Performance of Drift-Reducing Nozzles in Controlling Small Weed Seedlings with Contact Herbicides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiments
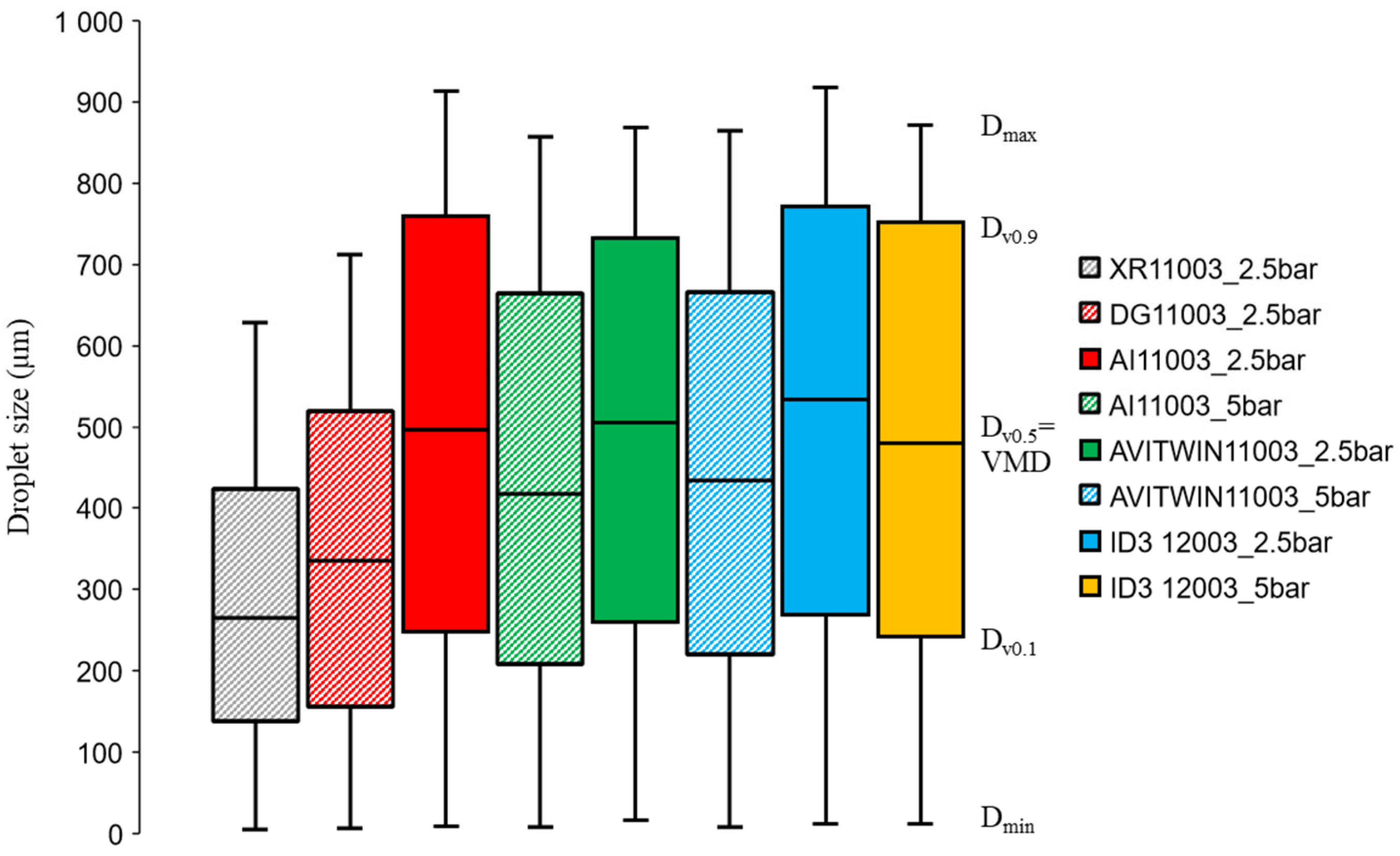
2.2. Droplet Size and Velocity Characteristics
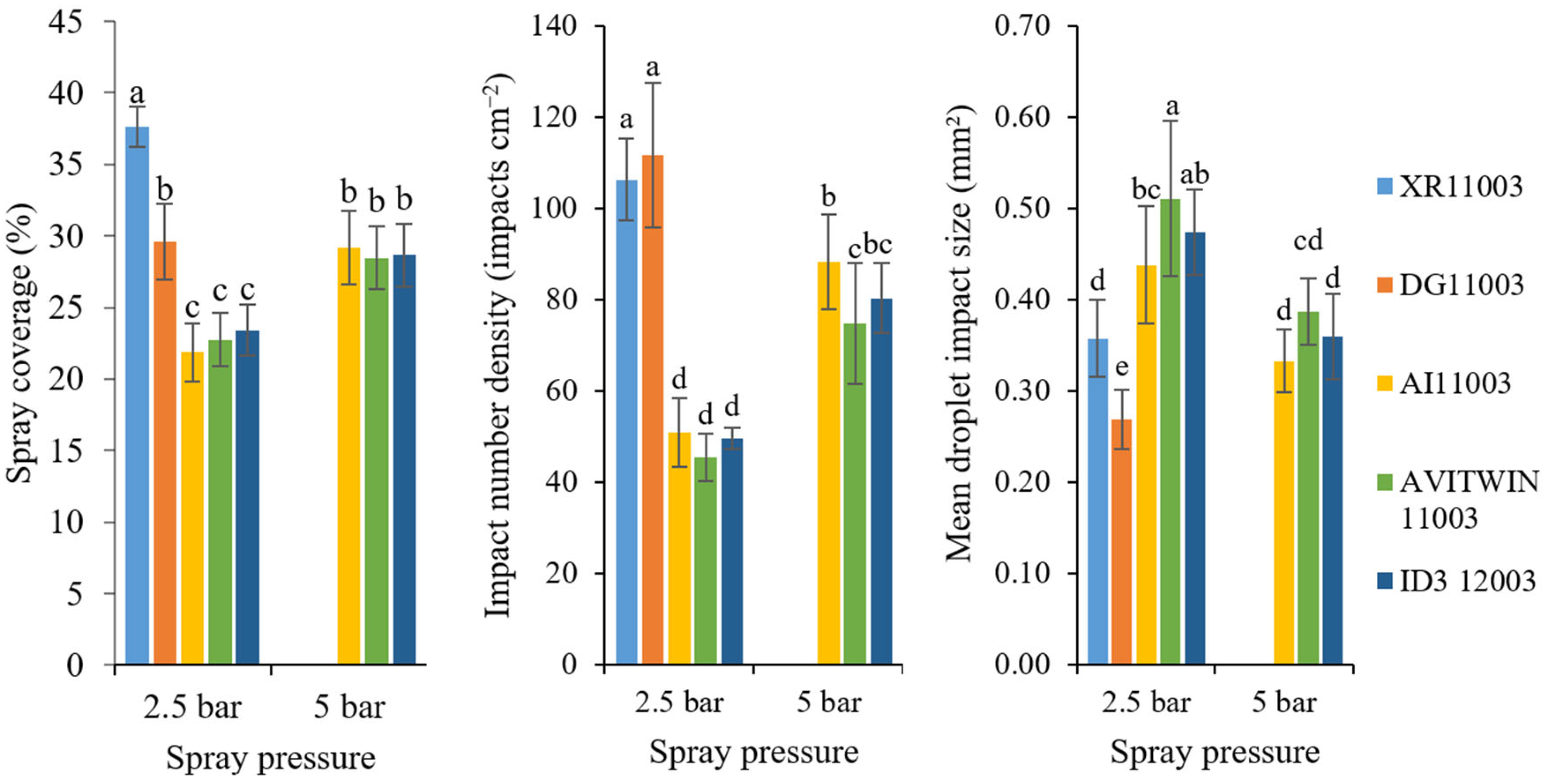
2.3. Spray Coverage Characteristics
2.4. Plant Response to Herbicide Application
2.5. Data Analysis
3. Results
3.1. Droplet Characteristics and Spray Coverage
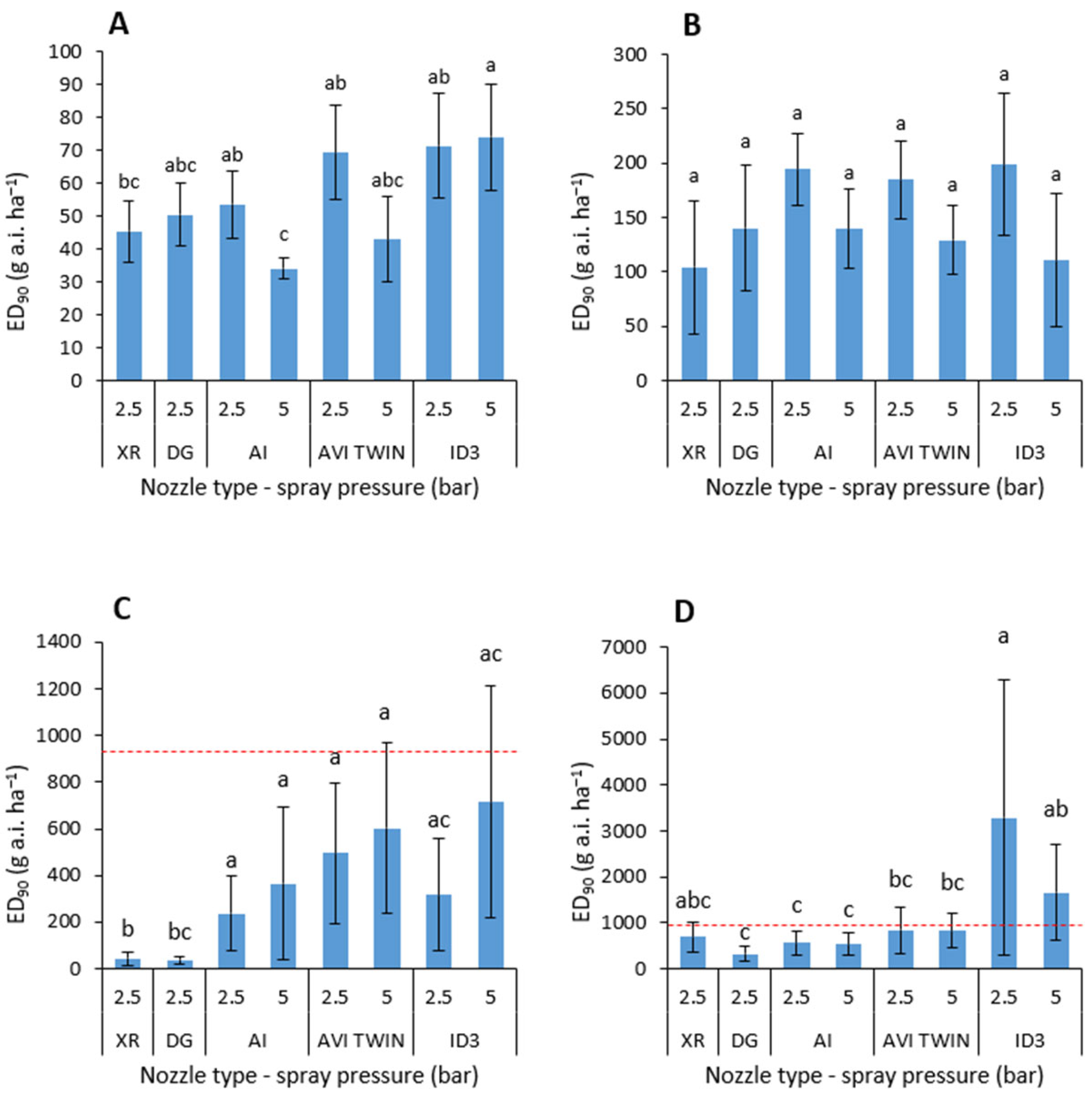
3.2. Herbicide Performance with Different Nozzle-Pressure Combinations
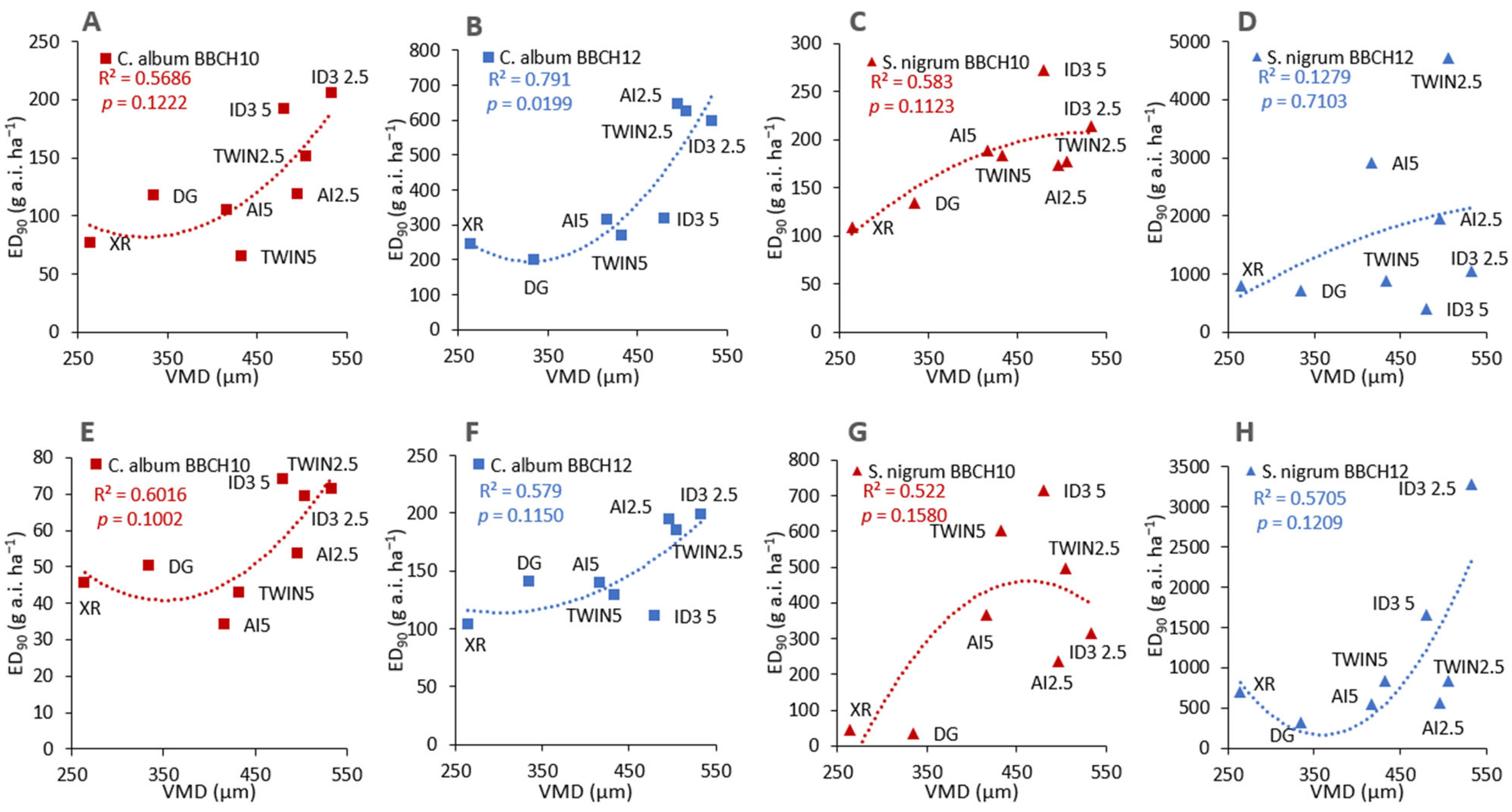
3.3. Relation between Herbicide Performance and Coverage/Droplet Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Schampheleire, M.; Baetens, K.; Nuyttens, D.; Spanoghe, P. Spray drift measurements to evaluate the Belgian drift mitigation measures in field crops. Crop Prot. 2008, 27, 577–589. [Google Scholar] [CrossRef]
