Effect of Varied Salinity on Marigold Flowers: Reduced Size and Quantity Despite Enhanced Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
2.2. Determination of Total Carotenoid, Polyphenol, and Flavonoid Content
2.3. Antioxidative Enzyme Activities
2.4. Statistical Analysis
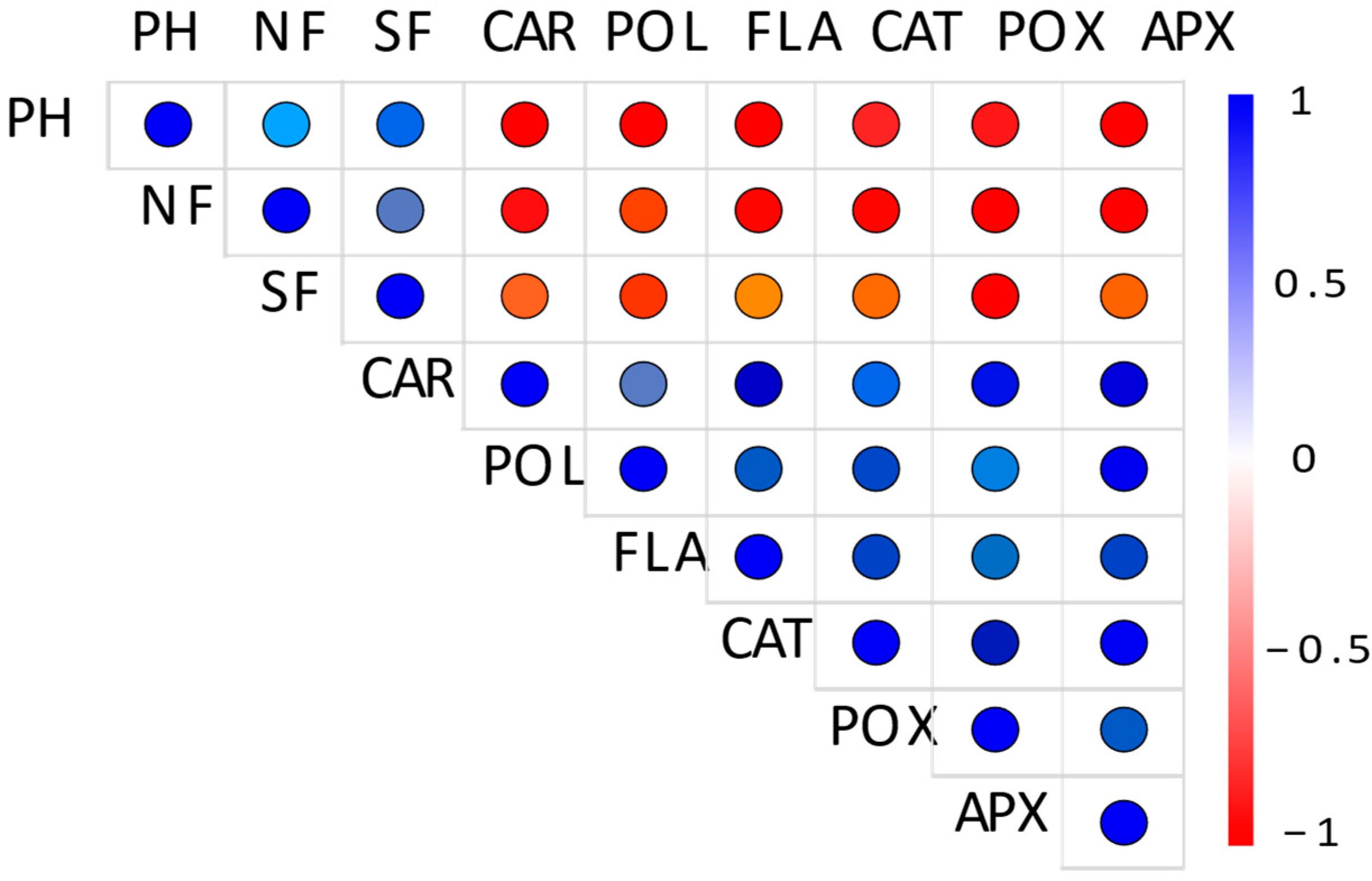
3. Results
3.1. Influence of Salinity on Plant Growth and Flower Production
3.2. Determination of Total Carotenoid, Polyphenol, and Flavonoid Content
3.3. Antioxidative Enzyme Activities
4. Discussion
4.1. Effects of Salinity on Plant Height and Flower Production
4.2. High Levels of Antioxidant Molecules on Flowers
4.3. The Role of Antioxidant Enzymatic Machinery on Flowers
4.4. Usefulness of Saline Conditions for French Marigold Plants: Implications for the Floriculture Industry
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yamaguchi, T.; Blumwald, E. Developing salt-tolerant crop plants: Challenges and opportunities. Trends Plant Sci. 2005, 10, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int. J. Mol. Sci. 2020, 21, 148. [Google Scholar] [CrossRef] [PubMed]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Vinci, G.; Marques, I.; Rodrigues, A.P.; Martins, S.; Leitão, A.E.; Semedo, M.C.; Silva, M.J.; Lidon, F.C.; DaMatta, F.M.; Ribeiro-Barros, A.I.; et al. Protective Responses at the Biochemical and Molecular Level Differ between a Coffea arabica L. Hybrid and Its Parental Genotypes to Supra-Optimal Temperatures and Elevated Air [CO2]. Plants 2022, 11, 2702. [Google Scholar] [CrossRef] [PubMed]
- Laamari, I.; Marques, I.; Ribeiro-Barros, A.I.; Zoubeir, B.; Abassi, M. Can saline preconditioning enhance plant survival in degraded soils? Physiological, biochemical, and molecular responses in Casuarina glauca saplings. Plant Ecol. 2023, 224, 905–919. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Tundis, R. Plant antioxidant for application in food and nutraceutical industries. Antioxidants 2019, 8, 453. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Vaseghi, G.; Pourfarzam, M.; Abdollahi, A. Are antioxidants helpful for disease prevention? Res. Pharm. Sci. 2010, 5, 5–12. [Google Scholar]
- Al Kharusi, L.; Al Yahyai, R.; Yaish, M.W. Antioxidant response to salinity in salt-tolerant and salt-susceptible cultivars of date palm. Agriculture 2019, 9, 8. [Google Scholar] [CrossRef]
- Fernandes, I.; Paulo, O.S.; Marques, I.; Sarjkar, I.; Sen, A.; Graça, I.; Pawlowski, K.; Ramalho, J.C.; Ribeiro-Barros, A.I. Salt Stress Tolerance in Casuarina glauca: Insights from the Branchlets Transcriptome. Plants 2022, 11, 2942. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zheng, J.; Zhang, X.; Hu, Q.; Qian, R. Salicylic acid alleviates the adverse effects of salt stress on Dianthus superbus (Caryophyllaceae) by activating photosynthesis, protecting morphological structure, and enhancing the antioxidant system. Front. Plant Sci. 2017, 8, 243823. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Plant Responses and Tolerance to Salt Stress: Physiological and Molecular Interventions. Int. J. Mol. Sci. 2022, 23, 4810. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Hasan, M.T.; Rahman, M.A.; Nuruzzaman, M.; Rahman, A.M.S.; Hasanuzzaman, M.; Haque, M.R.; Hossain, M.A.; Abdel Latef, A.A.H.; Murata, Y.; et al. Plant response to combined salinity and waterlogging stress: Current research progress and future prospects. Plant Stress 2023, 7, 100137. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. Changes in plant response to NaCl during development of rice (Oryza sativa L.) varieties differing in salinity resistance. J. Exp. Bot. 1995, 46, 1843–1852. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, W.-Y.; Yun, D.-J. A New Insight of Salt Stress Signaling in Plant. Mol. Cells 2016, 39, 447–459. [Google Scholar] [CrossRef]
- Oh, D.-H.; Hong, H.; Lee, S.Y.; Yun, D.-J.; Bohnert, H.J.; Dassanayake, M. Genome Structures and Transcriptomes Signify Niche Adaptation for the Multiple-Ion-Tolerant Extremophyte Schrenkiella parvula. Plant Physiol. 2014, 164, 2123–2138. [Google Scholar] [CrossRef]
- Boscaiu, M.; Estrelles, E.; Soriano, P.; Vicente, Ó. Effects of salt stress on the reproductive biology of the halophyte Plantago crassifolia. Biol. Plant. 2005, 49, 141–143. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Cassaniti, C.; Romano, D.; Hop, M.E.C.M.; Flowers, T.J. Growing floricultural crops with brackish water. Environ. Exp. Bot. 2013, 92, 165–175. [Google Scholar] [CrossRef]
- Villarino, G.H.; Mattson, N.S. Assessing tolerance to sodium chloride salinity in fourteen floriculture species. Horttechnology 2011, 21, 539–545. [Google Scholar] [CrossRef]
- Niu, G.; Wang, M.; Rodriguez, D.; Zhang, D. Response of Zinnia plants to saline water irrigation. HortScience 2012, 47, 793–797. [Google Scholar] [CrossRef]
- Marković, M.; Šoštarić, J.; Kojić, A.; Popović, B.; Bubalo, A.; Bošnjak, D.; Stanisavljević, A. Zinnia (Zinnia elegans L.) and Periwinkle (Catharanthus roseus (L.) G. Don) Responses to Salinity Stress. Water 2022, 14, 1066. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Tzionis, A.; Xylia, P.; Tzortzakis, N. Effects of salinity on tagetes growth, physiology, and shelf life of edible flowers stored in passive modified atmosphere packaging or treated with ethanol. Front. Plant Sci. 2018, 871, 1765. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gao, W.; Yun, X.; Qing, Z.; Zeng, J. Effect of Natural Antioxidants from Marigolds (Tagetes erecta L.) on the Oxidative Stability of Soybean Oil. Molecules 2022, 27, 2865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, S.; Zhang, H.; Chen, X.; Liang, F.; Qin, H.; Zhang, Y.; Cong, R.; Xin, H.; Zhang, Z. Carotenoid metabolite and transcriptome dynamics underlying flower color in marigold (Tagetes erecta L.). Sci. Rep. 2020, 10, 16835. [Google Scholar] [CrossRef] [PubMed]
