Effect of Partial Root-Zone Irrigation on Plant Growth, Root Morphological Traits and Leaf Elemental Stoichiometry of Tomato under Elevated CO2
Abstract
:1. Introduction
2. Materials and Methods
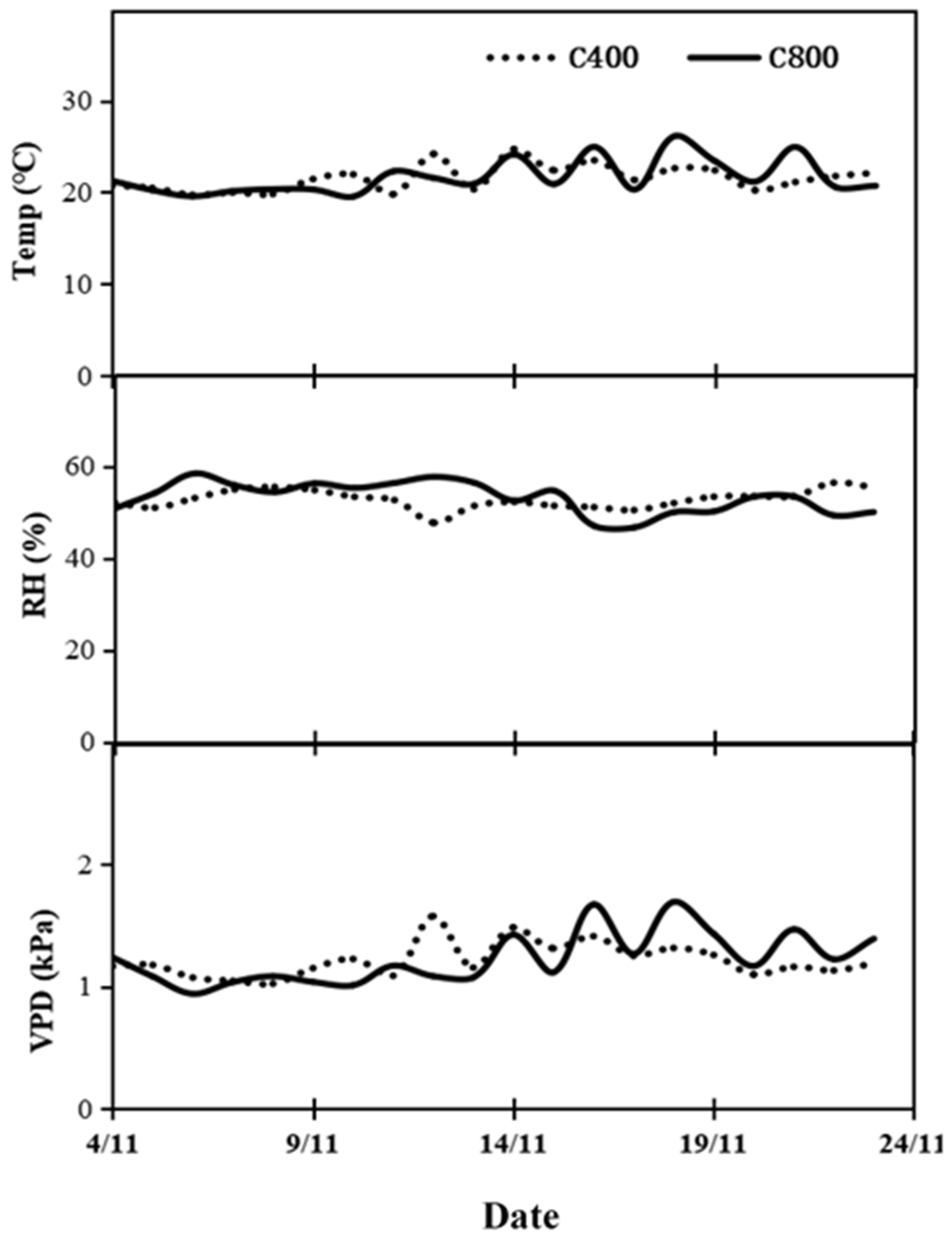
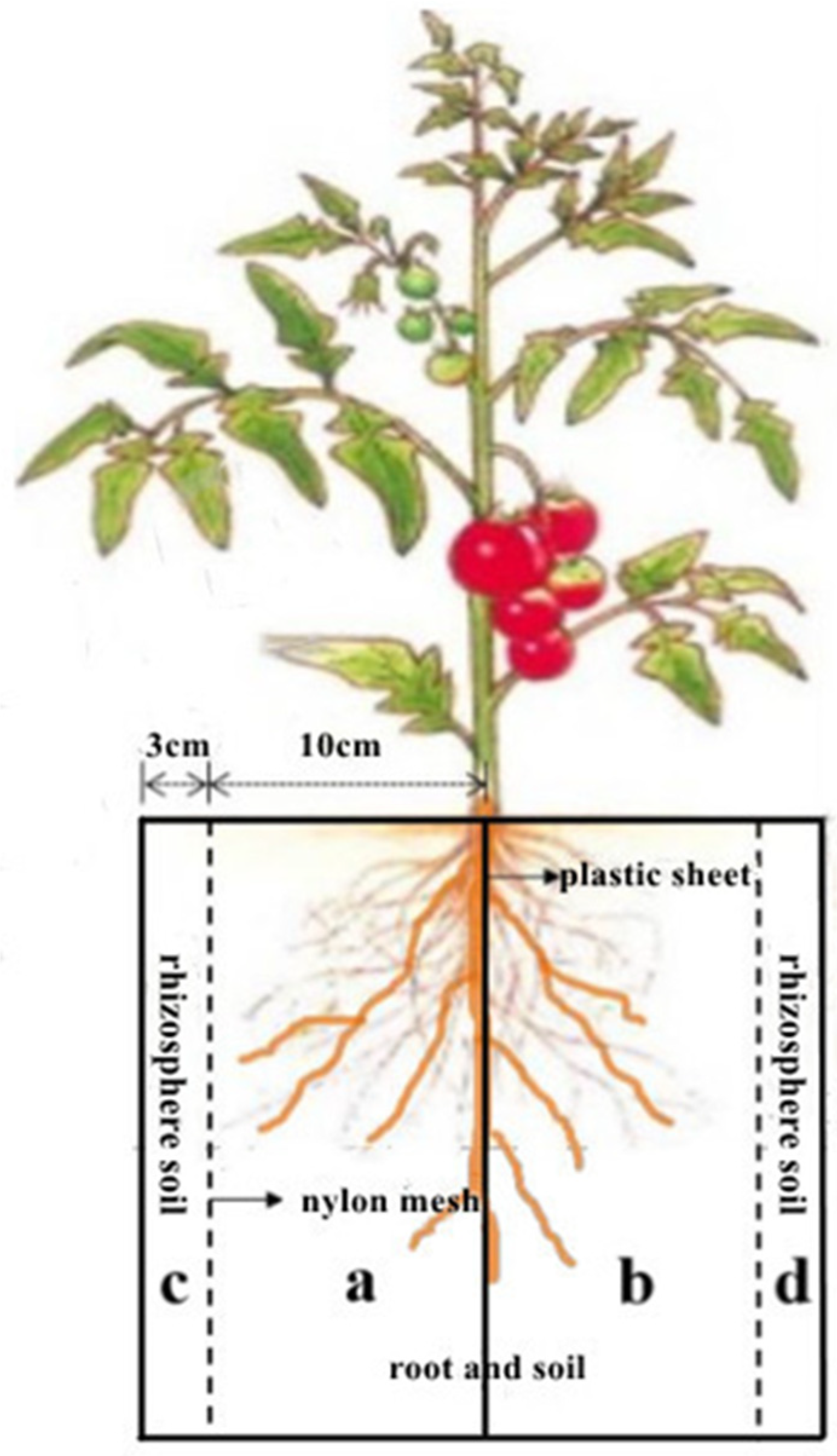
2.1. Treatments
2.2. Measurements
2.2.1. Harvest
2.2.2. Root Morphological Analyses
2.2.3. Plant Biomass, Water Use, Water Use Efficiency
2.2.4. Elemental Analyses
2.3. Statistical Analysis
3. Results
3.1. Plant Dry Matter Accumulation, Dry Biomass Allocation, Shoot Dry Weight, Plant Water Use and Plant Water Use Efficiency
3.2. Root Growth and Morphological Traits
3.3. Leaf Element Concentration
3.4. Leaf Element Uptakes
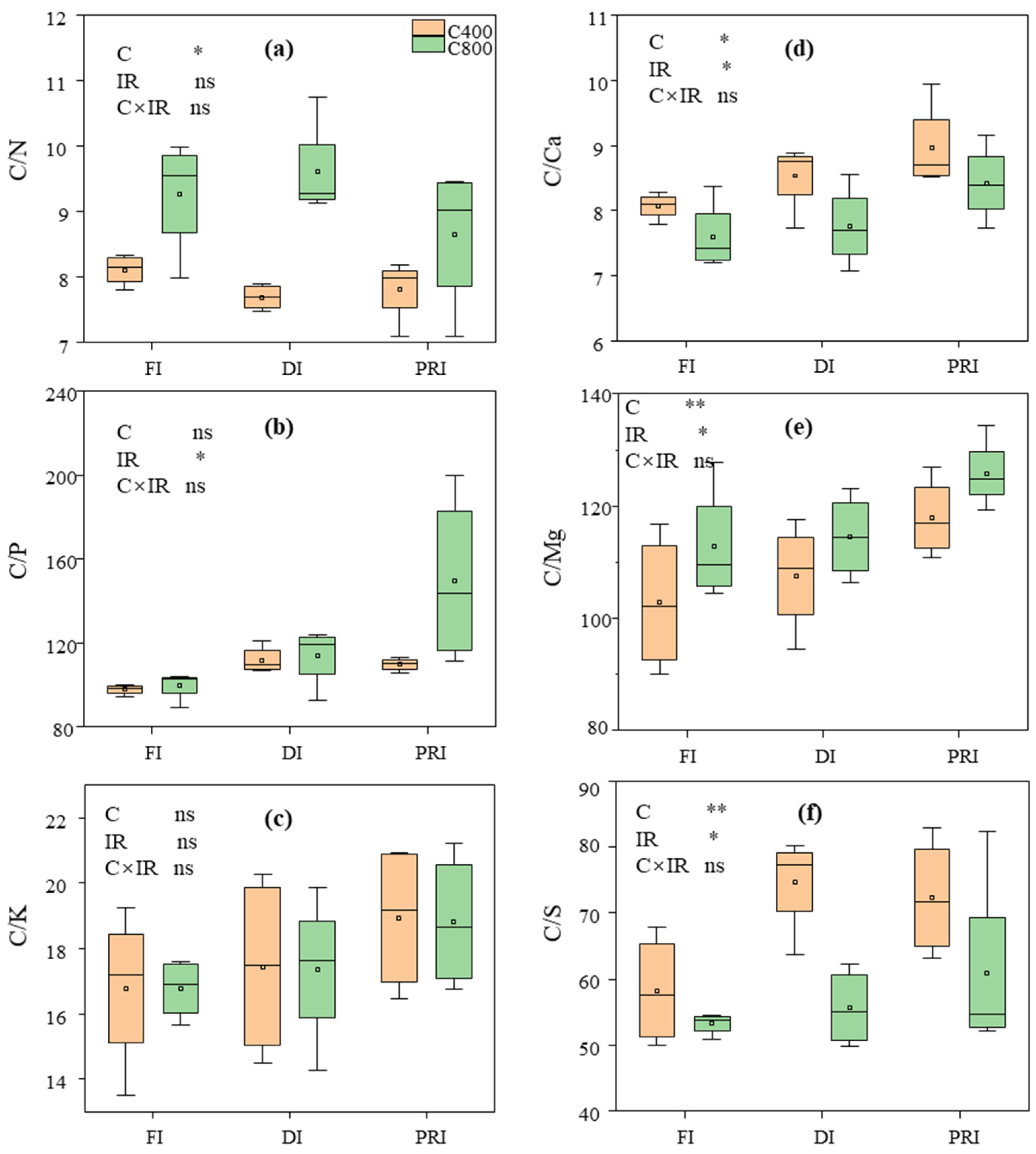
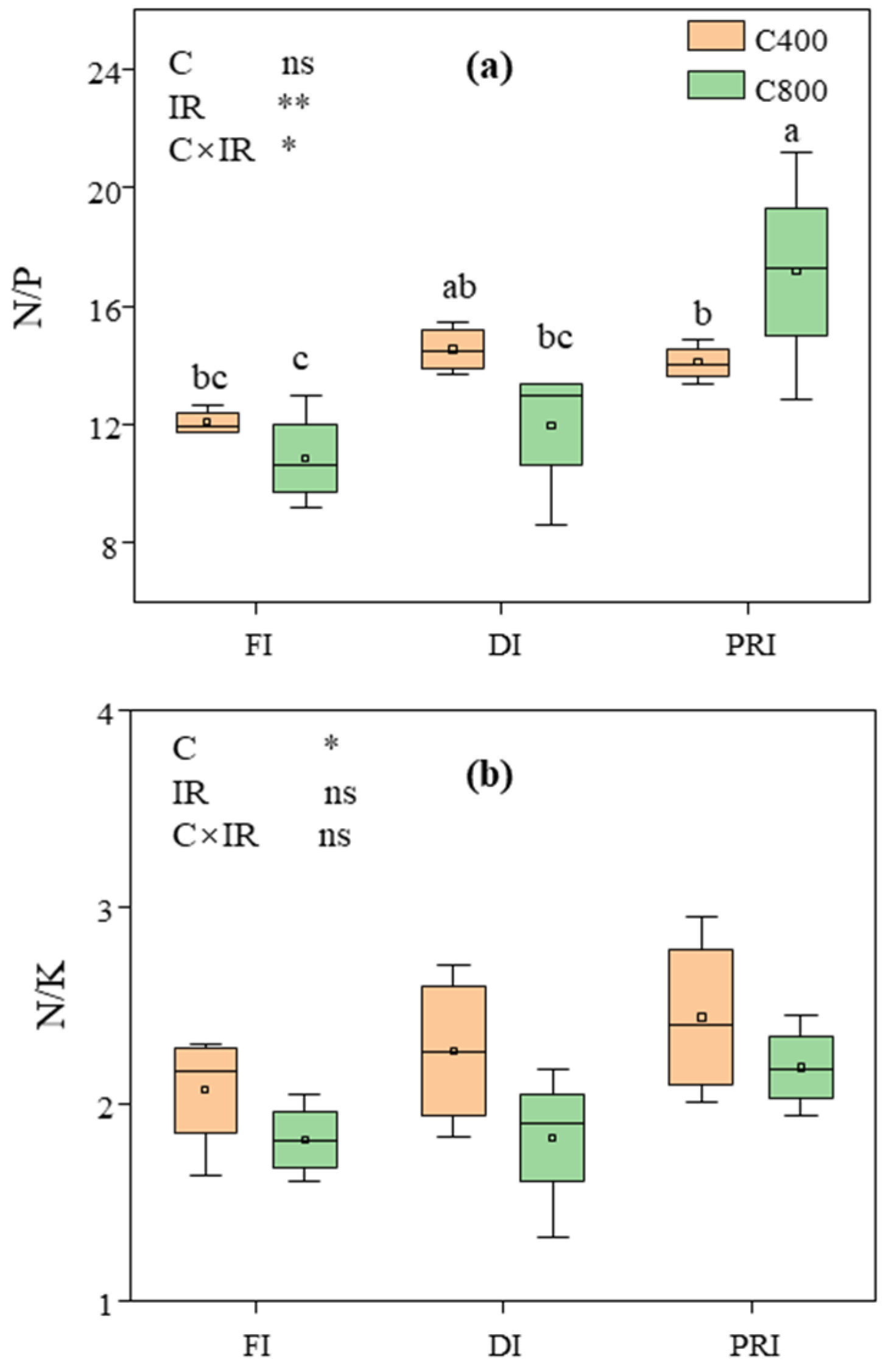
3.5. C/N, C/P, C/K, C/Ca, C/Mg, C/S, N/P and N/K in Tomato Leaves
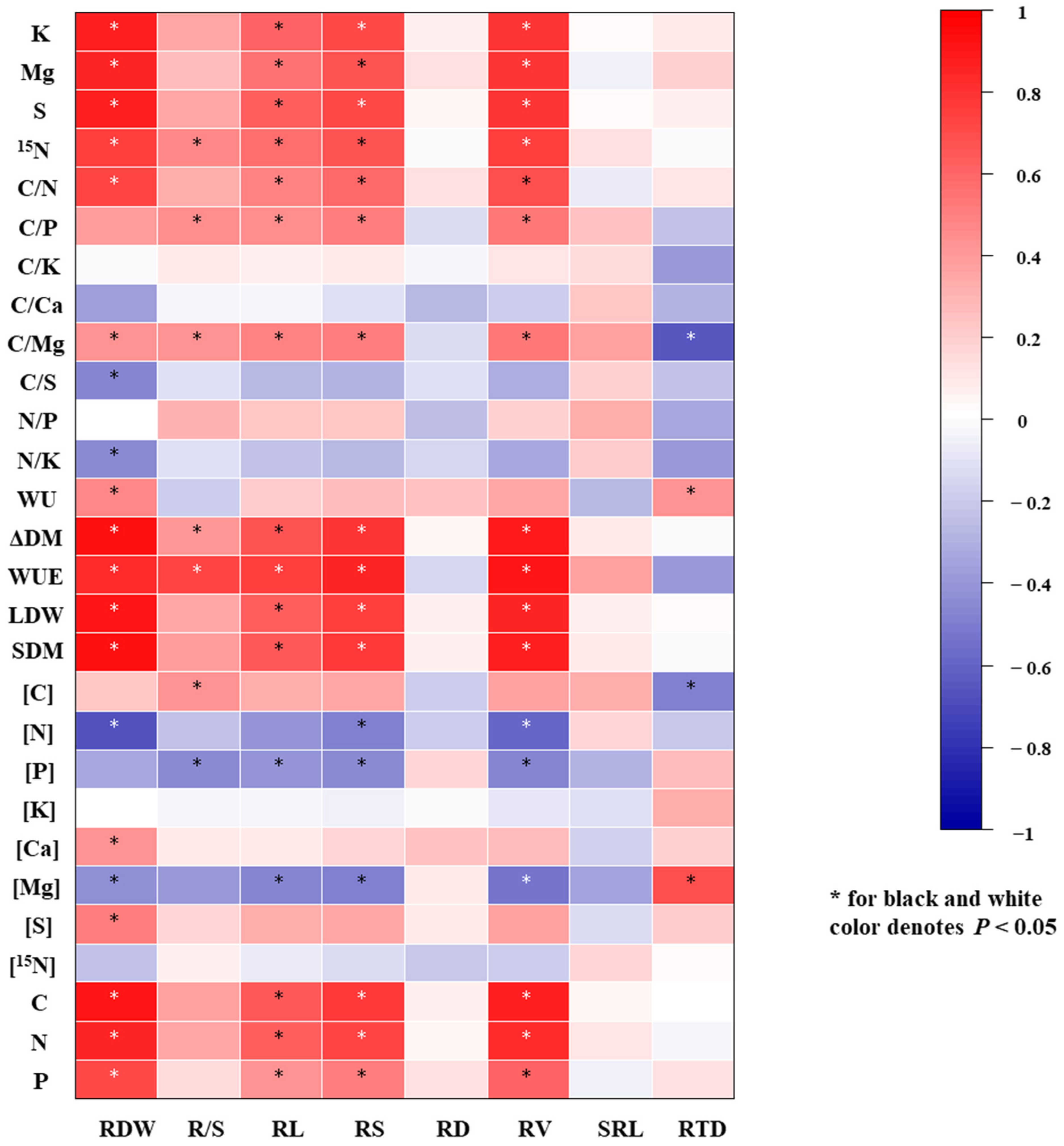
3.6. The Pearson Correlation Analysis between Root Morphology Traits and Tomato Growth, Element Concentrations, Element Uptakes and Stoichiometric Ratios
3.7. PCA Analysis of Tomato Parameters
4. Discussion
4.1. The Effects of PRI on Plant Biomass, Biomass Allocation and Water Use Efficiency under e[CO2]
4.2. The Effects of PRI on Root Morphological Traits of Tomato Leaves under e[CO2]
4.3. The Effects of PRI on Leaf Nutrient Concentration, Nutrient Uptake, and Nutrient Stoiochiometry of Tomato Leaves under e[CO2]
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; CoreWriting Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Long, S.P. 30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Glob. Chang. Biol. 2021, 27, 27–49. [Google Scholar] [CrossRef] [PubMed]
- Leakey, A.D.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Du, T.; Li, X.; Fang, L.; Liu, F. Interactive effects of CO2 concentration elevation and nitrogen fertilization on water and nitrogen use efficiency of tomato grown under reduced irrigation regimes. Agric. Water Manag. 2018, 202, 174–182. [Google Scholar] [CrossRef]