- Nuyttens, D.; De Schampheleire, M.; Baetens, K.; Brusselman, E.; Dekeyser, D.; Verboven, P. Drift from field crop sprayers using an integrated approach: Results of a five-year study. Trans. ASABE 2011, 54, 403–408. [Google Scholar] [CrossRef]
- De Schampheleire, M.; Nuyttens, D.; Dekeyser, D.; Verboven, P.; Spanoghe, P.; Cornelis, W.; Gabriels, D.; Steurbaut, W. Deposition of spray drift behind border structures. Crop Prot. 2009, 28, 1061–1075. [Google Scholar] [CrossRef]
- Machado, W.A.; Silva, S.M.; Carvalho, S.M.; Cunha, J.P.A.R. da Effect of nozzles, application rates, and adjuvants on spray deposition in wheat crops. Eng. Agríc. 2019, 39, 83–88. [Google Scholar] [CrossRef]
- Chen, P.; Douzals, J.P.; Lan, Y.; Cotteux, E.; Delpuech, X.; Pouxviel, G.; Zhan, Y. Characteristics of unmanned aerial spraying systems and related spray drift: A review. Front. Plant Sci. 2022, 13, 870956. [Google Scholar] [CrossRef] [PubMed]
- Grover, R.; Kerr, L.A.; Maybank, J.; Yoshida, K. Field measurement of droplet drift from ground sprayers. I. Sampling, analytical, and data integration techniques. Can. J. Plant Sci. 1978, 58, 611–622. [Google Scholar] [CrossRef]
