Foliar Application of Ascorbic Acid and Tocopherol in Conferring Salt Tolerance in Rapeseed by Enhancing K+/Na+ Homeostasis, Osmoregulation, Antioxidant Defense, and Glyoxalase System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Measurement of Growth Parameters
2.3. Estimation of Chlorophyll Content
2.4. Determination of Relative Water Content (RWC)
2.5. Estimation of Electrolyte Leakage (EL)
2.6. Determination of Proline (Pro) Content
2.7. Measurement of Na+ and K+ Content
2.8. Measurement of Malondialdehyde and Hydrogen Peroxide Content
2.9. Measurement of Ascorbate and Glutathione Pool
2.10. Determination of Protein and Enzyme Activity Assays
2.11. Statistical Analysis
3. Results
3.1. Plant Growth and Biomass
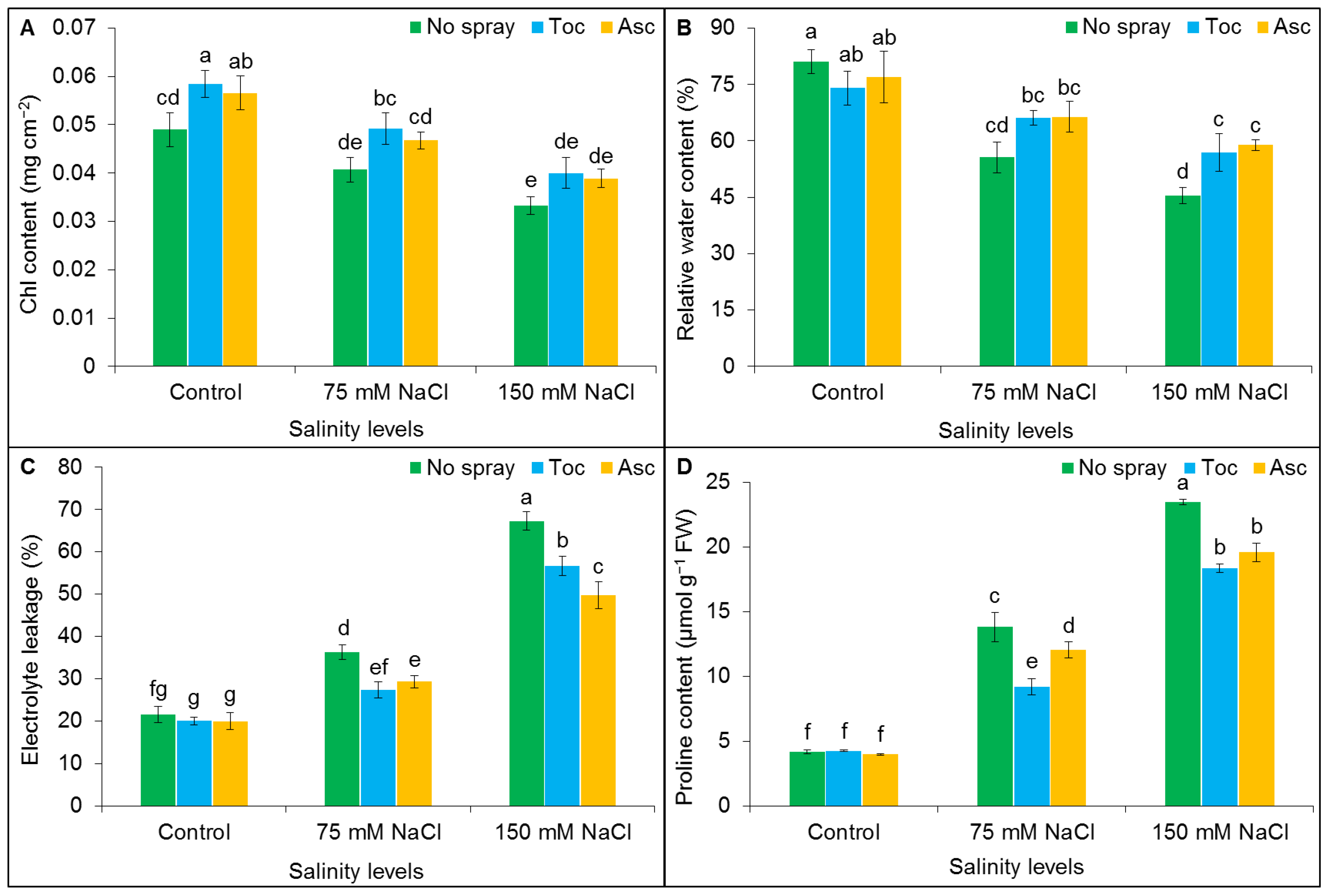
3.2. Chlorophyll Content, Relative Water Content, Electrolyte Leakage, and Proline Content
3.3. Contents of Na+ and K+
3.4. Malondialdehyde and H2O2 Contents
3.5. Ascorbate and Glutathione Content
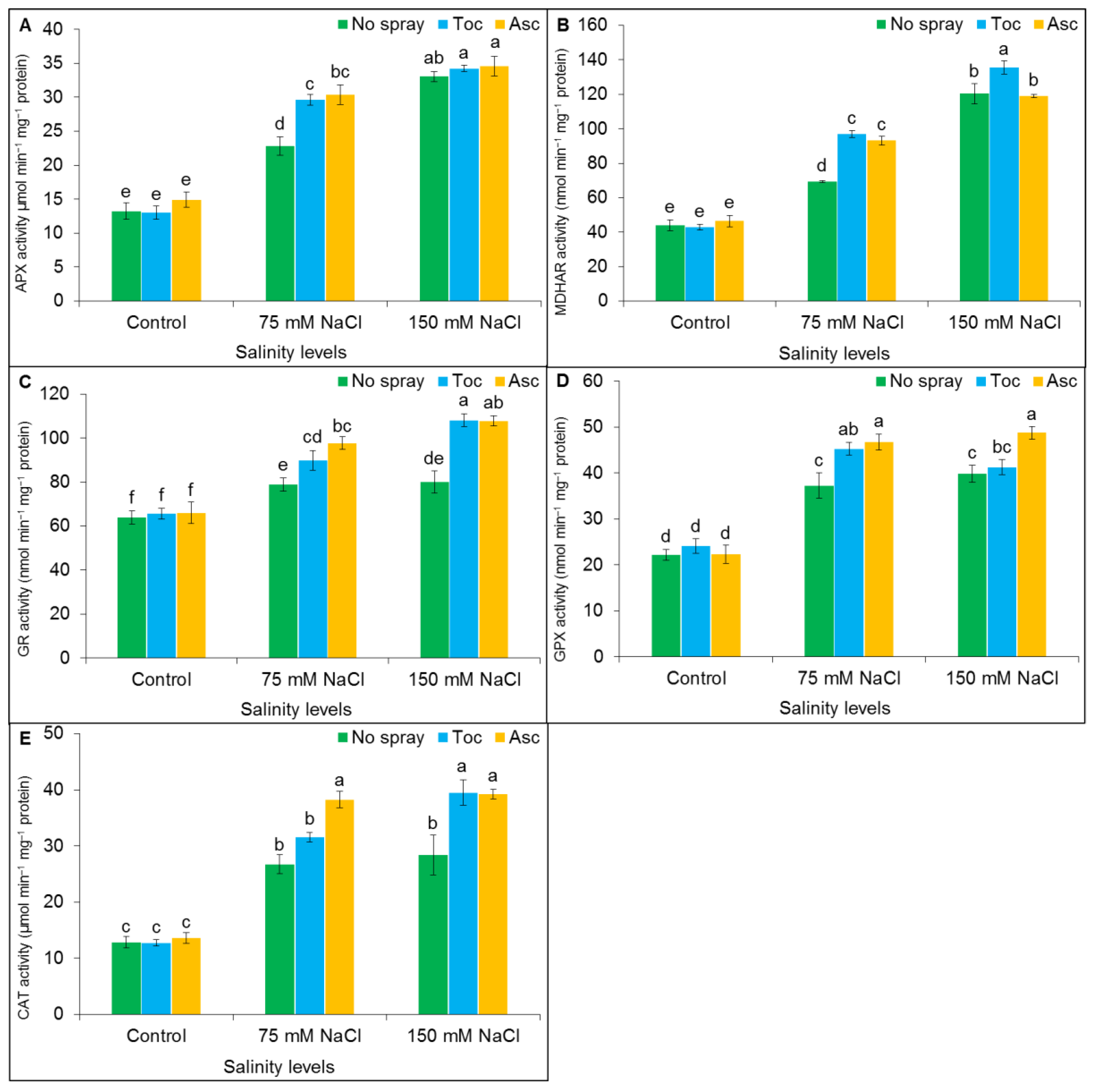
3.6. Antioxidant Enzyme Activities
3.7. Activities of Glyoxalase Enzymes
3.8. Yield and Yield-Contributing Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2020, 280, 111736. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Nara, U.; Kumar, A.; Choudhary, A.; Singh, H.; Thapa, S. Salinity tolerance mechanisms and their breeding implications. J. Genet. Eng. Biotechnol. 2021, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Hryvusevich, P.; Navaselsky, I.; Talkachova, Y.; Straltsova, D.; Keisham, M.; Viatoshkin, A.; Samokhina, V.; Smolich, I.; Sokolik, A.; Huang, X.; et al. Sodium influx and potassium efflux currents in sunflower root cells under high salinity. Front. Plant Sci. 2021, 11, 2181. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front. Plant Sci. 2016, 7, 609. [Google Scholar] [CrossRef] [Green Version]
- Hossen, M.S.; Karim, M.F.; Fujita, M.; Bhuyan, M.H.M.B.; Nahar, K.; Masud, A.A.C.; Mahmud, J.A.; Hasanuzzaman, M. Comparative physiology of Indica and Japonica rice under salinity and drought stress: An intrinsic study on osmotic adjustment, oxidative stress, antioxidant defense and methylglyoxal detoxification. Stresses 2022, 2, 156–178. [Google Scholar] [CrossRef]