- Yang, X.; Bornø, M.L.; Wei, Z.; Liu, F. Combined effect of partial root drying and elevated atmospheric CO2 on the physiology and fruit quality of two genotypes of tomato plants with contrasting endogenous ABA levels. Agric. Water Manag. 2021, 254, 106987. [Google Scholar] [CrossRef]
- McDonald, E.P.; Erickson, J.E.; Kruger, E.L. Can decreased transpiration limit plant nitrogen acquisition in elevated CO2? Funct. Plant Biol. 2002, 22, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef]
- Loladze, I. Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. eLife 2014, 3, e02245. [Google Scholar] [CrossRef]
- Müller, C.; Elliott, J.; Levermann, A. Fertilizing hidden hunger. Nat. Clim. Chang. 2014, 4, 540–541. [Google Scholar] [CrossRef]
- Loladze, I. Rising atmospheric CO2 and human nutrition: Toward globally imbalanced plant stoichiometry? Trends Ecol. Evol. 2002, 17, 457–461. [Google Scholar] [CrossRef]
- Kundu, P.; Goel, K.; Zinta, G. Nutritional imbalance in plants under rising atmospheric CO2. In Plant Nutrition and Food Security in the Era of Climate Change; Academic Press: Cambridge, MA, USA, 2022; pp. 513–536. [Google Scholar]
- Xing, K.; Zhao, M.; Niinemets, U.; Niu, S.; Tian, J.; Jiang, Y.; Chen, H.Y.H.; White, P.J.; Guo, D.; Ma, Z. Relationships Between Leaf Carbon and Macronutrients Across Woody Species and Forest Ecosystems Highlight How Carbon Is Allocated to Leaf Structural Function. Front. Plant Sci. 2021, 12, 674932. [Google Scholar] [CrossRef] [PubMed]
- Benlloch-Gonzalez, M.; Bochicchio, R.; Berger, J.; Bramley, H.; Palta, J.A. High temperature reduces the positive effect of elevated CO2 on wheat root system growth. Field Crops Res. 2014, 165, 71–79. [Google Scholar] [CrossRef]