- Nuyttens, D.; De Schampheleire, M.; Verboven, P.; Sonck, B. Comparison between indirect and direct spray drift assessment methods. Biosyst. Eng. 2010, 105, 2–12. [Google Scholar] [CrossRef]
- Sousa Alves, G.; Kruger, G.R.; da Cunha, J.P.A.R.; de Santana, D.G.; Pinto, L.A.T.; Guimarães, F.; Zaric, M. Dicamba spray drift as influenced by wind speed and nozzle type. Weed Technol. 2017, 31, 724–731. [Google Scholar] [CrossRef]
- Da Cunha, J.P.A.R.; França, J.A.L.; de Alvarenga, C.B.; Alves, G.S.; Antuniassi, U.R. Performance of air induction spray nozzle models under different operating conditions. Eng. Agríc. 2020, 40, 711–718. [Google Scholar] [CrossRef]
- Zwertvaegher, I.K.; Verhaeghe, M.; Brusselman, E.; Verboven, P.; Lebeau, F.; Massinon, M.; Nicolaï, B.M.; Nuyttens, D. The impact and retention of spray droplets on a horizontal hydrophobic surface. Biosyst. Eng. 2014, 126, 82–91. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Nuyttens, D.; Zhang, L.; Liu, Y.; Li, X. Evaluation of compact air-induction flat fan nozzles for herbicide applications: Spray drift and biological efficacy. Front. Plant Sci. 2023, 14, 1018626. [Google Scholar] [CrossRef] [PubMed]
