CrossRef]
- Fei, C.; Zhang, S.; Wei, W.; Liang, B.; Li, J.L.; Ding, X.D. Straw and optimized nitrogen fertilizer decreases phosphorus leaching risks in a long-term greenhouse soil. J. Soils Sediments 2020, 20, 1199–1207. [Google Scholar] [CrossRef]
- Koch, M.; Guppy, C.; Amelung, W.; Gypser, S.; Bol, R.; Seidel, S.; Siebers, N. Insights into 33phosphorus utilisation from fe- and Al-hydroxides in luvisol and ferralsol subsoils. Soil Res. 2019, 57, 447–458. [Google Scholar] [CrossRef]
- Stutter, M.I.; Shand, C.A.; George, T.S.; Blackwell, M.; Dixon, L.; Bol, R.; MacKay, R.L.; Richardson, A.E.; Condron, L.M.; Haygarth, P.M. Land use and soil factors affecting accumulation of phosphorus species in temperate soils. Geoderma 2015, 257–258, 29–39. [Google Scholar] [CrossRef]
- Jiang, B.F.; Gu, Y.C. A suggested fractionation scheme of inorganic phosphorus in calcareous soils. Sci. Agric. Sin. 1989, 22, 58–66. [Google Scholar] [CrossRef]
- Tiessen, H.; Moir, J. Characterization of Available P by Sequential Extraction; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. J. Soil Sci. Soc. Am. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Tandy, S.; Hawkins, J.M.B.; Dunham, S.J.; Hernandez-Allica, J.; Granger, S.J.; Yuan, H.M.; McGrath, S.P.; Blackwell, M.S. Investigation of the soil properties that affect Olsen P critical values in different soil types and impact on P fertiliser recommendations. Eur. J. Soil Sci. 2021, 72, 1802–1816. [Google Scholar] [CrossRef]
- Cao, N.; Zhi, M.L.; Zhao, W.P.; Pang, J.Y.; Hu, W.; Zhou, Z.G.; Meng, Y.L. Straw retention combined with phosphorus fertilizer promotes soil phosphorus availability by enhancing soil P-related enzymes and the abundance of phoC and phoD genes. Soil Tillage Res. 2022, 220, 105390. [Google Scholar] [CrossRef]
- Battisti, M.; Moretti, B.; Sacco, D.; Grignani, C.; Zavattaro, L. Soil Olsen P response to different phosphorus fertilization strategies in long-term experiments in NW Italy. Soil Use Manag. 2022, 38, 549–563. [Google Scholar] [CrossRef]