- Chaudhuri, U.N.; Kirkham, M.B.; Kanemasu, E.T. Root Growth of Winter Wheat under Elevated Carbon Dioxide and Drought. Crop Sci. 1990, 30, 853–857. [Google Scholar] [CrossRef]
- Uddin, S.; Low, M.; Parvin, S.; Fitzgerald, G.J.; Tausz-Posch, S.; Armstrong, R.; O’Leary, G.; Tausz, M. Elevated [CO2] mitigates the effect of surface drought by stimulating root growth to access sub-soil water. PLoS ONE 2018, 13, e0198928. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Zhang, J. Controlled alternate partial root-zone irrigation: Its physiological consequences and impact on water use efficiency. J. Exp. Bot. 2004, 55, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Liu, F.L.; Andersen, M.N.; Jensen, C.R. Improved plant nitrogen nutrition contributes to higher water use efficiency in tomatoes under alternate partial root-zone irrigation. Funct. Plant Biol. 2010, 37, 175–182. [Google Scholar] [CrossRef]
- Dodd, I.C. Soil moisture heterogeneity during deficit irrigation alters root-to-shoot signalling of abscisic acid. Funct. Plant Biol. 2007, 34, 439–448. [Google Scholar] [CrossRef]
- Wang, Y.S.; Liu, F.L.; Jensen, C.R. Comparative effects of partial root-zone irrigation and deficit irrigation on phosphorus uptake in tomato plants. J. Hortic. Sci. Biotechnol. 2012, 87, 600–604. [Google Scholar] [CrossRef]
- Kang, S.Z.; Liang, Z.S.; Hu, W.; Zhang, J.H. Water use efficiency of controlled alternate irrigation on root-divided maize plants. Agric. Water Manag. 1998, 38, 69–76. [Google Scholar] [CrossRef]
- Kang, S.Z.; Shi, W.J.; Cao, H.X.; Zhang, J.H. Alternate watering in soil vertical profile improved water use effciency of maize (Zea mays). Field Crops Res. 2002, 77, 31–41. [Google Scholar] [CrossRef]
- Wang, L.; de Kroon, H.; Bogemann, G.M.; Smits, A.J.M. Partial root drying effects on biomass production in Brassica napus and the significance of root responses. Plant Soil. 2005, 276, 313–326. [Google Scholar] [CrossRef]