- Parvin, K.; Nahar, K.; Hasanuzzaman, M.; Bhuyan, M.B.; Mohsin, S.M.; Fujita, M. Exogenous vanillic acid enhances salt tolerance of tomato: Insight into plant antioxidant defense and glyoxalase systems. Plant Physiol. Biochem. 2020, 150, 109–120. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Parvin, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.; Nahar, K.; Mohsin, S.M.; Fujita, M. Comparative physiological and biochemical changes in tomato (Solanum lycopersicum L.) under salt stress and recovery: Role of antioxidant defense and glyoxalase systems. Antioxidants 2019, 8, 350. [Google Scholar] [CrossRef] [Green Version]
- Mohsin, S.M.; Hasanuzzaman, M.; Parvin, K.; Fujita, M. Pretreatment of wheat (Triticum aestivum L.) seedlings with 2, 4-D improves tolerance to salinity-induced oxidative stress and methylglyoxal toxicity by modulating ion homeostasis, antioxidant defenses, and glyoxalase systems. Plant Physiol. Biochem. 2020, 152, 221–231. [Google Scholar] [CrossRef]
- Costa, S.F.; Martins, D.; Agacka-Mołdoch, M.; Czubacka, A.; de Sousa Araújo, S. Strategies to alleviate salinity stress in plants. In Salinity Responses and Tolerance in Plants; Kumar, V., Wani, S., Suprasanna, P., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2018; pp. 307–337. [Google Scholar]
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; El-Ezz, A.A.; Almoneafy, A.; Mahmoud, A.; Awad, M.; et al. Role of melatonin in plant tolerance to soil stressors: Salinity, pH and heavy metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, B. Exogenous ascorbic acid mediated abiotic stress tolerance in plants. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Hossain, M., Munné-Bosch, S., Burritt, D., Diaz-Vivancos, P., Fujita, M., Lorence, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 233–253. [Google Scholar]
- Bartoli, C.G.; Buet, A.; Grozeff, G.G.; Galatro, A.; Simontacchi, M. Ascorbate-glutathione cycle and abiotic stress tolerance in plants. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Hossain, M., Munné-Bosch, S., Burritt, D., Diaz-Vivancos, P., Fujita, M., Lorence, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 177–200. [Google Scholar]
- Billah, M.; Rohman, M.M.; Hossain, N.; Uddin, M.S. Exogenous ascorbic acid improved tolerance in maize (Zea mays L.) by increasing antioxidant activity under salinity stress. Afr. J. Agric. Res. 2017, 12, 1437–1446. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.M.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Alleviation of osmotic stress in Brassica napus, B. campestris, and B. juncea by ascorbic acid application. Biol. Plant. 2014, 58, 697–708. [Google Scholar] [CrossRef]