- Debicka, M.; Kocowicz, A.; Weber, J.; Jamroz, E. Organic matter effects on phosphorus sorption in sandy soils. Arch. Agron. Soil Sci. 2016, 62, 840–855. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Yang, X. Effect of organic matter on phosphorus adsorption and desorption in a black soil from northeast china. Soil Tillage Res. 2019, 187, 85–91. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods of Soil Agricultural Chemistry. China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Page, A.L.; Miller, R.H.; Dennis, R.K. Methods of Soil Analysis. Part 2 Chemical Methods; Soil Science Society of America Inc.: Madison, WI, USA, 1982. [Google Scholar]
- Withers, P.; Edwards, A.C.; Foy, R.H. Phosphorus cycling in uk agriculture and implications for phosphorus loss from soil. Soil Use Manag. 2010, 17, 139–149. [Google Scholar] [CrossRef]
- Shen, P.; Xu, M.G.; Zhang, H.M.; Yang, X.Y.; Huang, S.M.; Zhang, S.X.; He, X.H. Long-term response of soil Olsen P and organic C to the depletion or addition of chemical and organic fertilizers. Catena 2014, 118, 20–27. [Google Scholar] [CrossRef]
- Zhang, W.W.; Wang, Q.; Wu, Q.H.; Zhang, S.X.; Zhu, P.; Peng, C.; Huang, S.M.; Wang, B.R.; Zhang, H.M. The response of soil Olsen-P to the P budgets of three typical cropland soil types under long-term fertilization. PLoS ONE 2020, 15, e0230178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, B.; Liang, Y.C.; Li, Z.J.; Han, F.X. Phosphorus adsorption and bioavailability in a paddy soil amended with pig manure compost and decaying rice straw. Commun. Soil Sci. Plant Anal. 2009, 40, 2185–2199. [Google Scholar] [CrossRef]
- Hua, K.K.; Zhu, B.; Li, C.C. Pathways of dissolved unreactive phosphorus loss under long-term crop straw and manure application. Nutr. Cycl. Agroecosystems 2021, 120, 161–175. [Google Scholar] [CrossRef]
- Messiga, A.J.; Ziadi, N.; Angers, D.A.; Morel, C.; Parent, L.E. Tillage practices of a clay loam soil affect soil aggregation and associated C and P concentration. Geoderma 2011, 164, 225–231. [Google Scholar] [CrossRef]
- Lan, Z.M.; Lin, X.J.; Wang, F.; Zhang, H.; Chen, C.R. Phosphorus availability and rice grain yield in a paddy soil in response to long-term fertilization. Biol. Fertil. Soils 2012, 48, 579–588. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yang, R.; Gao, R.; Wei, H.A.; Chen, A.l.; Li, Y. Effects of long-term phosphorus fertilization and straw incorporation on phosphorus fractions in subtropical paddy soil. J. Integr. Agric. 2015, 14, 365–373. [Google Scholar] [CrossRef]