- Dodd, I.C. Rhizosphere manipulations to maximize ‘crop per drop’ during deficit irrigation. J. Exp. Bot. 2009, 60, 2454–2459. [Google Scholar] [CrossRef]
- Birch, H.F. The Effec tOf Soi lDrying On Humus Decomposition and Nitrogen availability. Plant Soil. 1958, 1, 9–31. [Google Scholar] [CrossRef]
- Wang, Y.S.; Liu, F.L.; Jensen, C.R. Comparative effects of deficit irrigation and alternate partial root-zone irrigation on xylem pH, ABA and ionic concentrations in tomatoes. J. Exp. Bot. 2012, 63, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Bünemann, E.K.; Keller, B.; Hoop, D.; Jud, K.; Boivin, P.; Frossard, E. Increased availability of phosphorus after drying and rewetting of a grassland soil: Processes and plant use. Plant Soil. 2013, 370, 511–526. [Google Scholar] [CrossRef]
- Wei, G.; Zhang, M.; Cui, B.; Wei, Z.; Liu, F. Ammonium nitrogen combined with partial root-zone drying enhanced fruit quality of tomato under elevated atmospheric CO2. Sci. Hortic. 2024, 323, 112514. [Google Scholar] [CrossRef]
- Darby, L.A.; Ritchie, D.B.; Taylor, I.B. Isogenic Lines of the Tomato ‘Ailsa Craig’; Annual Report Glasshouse Crops Research Institute, tgrc.ucdavis.edu; Dorset Press: Dorchester, UK, 1977; pp. 168–184. [Google Scholar]
- Liu, X.; Ma, Y.; Manevski, K.; Andersen, M.N.; Li, Y.; Wei, Z.; Liu, F. Biochar and alternate wetting-drying cycles improving rhizosphere soil nutrients availability and tobacco growth by altering root growth strategy in Ferralsol and Anthrosol. Sci. Total Environ. 2021, 806, 150513. [Google Scholar] [CrossRef] [PubMed]
- Pazzagli, P.T.; Weiner, J.; Liu, F. Effects of CO2 elevation and irrigation regimes on leaf gas exchange, plant water relations, and water use efficiency of two tomato cultivars. Agric. Water Manag. 2016, 169, 26–33. [Google Scholar] [CrossRef]