- Ortiz-Espín, A.; Sánchez-Guerrero, A.; Sevilla, F.; Jiménez, A. The role of ascorbate in plant growth and development. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Hossain, M., Munné-Bosch, S., Burritt, D., Diaz-Vivancos, P., Fujita, M., Lorence, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 25–45. [Google Scholar]
- El-Beltagi, H.S.; Mohamed, H.I.; Sofy, M.R. Role of ascorbic acid, glutathione and proline applied as singly or in sequence combination in improving chickpea plant through physiological change and antioxidant defense under different levels of irrigation intervals. Molecules 2020, 25, 1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noreen, S.; Sultan, M.; Akhter, M.S.; Shah, K.H.; Ummara, U.; Manzoor, H.; Ulfat, M.; Alyemeni, M.N.; Ahmad, P. Foliar fertigation of ascorbic acid and zinc improves growth, antioxidant enzyme activity and harvest index in barley (Hordeum vulgare L.) grown under salt stress. Plant Physiol. Biochem. 2021, 158, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.; Akram, N.A.; Ashraf, M.; Al-Qurainy, F.; Ahmad, P. Alpha-Tocopherol-Induced regulation of growth and metabolism in plants under non-stress and stress conditions. J. Plant Growth Regul. 2019, 38, 1325–1340. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Role of tocopherol (vitamin E) in plants: Abiotic stress tolerance and beyond. In Emerging Technologies and Management of Crop Stress Tolerance; Ahmad, P., Rasool, S., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 267–289. [Google Scholar]
- Srinivasan, A.; Vijayakumar, S.; Raman, K.; Srivastava, S. Rational metabolic engineering for enhanced alpha-tocopherol production in Helianthus annuus cell culture. Biochem. Eng. J. 2019, 151, 107256. [Google Scholar] [CrossRef]
- Ali, Q.; Ali, S.; Iqbal, N.; Javed, M.T.; Rizwan, M.; Khaliq, R.; Shahid, S.; Perveen, R.; Alamri, S.A.; Alyemeni, M.N.; et al. Alpha-tocopherol fertigation confers growth physio-biochemical and qualitative yield enhancement in field grown water deficit wheat (Triticum aestivum L.). Sci. Rep. 2019, 9, 12924. [Google Scholar] [CrossRef] [Green Version]
- Lalarukh, I.; Shahbaz, M. Response of antioxidants and lipid peroxidation to exogenous application of alpha-tocopherol in sunflower (Helianthus annuus L.) under salt stress. Pak. J. Bot. 2020, 52, 75–83. [Google Scholar] [CrossRef]