- Gou, X.; Cai, Y.; Wang, C.; Li, B.; Zhang, Y.; Tang, X. Effects of different long-term cropping systems on phosphorus adsorption and desorption characteristics in red soils. J. Soil Sediments 2020, 20, 1371–1382. [Google Scholar] [CrossRef]
- Moshi, A.O.; Wild, A.; Greenland, D.J. Effect of organic matter on the charge and phosphate adsorption characteristics of kikuyu red clay from kenya. Geoderma 1974, 11, 275–285. [Google Scholar] [CrossRef]
- Soma, D.M.; Kiba, D.I.; Ewusi-Mensah, N.; Gnankambary, Z.; Lompo, F.; Sedogo, M.P.; Abaidoo, R.C. Changes in sorghum production, soil p forms and p use efficiency following long-term application of manure, compost and straw in a ferric lixisol. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2017, 68, 401–411. [Google Scholar] [CrossRef]
- Bera, R.; Seal, A.; Bhattacharyya, P.; Mukhopadhyay, K.; Giri, R. Phosphate sorption desorption characteristics of some ferruginous soils of tropical region in Eastern India. Environ. Geol. 2006, 51, 399–407. [Google Scholar] [CrossRef]
- Arias, M.; Da Silva-Carballal, J.; Garcı’a-Rı´o, L.; Mejuto, J.; Nu´ñez, A. Retention of phosphorus by iron and aluminum-oxides-coated quartz particles. J. Colloid Interface Sci. 2006, 295, 65–70. [Google Scholar] [CrossRef]
- Miller, J.J.; Beasley, B.W.; Drury, C.F.; Zebarth, B.J. Available nitrogen and phosphorus in soil amended with fresh or composted cattle manure containing straw or wood-chip bedding. Can. J. Soil Sci. 2010, 90, 341–354. [Google Scholar] [CrossRef]
- Zhan, X.Y.; Zhang, L.; Zhou, B.K.; Zhu, P.; Zhang, S.X.; Xu, M.G. Changes in Olsen phosphorusconcentration and its response to phosphorus balance in black soils under different Long-Term fertilization patterns. PLoS ONE 2015, 10, e0131713. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.J.; Zhang, G.; Wang, Y.; Wang, C.; Teng, Y.; Peter, C. Nitrogen and phosphorus leaching losses from intensively managed paddy fields with straw retention. Agric. Water Manag. 2014, 141, 66–73. [Google Scholar] [CrossRef]
- Ge´rard, F. Clay minerals, iron/aluminum oxides, and their contribution to phosphate sorption in soils A-myth revisited. Geoderma 2016, 262, 213–226. [Google Scholar] [CrossRef]