- Knoche, M. Effect of droplet size and carrier volume on performance of foliage-applied herbicides. Crop Prot. 1994, 13, 163–178. [Google Scholar] [CrossRef]
- Gossen, B.D.; Peng, G.; Wolf, T.M.; McDonald, M.R. Improving spray retention to enhance the efficacy of foliar-applied disease- and pest-management products in field and row crops. Can. J. Plant Pathol. 2008, 30, 505–516. [Google Scholar] [CrossRef]
- Wolf, R.; Daggupati, N. Nozzle type effect on soybean canopy penetration. Appl. Eng. Agric. 2009, 25, 23–30. [Google Scholar] [CrossRef]
- Guler, H.; Zhu, H.; Ozkan, H.E.; Ling, P. Characterization of hydraulic nozzles for droplet size and spray coverage. Atomiz. Spr. 2012, 22, 627–645. [Google Scholar] [CrossRef]
- Ferguson, J.C.; Chechetto, R.G.; Hewitt, A.J.; Chauhan, B.S.; Adkins, S.W.; Kruger, G.R.; O’Donnell, C.C. Assessing the deposition and canopy penetration of nozzles with different spray qualities in an oat (Avena sativa L.) canopy. Crop Prot. 2016, 81, 14–19. [Google Scholar] [CrossRef]
- Kudsk, P. Optimising herbicide performance. In Weed Management Handbook, 9th ed.; Naylor, R., Ed.; Blackwell Science: Oxford, UK, 2002; pp. 323–344. [Google Scholar]
- Lauwers, M.; Nuyttens, D.; De Cauwer, B.; Pieters, J. Hyperspectral classification of poisonous solanaceous weeds in processing Phaseolus vulgaris L. and Spinacia oleracea L. Comput. Electron. Agr. 2022, 196, 106908. [Google Scholar] [CrossRef]
- Nuyttens, D.; Baetens, K.; De Schampheleire, M.; Sonck, B. PDPA laser-based characterisation of agricultural spray nozzles. Agric. Eng. Int. CIGR Ejournal 2006, 8, 1–18. [Google Scholar]
- Nuyttens, D.; Baetens, K.; De Schampheleire, M.; Sonck, B. Effect of nozzle type, size and pressure on spray droplet characteristics. Biosyst. Eng. 2007, 97, 333–345. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Evironment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; ISBN 3-900051-07-0.