- Hameed, A.; Akram, N.A.; Saleem, M.H.; Ashraf, M.; Ahmed, S.; Ali, S.; Alsahli, A.A.; Alyemeni, M.N. Seed treatment with α-tocopherol regulates growth and key physio-biochemical attributes in carrot (Daucus carota L.) plants under water limited regimes. Agronomy 2021, 11, 469. [Google Scholar] [CrossRef]
- Naqve, M.; Wang, X.; Shahbaz, M.; Fiaz, S.; Naqvi, W.; Naseer, M.; Mahmood, A.; Ali, H. Foliar spray of alpha-tocopherol modulates antioxidant potential of okra fruit under salt stress. Plants 2021, 10, 1382. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teari, D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplast: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.; Murphy, T.M.; Lin, C.H. Hydrogen peroxide induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct. Plant Biol. 2003, 30, 189–198. [Google Scholar] [CrossRef]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 2005, 56, 3041–3049. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Khan, M.I.R.; Fujita, M. Silicon-mediated regulation of antioxidant defense and glyoxalase systems confers drought stress tolerance in Brassica napus L. S. Afr. J. Bot. 2018, 115, 50–57. [Google Scholar] [CrossRef]
- Paradiso, A.; Berardino, R.; de Pinto, M.; di Toppi, L.S.; Storelli, F.T.; de Gara, L. Increase in ascorbate-glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant Cell Physiol. 2008, 49, 362–374. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Hossain, M.A.; Nakano, Y.; Asada, K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984, 25, 385–395. [Google Scholar] [CrossRef]
- Elia, A.C.; Galarini, R.; Taticchi, M.I.; Dorr, A.J.M.; Mantilacci, L. Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol. Environ. Saf. 2003, 55, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Addinsoft, XLSTAT v. 2021.2.2; Data Analysis and Statistics Software for Microsoft Excel; Addinsoft: Paris, French, 2021.
- Lalarukh, I.; Shahbaz, M. Alpha-tocopherol induced modulations in morpho-physiological attributes of sunflower (Helianthus annuus) grown under saline environment. Int. J. Agric. Biol. 2018, 20, 661–668. [Google Scholar] [CrossRef]