- Lü, D.A.; Yan, B.W.; Wang, L.X.; Deng, Z.Q.; Zhang, Y.B. Changes in phosphorus fractions and nitrogen forms during composting of pig manure with rice straw. J. Integr. Agric. 2013, 12, 1855–1864. [Google Scholar] [CrossRef]
- Fang, H.; Cui, Z.; He, G.; Huang, L.; Chen, M. Phosphorus adsorption onto clay minerals and iron oxide with consideration of heterogeneous particle morphology. Sci. Total Environ. 2017, 605–606, 357–367. [Google Scholar] [CrossRef] [PubMed]

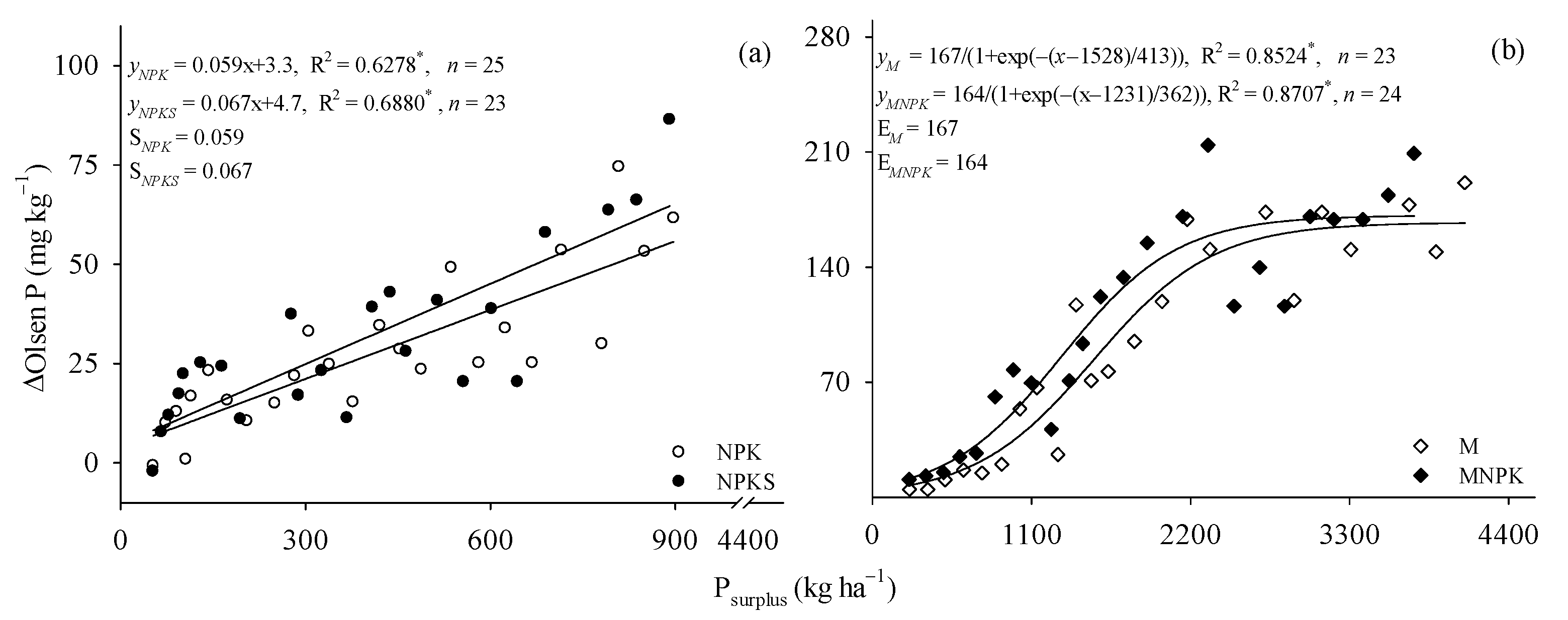
| Treatment | 2000 (year) | 2013 (year) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔResin -P (1) (mg kg−1) | ΔNaHCO3 -Pi (mg kg−1) | ΔNaHCO3 -Po (mg kg−1) | ΔNaOH -Pi (mg kg−1) | ΔNaOH -Po (mg kg−1) | ΔDHCl -Pi (mg kg−1) | ΔCHCl -Pi (mg kg−1) | ΔCHCl -Po (mg kg−1) | Δresidual -P (mg kg−1) | Δresin -P (mg kg−1) | ΔNaHCO3 -Pi (mg kg−1) | ΔNaHCO3 -Po (mg kg−1) | ΔNaOH -Pi (mg kg−1) | ΔNaOH -Po (mg kg−1) | ΔDHCl -Pi (mg kg−1) | ΔCHCl -Pi (mg kg−1) | ΔCHCl -Po (mg kg−1) | Δresidual -P (mg kg−1) | |
| NPK(2) | 3.6 ± 1.0 bB | 54.3 ± 6.6 cB | 4.5 ± 1.6 cB | 81.2 ± 10.9 cB | 1.4 ± 0.6 cB | 4.3 ± 0.7 dB | 31.6 ± 4.4 bB | 9.1 ± 1.8 aB | 3.6 ± 0.5 cB | 99.5 ± 12.8 aA | 116.8 ± 21.5 cA | 8.7 ± 2.8 bA | 133.9 ± 21.8 cA | 12.8 ± 3.3 bA | 57.0 ± 6.5 cA | 103.1 ± 8.9 bA | 17.5 ± 3.6 bA | 11.6 ± 2.8 cA |
| NPKS | 20.8 ± 2.9 aB | 44.3 ± 6.5 cB | 11.8 ± 2.1 bA | 112.6 ± 9.8 bB | 1.4 ± 0.4 cB | 10.0 ± 1.2 cB | 63.2 ± 10.0 aB | 10.0 ± 2.1 aB | 20.0 ± 5.3 bA | 107.7 ± 10.8 aA | 143.7 ± 9.9 cA | 9.0 ± 2.2 bA | 176.7 ± 20.6 bA | 2.6 ± 0.6 cA | 55.6 ± 8.9 cA | 121.4 ± 11.5 abA | 21.6 ± 5.5 abA | 15.0 ± 3.5 cA |
| M | 31.6 ± 12.1 aB | 78.6 ± 9.0 bB | 22.9 ± 4.2 aA | 182.1 ± 31.7 aB | 3.8 ± 1.0 bB | 48.3 ± 7.2 bB | 68.1 ± 5.9 aB | 11.1 ± 2.9 aB | 31.6 ± 6.8 aB | 98.5 ± 7.5 aA | 199.6 ± 26.6 bA | 15.8 ± 2.5 aA | 366.0 ± 44.5 aA | 21.4 ± 6.7 abA | 98.2 ± 9.0 bA | 119.6 ± 20.0 abA | 23.4 ± 3.8 abA | 61.4 ± 8.8 bA |
| MNPK | 41.6 ± 11.9 aB | 108.6 ± 11.5 aB | 27.1 ± 5.1 aA | 282.2 ± 34.7 aB | 8.6 ± 2.1 aB | 68.4 ± 5.1 aB | 78.1 ± 9.9 aB | 15.0 ± 5.1 aB | 45.7 ± 7.7 aB | 118.6 ± 9.9 aA | 269.6 ± 32.1 aA | 19.9 ± 3.6 faA | 466.0 ± 34.8 aA | 31.4 ± 7.6 aA | 148.2 ± 9.9 aA | 139.7 ± 10.7 aA | 28.3 ± 6.4 aA | 101.4 ± 8.9 aA |