- Ritz, C.; Streibig, J.C. Bioassay analysis using R. J. Stat. Softw. 2005, 12. [Google Scholar] [CrossRef]
- Streibig, J.C.; Rudemo, M.; Jensen, J.E. Dose-response curves and statistical models. In Herbicide Bioassays; Streibig, J.C., Kudsk, P., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 29–55. [Google Scholar]
- Van Der Vaart, A. Asymptotic Statistics; Cambridge University Press: Cambridge, UK, 1998; 439p. [Google Scholar]
- Creech, C.F. Herbicide Application Technology Impacts on Herbicide Spray Characteristics and Performance. Ph.D. Thesis, University of Nebraska, Lincoln, NE, USA, 2015. [Google Scholar]
- Ramsdale, B.K.; Messersmith, C.G. Nozzle, spray volume, and adjuvant effects on carfentrazone and imazamox efficacy. Weed Technol. 2001, 15, 485–491. [Google Scholar] [CrossRef]
- Antoons, S. Invloed van Tank-Mix Hulpstoffen Op Effectiviteit van Bladherbiciden, Faculteit Bio-Ingenieurswetenschappen; Universiteit Gent: Gent, België, 2017. [Google Scholar]
- Miller, P.; Powell, E.; Orson, J.; Kudsk, P.; Mathiassen, S. Defining the size of target for air induction nozzles (Project Report 317); British Crop Protection Council: Hampshire, UK, 2003; 35p. [Google Scholar]
- Stainier, C.; Destain, M.-F.; Schiffers, B.; Lebeau, F. Droplet size spectra and drift effect of two phenmedipham formulations and four adjuvants mixtures. Crop Prot. 2006, 25, 1238–1243. [Google Scholar] [CrossRef]
- Jensen, P.K.; Jorgensen, L.N.; Kirknel, E. Biological efficacy of herbicides and fungicides applied with low-drift and twin-fluid nozzles. Crop Prot. 2001, 20, 57–64. [Google Scholar] [CrossRef]
- Peters, T.; Thostenson, A.; Nowatzki, J.; Hofman, V.; Wilson, J. Selecting Spray Nozzles to Reduce Particle Drift; North Dakota State University: Fargo, ND, USA, 2017. [Google Scholar]
- Spanoghe, P. Effect van Additieven en Adjuvantia op de Efficiëntie van de Spuittoepassing van Gewasbeschermingsmiddelen; Universiteit Gent: Gent, België, 2005. [Google Scholar]
- Brown, L.; Soltani, N.; Shropshire, C.; Spieser, H.; Sikkema, P.H. Efficacy of four corn (Zea mays L.) herbicides when applied with flat fan and air induction nozzles. Weed Biol. Manag. 2007, 7, 55–61. [Google Scholar] [CrossRef]
- Liao, J.; Luo, X.; Wang, P.; Zhou, Z.; O’Donnell, C.C.; Zang, Y.; Hewitt, A.J. Analysis of the influence of different parameters on droplet characteristics and droplet size classification categories for air induction nozzle. Agronomy 2020, 10, 256. [Google Scholar] [CrossRef]
- Fogg, G.E.; Pearsall, W.H. Quantitative studies on the wetting of leaves by water. Proc. R. Soc. London. Ser. B Biol. Sci. 1947, 134, 503–522. [Google Scholar] [CrossRef]
- Harr, J.; Guggenheim, R.; Schulke, G.; Falk, R.H. The Leaf Surface of Major Weeds; Sandoz Agro, Ltd.: Greensboro, NC, USA, 1991; 11p. [Google Scholar]
- Ramsdale, B.K.; Messersmith, C.G. Drift-reducing nozzle effects on herbicide performance. Weed Technol. 2001, 15, 453–460. [Google Scholar] [CrossRef]