- Ali, Q.; Javed, M.T.; Haider, M.Z.; Habib, N.; Rizwan, M.; Perveen, R.; Ali, S.; Alyemeni, M.N.; El-Serehy, H.A.; Al-Misned, F.A. α-Tocopherol foliar spray and translocation mediates growth, photosynthetic pigments, nutrient uptake, and oxidative defense in maize (Zea mays L.) under drought stress. Agronomy 2020, 10, 1235. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Semida, W.M.; Rady, M.M.; Mohamed, G.F.; Hemida, K.A.; Alhammad, B.A.; Hassan, M.M.; Shami, A. Sequential application of antioxidants rectifies ion imbalance and strengthens antioxidant systems in salt-stressed cucumber. Plants 2020, 9, 1783. [Google Scholar] [CrossRef]
- Sadiq, M.; Akram, N.A.; Ashraf, M. Foliar applications of alpha-tocopherol improve the composition of fresh pods of Vigna radiata subjected to water deficiency. Turk. J. Bot. 2017, 41, 1–9. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Trebst, A.; Depka, B.; Holländer-Czytko, H. A Specific role for tocopherol and of chemical singlet oxygen quenchers in the maintenance of photosystem II structure and function in Chlamydomonas reinhardtii. FEBS Lett. 2002, 516, 156–160. [Google Scholar] [CrossRef]
- EL-Afry, M.M.; EL-Okkiah, S.A.F.; EL-Kady, E.A.F.; EL-Yamanee, G.S.A. Exogenous application of ascorbic acid for alleviation the adverse effects of salinity stress in flax (Linum usitatissimum L.). Middle East J. Agric. Res. 2018, 7, 716–739. [Google Scholar]
- Sharmin, S.; Lipka, U.; Polle, A.; Eckert, C. The influence of transpiration on foliar accumulation of salt and nutrients under salinity in poplar (Populus × canescens). PLoS ONE 2021, 16, e0253228. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Qiu, D.; Gao, H.; Wen, H.; Wu, Y.; Pang, Y.; Wang, X.; Qin, Y. Over-expression of a γ-tocopherol methyltransferase gene in vitamin E pathway confers PEG-simulated drought tolerance in alfalfa. BMC Plant Biol. 2020, 20, 226. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Yusuf, M.A.; Singh, P.; Sardar, M.; Sarin, N.B. Modulation of antioxidant machinery in α-tocopherol-enriched transgenic Brassica juncea plants tolerant to abiotic stress conditions. Protoplasma 2013, 250, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Amjad, S.F.; Saleem, M.H.; Yasmin, H.; Imran, M.; Riaz, M.; Ali, Q.; Joyia, F.A.; Mobeen; Ahmed, S.; et al. Foliar application of ascorbic acid enhances salinity stress tolerance in barley (Hordeum vulgare L.) through modulation of morpho-physio-biochemical attributes, ions uptake, osmo-protectants and stress response genes expression. Saudi J. Biol. Sci. 2021, 28, 4276–4290. [Google Scholar] [CrossRef] [PubMed]