- Elhani, S.; Haddadi, M.; Csákvári, E.; Zantar, S.; Hamim, A.; Villányi, V.; Douaik, A.; Bánfalvi, Z. Effects of partial root-zone drying and deficit irrigation on yield, irrigation water-use efficiency and some potato (Solanum tuberosum L.) quality traits under glasshouse conditions. Agric. Water Manag. 2019, 224, 105745. [Google Scholar] [CrossRef]
- Reddy, A.R.; Rasineni, G.K.; Raghavendra, A.S. The impact of global elevated CO2 concentration on photosynthesis and plant productivity. Curr. Sci. 2010, 99, 46–57. [Google Scholar]
- Fleisher, D.H.; Timlin, D.J.; Reddy, V.R. Interactive Effects of Carbon Dioxide and Water Stress on Potato Canopy Growth and Development. Agron. J. 2008, 100, 711–719. [Google Scholar] [CrossRef]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Aust. J. Plant Physiol. 2000, 27, 595–607. [Google Scholar]
- Kizildeniz, T.; Pascual, I.; Irigoyen, J.J.; Morales, F. Future CO2, warming and water deficit impact white and red Tempranillo grapevine: Photosynthetic acclimation to elevated CO2 and biomass allocation. Physiol. Plant 2021, 172, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, R. Distribution of dry matter in the plant. Neth. J. Agric. Sci. 1962, 10, 361–376. [Google Scholar] [CrossRef]
- Benlloch-Gonzalez, M.; Berger, J.; Bramley, H.; Rebetzke, G.; Palta, J.A. The plasticity of the growth and proliferation of wheat root system under elevated CO2. Plant Soil. 2013, 374, 963–976. [Google Scholar] [CrossRef]
- Madhu, M.; Hatfield, J.L. Dynamics of Plant Root Growth under Increased Atmospheric Carbon Dioxide. Agron. J. 2013, 105, 657–669. [Google Scholar] [CrossRef]
- Rogers, H.H.; Peterson, C.M.; Mccrimmon, J.N.; CURE, J.D. Response of plant roots to elevated atmospheric carbondioxide. Plant Cell Environ. 1992, 15, 749–752. [Google Scholar] [CrossRef]
- Mingo, D.M.; Theobald, J.C.; Bacon, M.A.; Davies, W.J.; Dodd, I.C. Biomass allocation in tomato (Lycopersicon esculentum) plants grown under partial rootzone drying: Enhancement of root growth. Funct. Plant Biol. 2004, 31, 971–978. [Google Scholar] [CrossRef]