- Burlec, A.F.; Pecio, Ł.; Kozachok, S.; Mircea, C.; Corciovă, A.; Vereştiuc, L.; Cioancă, O.; Oleszek, W.; Hăncianu, M. Phytochemical profile, antioxidant activity, and cytotoxicity assessment of Tagetes erecta L. flowers. Molecules 2021, 26, 1201. [Google Scholar] [CrossRef]
- Kumar, A.; Gautam, R.D.; Kumar, A.; Singh, S.; Singh, S. Understanding the Effect of Different Abiotic Stresses on Wild Marigold (Tagetes minuta L.) and Role of Breeding Strategies for Developing Tolerant Lines. Front. Plant Sci. 2022, 12, 3332. [Google Scholar] [CrossRef]
- Cicevan, R.; Sestras, A.F.; Plazas, M.; Boscaiu, M.; Vilanova, S.; Gramazio, P.; Vicente, O.; Prohens, J.; Sestras, R.E. Biological Traits and Genetic Relationships Amongst Cultivars of Three Species of Tagetes (Asteraceae). Plants 2022, 11, 760. [Google Scholar] [CrossRef]
- Cicevan, R.; Al Hassan, M.; Sestras, A.F.; Prohens, J.; Vicente, O.; Sestras, R.E.; Boscaiu, M. Screening for drought tolerance in cultivars of the ornamental genus Tagetes (Asteraceae). PeerJ 2016, 2016, e2133. [Google Scholar] [CrossRef]
- Koksal, N.; Alkan-Torun, A.; Kulahlioglu, I.; Ertargin, E.; Karalar, E. Ion uptake of marigold under saline growth conditions. Springerplus 2016, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gautam, R.D.; Singh, S.; Chauhan, R.; Kumar, A.; Singh, S. Comparative study of the effects of different soluble salts on seed germination of wild marigold (Tagetes minuta L.). J. Appl. Res. Med. Aromat. Plants 2022, 31, 100421. [Google Scholar] [CrossRef]
- Tarchoune, I.; Sgherri, C.; Izzo, R.; Lachaal, M.; Ouerghi, Z.; Navari-Izzo, F. Antioxidative responses of Ocimum basilicum to sodium chloride or sodium sulphate salinization. Plant Physiol. Biochem. PPB 2010, 48, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Lao, M.T. The effects of salt stress on ornamental plants and integrative cultivation practices. Sci. Hortic. 2018, 240, 430–439. [Google Scholar] [CrossRef]
- Baranova, E.N.; Gulevich, A.A. Asymmetry of Plant Cell Divisions under Salt Stress. Symmetry 2021, 13, 1811. [Google Scholar] [CrossRef]
- Bano, C.; Amist, N.; Singh, N.B. Morphological and Anatomical Modifications of Plants for Environmental Stresses. Mol. Plant Abiotic Stress Biol. Biotechnol. 2019, 29–44. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Ventura, Y.; Myrzabayeva, M.; Alikulov, Z.; Omarov, R.; Khozin-Goldberg, I.; Sagi, M. Effects of salinity on flowering, morphology, biomass accumulation and leaf metabolites in an edible halophyte. AoB Plants 2014, 6, plu053. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Van Elteren, J.; Albacete, A.; Quinet, M.; Martínez-Andújar, C.; Kinet, J.M.; Pérez-Alfocea, F.; Lutts, S. Impact of salinity on early reproductive physiology of tomato (Solanum lycopersicum) in relation to a heterogeneous distribution of toxic ions in flower organs. Funct. Plant Biol. 2009, 36, 125–136. [Google Scholar] [CrossRef]