- Orabi, S.A.; Abdelhamid, M.T. Protective role of a-tocopherol on two Vicia faba cultivars against seawater-induced lipid peroxidation by enhancing capacity of anti-oxidative system. J. Saudi Soc. Agric. Sci. 2016, 15, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [Green Version]
- Nahar, K.; Hasanuzzaman, M.; Rahman, A.; Alam, M.M.; Mahmud, J.A.; Suzuki, T.; Fujita, M. Polyamines confer salt tolerance in mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. Front. Plant Sci. 2016, 7, 1104. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Rahman, A.; Inafuku, M.; Oku, H.; Fujita, M. Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int. J. Mol. Sci. 2017, 18, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Z.; Li, J.; Jia, X.; Yang, F.; Wang, Z. Assimilation and translocation of dry matter and phosphorus in rice genotypes affected by salt-alkaline stress. Sustainability 2016, 8, 568. [Google Scholar] [CrossRef] [Green Version]
- Semida, W.M.; Taha, R.S.; Abdelhamid, M.T.; Rady, M.M. Foliar-applied α-tocopherol enhances salt-tolerance in Vicia faba L. plants grown under saline conditions. S. Afr. J. Bot. 2014, 95, 24–31. [Google Scholar] [CrossRef] [Green Version]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanuzzaman, M.; Raihan, M.R.H.; Alharby, H.F.; Al-Zahrani, H.S.; Alsamadany, H.; Alghamdi, K.M.; Ahmed, N.; Nahar, K. Foliar Application of Ascorbic Acid and Tocopherol in Conferring Salt Tolerance in Rapeseed by Enhancing K+/Na+ Homeostasis, Osmoregulation, Antioxidant Defense, and Glyoxalase System. Agronomy 2023, 13, 361. https://doi.org/10.3390/agronomy13020361
Hasanuzzaman M, Raihan MRH, Alharby HF, Al-Zahrani HS, Alsamadany H, Alghamdi KM, Ahmed N, Nahar K. Foliar Application of Ascorbic Acid and Tocopherol in Conferring Salt Tolerance in Rapeseed by Enhancing K+/Na+ Homeostasis, Osmoregulation, Antioxidant Defense, and Glyoxalase System. Agronomy. 2023; 13(2):361. https://doi.org/10.3390/agronomy13020361
Chicago/Turabian StyleHasanuzzaman, Mirza, Md. Rakib Hossain Raihan, Hesham F. Alharby, Hassan S. Al-Zahrani, Hameed Alsamadany, Khalid M. Alghamdi, Naznin Ahmed, and Kamrun Nahar. 2023. "Foliar Application of Ascorbic Acid and Tocopherol in Conferring Salt Tolerance in Rapeseed by Enhancing K+/Na+ Homeostasis, Osmoregulation, Antioxidant Defense, and Glyoxalase System" Agronomy 13, no. 2: 361. https://doi.org/10.3390/agronomy13020361