- Chen, C.; Xu, F.; Zhu, J.R.; Wang, R.F.; Xu, Z.H.; Shu, L.Z.; Xu, W.W. Nitrogen forms affect root growth, photosynthesis, and yield of tomato under alternate partial root-zone irrigation. J. Plant Nutr. Soil. Sci. 2015, 179, 104–112. [Google Scholar] [CrossRef]
- Wang, H.; Inukai, Y.; Yamauchi, A. Root Development and Nutrient Uptake. Crit. Rev. Plant Sci. 2007, 25, 279–301. [Google Scholar] [CrossRef]
- Ogawa, A.; Kawashima, C.; Yamauchi, A. Sugar Accumulation along the Seminal Root Axis, as Affected by Osmotic Stress in Maize: A Possible Physiological Basis for Plastic Lateral Root Development. Plant Prod. Sci. 2015, 8, 173–180. [Google Scholar] [CrossRef]
- Ristova, D.; Busch, W. Natural variation of root traits: From development to nutrient uptake. Plant Physiol. 2014, 166, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Sinha, R.; Singla-Pareek, S.L.; Pareek, A.; Singh, A.K. Shaping the root system architecture in plants for adaptation to drought stress. Physiol. Plant 2022, 174, e13651. [Google Scholar] [CrossRef] [PubMed]
- Birouste, M.; Zamora-Ledezma, E.; Bossard, C.; Pérez-Ramos, I.M.; Roumet, C. Measurement of fine root tissue density: A comparison of three methods reveals the potential of root dry matter content. Plant Soil. 2013, 374, 299–313. [Google Scholar] [CrossRef]
- Kong, D.; Wang, J.; Wu, H.; Valverde-Barrantes, O.J.; Wang, R.; Zeng, H.; Kardol, P.; Zhang, H.; Feng, Y. Nonlinearity of root trait relationships and the root economics spectrum. Nat. Commun. 2019, 10, 2203. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, C.M.; Lindahl, B.; Wardle, D.A.; Sundqvist, M.K.; Gundale, M.J.; Fanin, N.; Kardol, P. Root trait-microbial relationships across tundra plant species. New Phytol. 2021, 229, 1508–1520. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Zhang, Y.; Wang, B.; Ran, Q.; Zhang, J. The bHLH family member ZmPTF1 regulates drought tolerance in maize by promoting root development and abscisic acid synthesis. J. Exp. Bot. 2019, 70, 5471–5486. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, W.; Wang, Q.; Cao, Y.; Xu, F.; Dodd, I.C.; Xu, W. ABA regulation of root growth during soil drying and recovery can involve auxin response. Plant Cell Environ. 2022, 45, 871–883. [Google Scholar] [CrossRef]
- Wasaya, A.; Zhang, X.; Fang, Q.; Yan, Z. Root Phenotyping for Drought Tolerance: A Review. Agronomy 2018, 8, 241. [Google Scholar] [CrossRef]
- Ho, M.D.; Rosas, J.C.; Brown, K.M.; Lynch, J.P. Root architectural tradeoffs for water and phosphorus acquisition. Funct. Plant Biol. 2005, 32, 737–748. [Google Scholar] [CrossRef]
- Nie, M.; Lu, M.; Bell, J.; Raut, S.; Pendall, E. Altered root traits due to elevated CO2: A meta-analysis. Glob. Ecol. Biogeogr. 2013, 22, 1095–1105. [Google Scholar] [CrossRef]
- Pokorný, R.; Tomášková, I.; Marek, M.V. Response of Norway spruce root system to elevated atmospheric CO2 concentration. Acta Physiol. Plant. 2013, 35, 1807–1816. [Google Scholar] [CrossRef]
- Wang, N.; Gao, G.; Wang, Y.; Wang, D.; Wang, Z.; Gu, J. Coordinated responses of leaf and absorptive root traits under elevated CO2 concentration in temperate woody and herbaceous species. Environ. Exp. Bot. 2020, 179, 104199. [Google Scholar] [CrossRef]
- Li, X.; Jiang, D.; Liu, F. Soil warming enhances the hidden shift of elemental stoichiometry by elevated CO2 in wheat. Sci. Rep. 2016, 6, 23313. [Google Scholar] [CrossRef] [PubMed]
- Taub, D.R.; Wang, X. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J. Integr. Plant Biol. 2008, 50, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.E.; LeCain, D.R.; McCormack, M.L.; Pendall, E.; Carlson, M.; Blumenthal, D.M.; Lamb, E. Root responses to elevated CO2, warming and irrigation in a semi-arid grassland: Integrating biomass, length and life span in a 5-year field experiment. J. Ecol. 2018, 106, 2176–2189. [Google Scholar] [CrossRef]