- Kaashyap, M.; Ford, R.; Mann, A.; Varshney, R.K.; Siddique, K.H.M.; Mantri, N. Comparative Flower Transcriptome Network Analysis Reveals DEGs Involved in Chickpea Reproductive Success during Salinity. Plants 2022, 11, 434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fatima, M.; Zhou, P.; Ma, Q.; Ming, R. Analysis of MADS-box genes revealed modified flowering gene network and diurnal expression in pineapple. BMC Genom. 2020, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, G.; Al-Babili, S.; Von Lintig, J. Carotenoid oxygenases: Cleave it or leave it. Trends Plant Sci. 2003, 8, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Deng, Y.J.; Wang, Y.H.; Lou, Y.R.; He, L.F.; Liu, H.; Li, T.; Yan, Z.M.; Zhuang, J.; Xiong, A.S. Changes in Carotenoid Concentration and Expression of Carotenoid Biosynthesis Genes in Daucus carota Taproots in Response to Increased Salinity. Horticulturae 2022, 8, 650. [Google Scholar] [CrossRef]
- Stepien, P.; Johnson, G.N. Contrasting Responses of Photosynthesis to Salt Stress in the Glycophyte Arabidopsis and the Halophyte Thellungiella: Role of the Plastid Terminal Oxidase as an Alternative Electron Sink. Plant Physiol. 2009, 149, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Frary, A.; Göl, D.; Keleş, D.; Ökmen, B.; Pinar, H.; Şiǧva, H.T.; Yemenicioǧlu, A.; Doǧanlar, S. Salt tolerance in Solanum pennellii: Antioxidant response and related QTL. BMC Plant Biol. 2010, 10, 58. [Google Scholar] [CrossRef]
- Jan, R.; Kim, N.; Lee, S.H.; Khan, M.A.; Asaf, S.; Lubna; Park, J.R.; Asif, S.; Lee, I.J.; Kim, K.M. Enhanced Flavonoid Accumulation Reduces Combined Salt and Heat Stress Through Regulation of Transcriptional and Hormonal Mechanisms. Front. Plant Sci. 2021, 12, 796956. [Google Scholar] [CrossRef]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef] [PubMed]
- Moliner, C.; Barros, L.; Dias, M.I.; López, V.; Langa, E.; Ferreira, I.C.F.R.; Gómez-Rincón, C. Edible flowers of tagetes erecta l. As functional ingredients: Phenolic composition, antioxidant and protective effects on Caenorhabditis elegans. Nutrients 2018, 10, 2002. [Google Scholar] [CrossRef] [PubMed]
- Chisowa, E.H.; Hall, D.R.; Farman, D.I. Chemical composition of the essential oil of Tagetes minuta L. from Zambia. J. Essent. Oil Res. 1998, 10, 183–184. [Google Scholar] [CrossRef]
- Guo, J.; Shan, C.; Zhang, Y.; Wang, X.; Tian, H.; Han, G.; Zhang, Y.; Wang, B. Mechanisms of Salt Tolerance and Molecular Breeding of Salt-Tolerant Ornamental Plants. Front. Plant Sci. 2022, 13, 854116. [Google Scholar] [CrossRef]
- Kazan, K.; Lyons, R. The link between flowering time and stress tolerance. J. Exp. Bot. 2016, 67, 47–60. [Google Scholar] [CrossRef]
- Zrig, A.; AbdElgawad, H.; Touneckti, T.; Ben Mohamed, H.; Hamouda, F.; Khemira, H. Potassium and calcium improve salt tolerance of Thymus vulgaris by activating the antioxidant systems. Sci. Hortic. 2021, 277, 109812. [Google Scholar] [CrossRef]