- Nansen, C.; Ferguson, J.C.; Moore, J.; Groves, L.; Emery, R.; Garel, N.; Hewitt, A. Optimizing pesticide spray coverage using a novel web and smartphone tool, SnapCard. Agron. Sustain. Dev. 2015, 35, 1075–1085. [Google Scholar] [CrossRef]
- Ferguson, J.C.; Hewitt, A.J.; O’Donnell, C.C. Pressure, droplet size classification, and nozzle arrangement effects on coverage and droplet number density using air-inclusion dual fan nozzles for pesticide applications. Crop Prot. 2016, 89, 231–238. [Google Scholar] [CrossRef]





| Nozzle Type | Manufacturer | Description of Nozzle Type | Drift Reduction Class (%) 2 | Spray Pressure (bar) | |
|---|---|---|---|---|---|
| 2.5 | 5.0 | ||||
| XR 110 03 | TeeJet | Standard single 110° flat-fan | 0 | X | - 1 |
| DG 110 03 | TeeJet | Pre-orifice single 110° flat-fan | 50 | X | - 1 |
| AI 110 03 | TeeJet | Air induction single 110° flat-fan | 50 | X | X |
| AVI TWIN 110 03 | Albuz | Symmetric air induction dual 110° flat-fan with 30° forward and backward angle | 75 | X | X |
| ID3 120 03 | Lechler | Air induction single 120° flat-fan | 90 | X | X |
| Herbicide (Formulated Product) | Herbicide Mode of Action (HRAC/WSSA-Group/Legacy HRAC) | Max. Field Dose 2 (g a.i. ha−1) | Herbicide Doses (g a.i. ha−1) |
|---|---|---|---|
| Bentazon 1 (Basagran, 87%, SG, BASF, Waterloo, Belgium) | Photosystem II-inhibitor (6/C3) | 960 (beans and peas) | 0.0–18.6–41.0–90.2–198.3–436.4–960.0 |
| Phenmedipham (Astrix, 160 g L−1, EC, UPL Europe, Ltd., Warrington, UK) | Photoystem II-inhibitor (5/C1) | 320 (spinach) | 0–20–40–80–160–320–640 |
| Experimental Period | Mean DayTime/Night-Time Temperature (°C) 3 | Mean Day/Night Relative Humidity (%) | Mean Daytime Light Intensity (lux) | |
|---|---|---|---|---|
| Pre-application | 10/07/20 1–19/07/20 | 24.3/13.8 | 56.2/84.6 | 7745.7 |
| Day of application | 19/07/20 11 h–12 h 30 (phenmedipham) | 20.9/- | 61.7/- | 22,161.8 |
| 19/07/20 13 h–15 h 20 (bentazon) | 22.3/- | 51.3/- | 20,485.9 | |
| Post-application | 19/07/20–01/08/20 2 (phenmedipham) | 29.8/16.7 | 50.9/85.8 | 7711.6 |
| 19/07/20–31/07/20 2 (bentazon) | 30.4/17.0 | 50.1/85.6 | 7652.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Cauwer, B.; De Meuter, I.; De Ryck, S.; Dekeyser, D.; Zwertvaegher, I.; Nuyttens, D. Performance of Drift-Reducing Nozzles in Controlling Small Weed Seedlings with Contact Herbicides. Agronomy 2023, 13, 1342. https://doi.org/10.3390/agronomy13051342
De Cauwer B, De Meuter I, De Ryck S, Dekeyser D, Zwertvaegher I, Nuyttens D. Performance of Drift-Reducing Nozzles in Controlling Small Weed Seedlings with Contact Herbicides. Agronomy. 2023; 13(5):1342. https://doi.org/10.3390/agronomy13051342
Chicago/Turabian StyleDe Cauwer, Benny, Ilke De Meuter, Sander De Ryck, Donald Dekeyser, Ingrid Zwertvaegher, and David Nuyttens. 2023. "Performance of Drift-Reducing Nozzles in Controlling Small Weed Seedlings with Contact Herbicides" Agronomy 13, no. 5: 1342. https://doi.org/10.3390/agronomy13051342