| Treatment | ΔResin -P (%) | ΔNaHCO3 -Pi (%) | ΔNaHCO3 -Po (%) | ΔNaOH -Pi (%) | ΔNaOH -Po (%) | ΔDHCl -Pi (%) | ΔCHCl -Pi (%) | ΔCHCl -Po (%) | ΔResidual -P (%) | ΔResin -P % (%) | ΔNaHCO3 -Pi (%) | ΔNaHCO3 -Po (%) | ΔNaOH -Pi (%) | ΔNaOH -Po (%) | ΔDHCl -Pi (%) | ΔCHCl -Pi (%) | ΔCHCl -Po (%) | ΔResidual -P (%) |
| NPK | 0.2 ± 0.04 bB | 7.3 ± 2.0 aB | 0.2 ± 0.02 cB | 6.9 ± 1.1 cA | −1.9 ± 0.4 aA | 0.1 ± 0.02 cB | −5.6 ± 0.7 aA | −0.1 ± 0.02 aA | −7.2 ± 1.1 adA | 8.9 ± 1.1 aA | 12.1 ± 2.1 aA | 0.4 ± 0.1 aA | 3.6 ± 0.7 cB | −2.6 ± 0.8 aA | 3.6 ± 0.3 bA | −9.9 ± 1.0 aB | −0.23 ± 0.1 bB | −15.8 ± 4.7 aB |
| NPKS | 2.2 ± 0.08 aB | 4.8 ± 0.8 bB | 0.9 ± 0.2 bA | 7.6 ± 1.2 cA | −2.6 ± 0.5 bA | 0.5 ± 0.03 bB | −5.6 ± 0.9 aA | −0.6 ± 0.04 bB | −7.2 ± 2.0 aA | 9.4 ± 2.5 aA | 10.0 ± 1.8 aA | −0.02 ± 0.01 cB | 6.9 ± 0.8 bA | −4.6 ± 0.9 bB | 3.2 ± 0.4 bA | −9.0 ± 1.3 aB | 0.02 ± 0.01 aA | −16.0 ± 1.9 aB |
| M | 2.7 ± 0.9 aB | 7.0 ± 1.1 aB | 1.5 ± 0.1 aA | 9.8 ± 0.7 bB | −3.3 ± 1.0 bB | 4.0 ± 0.7 aA | −11.1 ± 1.0 bA | −1.4 ± 0.3 cB | −9.2 ± 2.4 abA | 6.1 ± 1.6 aA | 12.7 ± 1.7 aA | 0.01 ± 0.02 bB | 13.6 ± 1.2 aA | −3.2 ± 0.6 abA | 4.4 ± 1.3 abA | −16.1 ± 1.7 bB | −0.8 ± 0.1 cA | −16.8 ± 2.2 aB |
| MNPK | 2.9 ± 0.8 aB | 8.0 ± 0.9 aB | 1.4 ± 0.2 aA | 13.8 ± 0.9 aA | −3.6 ± 1.1 bA | 4.7 ± 1.0 aA | −14.5 ± 2.6 bA | −1.6 ± 0.3 cB | −11.1 ± 1.7 bA | 7.0 ± 0.9 aA | 14.1 ± 2.4 aA | 0.01 ± 0.01 bB | 13.9 ± 2.0 aA | −3.3 ± 1.1 abA | 5.8 ± 1.1 aA | −18.5 ± 2.8 bB | −1.0 ± 0.2 cA | −17.0 ± 2.8 aB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, F.; Sun, N.; Wang, B.; Cai, Z.; Xu, M. Significant Effects of Long-Term Application of Straw and Manure Combined with NPK Fertilizers on Olsen P and PAC in Red Soil. Agronomy 2023, 13, 1647. https://doi.org/10.3390/agronomy13061647
Sun F, Sun N, Wang B, Cai Z, Xu M. Significant Effects of Long-Term Application of Straw and Manure Combined with NPK Fertilizers on Olsen P and PAC in Red Soil. Agronomy. 2023; 13(6):1647. https://doi.org/10.3390/agronomy13061647
Chicago/Turabian StyleSun, Fengxia, Nan Sun, Boren Wang, Zejiang Cai, and Minggang Xu. 2023. "Significant Effects of Long-Term Application of Straw and Manure Combined with NPK Fertilizers on Olsen P and PAC in Red Soil" Agronomy 13, no. 6: 1647. https://doi.org/10.3390/agronomy13061647