- Jahan, M.S.; Li, G.; Xie, D.; Farag, R.; Hasan, M.M.; Alabdallah, N.M.; Al-Harbi, N.A.; Al-Qahtani, S.M.; Zeeshan, M.; Nasar, J.; et al. Melatonin Mitigates Salt-Induced Growth Inhibition Through the Regulation of Carbohydrate and Nitrogen Metabolism in Tomato Seedlings. J. Soil Sci. Plant Nutr. 2023, 23, 4290–4308. [Google Scholar] [CrossRef]

| 0 mM NaCl | 50 mM NaCl | 100 mM NaCl | 300 mM NaCl | ||
|---|---|---|---|---|---|
| Carotenoids | Aurora Orange | 1.23 ± 0.10 c | 1.29 ± 0.15 c | 2.64 ± 0.71 b | 3.44 ± 0.91 a |
| Fireball | 1.28 ± 0.12 d | 1.42 ± 0.19 c | 2.59 ± 0.81 b | 3.19 ± 0.95 a | |
| Safari Scarlet | 1.27 ± 0.14 d | 1.57 ± 0.21 c | 3.11 ± 0.90 b | 3.74 ± 0.92 a | |
| Polyphenols | Aurora Orange | 23.21 ± 0.66 d | 33.99 ± 0.55 c | 44.61 ± 0.51 b | 55.45 ± 1.01 a |
| Fireball | 22.25 ± 0.55 d | 33.98 ± 0.66 c | 44.50 ± 0.44 b | 55.66 ± 0.99 a | |
| Safari Scarlet | 24.21 ± 0.77 d | 34.99 ± 0.88 c | 45.66 ± 0.90 b | 58.11 ± 1.75 a | |
| Flavonoids | Aurora Orange | 4.31 ± 0.34 d | 6.32 ± 0.44 c | 8.11 ± 0.61 b | 10.41 ± 1.92 a |
| Fireball | 4.23 ± 0.31 d | 6.23 ± 0.55 c | 8.59 ± 0.55 b | 11.23 ± 1.95 a | |
| Safari Scarlet | 6.29 ± 0.55 d | 7.44 ± 0.61 c | 9.11 ± 1.78 b | 14.74 ± 2.11 a |
| 0 mM NaCl | 50 mM NaCl | 100 mM NaCl | 300 mM NaCl | ||
|---|---|---|---|---|---|
| Aurora Orange | 1.23 ± 0.10 c | 1.29 ± 0.25 c | 2.64 ± 0.71 b | 3.44 ± 0.91 a | |
| CAT | Fireball | 1.28 ± 0.12 d | 1.42 ± 0.19 c | 2.59 ± 0.81 b | 3.19 ± 0.95 a |
| Safari Scarlet | 1.37 ± 0.14 d | 1.57 ± 0.21 c | 3.11 ± 0.90 b | 3.74 ± 0.92 a | |
| POX | Aurora Orange | 23.21 ± 0.66 d | 33.99 ± 0.55 c | 44.61 ± 0.51 b | 55.45 ± 1.01 a |
| Fireball | 22.25 ± 0.55 d | 33.98 ± 0.66 c | 44.50 ± 0.44 b | 55.66 ± 0.99 a | |
| Safari Scarlet | 24.21 ± 0.77 d | 34.99 ± 0.88 c | 45.66 ± 0.90 b | 58.11 ± 1.75 a | |
| APX | Aurora Orange | 4.31 ± 0.34 d | 6.32 ± 0.44 c | 8.11 ± 0.61 b | 10.41 ± 1.92 a |
| Fireball | 4.23 ± 0.31 d | 6.23 ± 0.55 c | 8.59 ± 0.55 b | 11.23 ± 1.95 a | |
| Safari Scarlet | 6.29 ± 0.55 d | 7.44 ± 0.61 c | 9.11 ± 1.78 b | 14.74 ± 2.11 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzman, M.R.; Marques, I. Effect of Varied Salinity on Marigold Flowers: Reduced Size and Quantity Despite Enhanced Antioxidant Activity. Agronomy 2023, 13, 3076. https://doi.org/10.3390/agronomy13123076
Guzman MR, Marques I. Effect of Varied Salinity on Marigold Flowers: Reduced Size and Quantity Despite Enhanced Antioxidant Activity. Agronomy. 2023; 13(12):3076. https://doi.org/10.3390/agronomy13123076
Chicago/Turabian StyleGuzman, María Rita, and Isabel Marques. 2023. "Effect of Varied Salinity on Marigold Flowers: Reduced Size and Quantity Despite Enhanced Antioxidant Activity" Agronomy 13, no. 12: 3076. https://doi.org/10.3390/agronomy13123076