- Jungk, A. Root hairs and the acquisition of plant nutrients from soil. J. Plant Nutr. Soil. Sci. 2001, 164, 121–129. [Google Scholar] [CrossRef]
- McGrath, J.M.; Lobell, D.B. Reduction of transpiration and altered nutrient allocation contribute to nutrient decline of crops grown in elevated CO2 concentrations. Plant Cell Environ. 2013, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Ito, O.; Engelaar, W.M.H.G. Uptake, metabolism and distribution of nitrogen in crop plants traced by enriched and natural 15N: Progress over the last 30 years. Phytochem. Rev. 2003, 2, 121–132. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Rubio Asensio, J.S.; Cousins, A.B. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 2010, 328, 899–903. [Google Scholar] [CrossRef]
- Ullah, S.; Zhao, Q.; Wu, K.; Ali, I.; Liang, H.; Iqbal, A.; Wei, S.; Cheng, F.; Ahmad, S.; Jiang, L.; et al. Biochar application to rice with 15N-labelled fertilizers, enhanced leaf nitrogen concentration and assimilation by improving morpho-physiological traits and soil quality. Saudi J. Biol. Sci. 2021, 28, 3399–3413. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, C.-S.; Li, J.; Christie, P.; He, X.-H.; Ju, X.-T. Natural 15N Abundance in Winter Wheat Amended with Urea and Compost: A Long-Term Experiment. Pedosphere 2013, 23, 835–843. [Google Scholar] [CrossRef]
- Fratte, M.D.; Pierce, S.; Zanzottera, M.; Cerabolini, B.E.L. The association of leaf sulfur content with the leaf economics spectrum and plant adaptive strategies. Funct. Plant Biol. 2021, 48, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Gusewell, S.; Koerselman, W. Variation in nitrogen and phosphorus concentrations of wetland plants. Perspect. Plant Ecol. Evol. Syst. 2002, 5, 37–61. [Google Scholar] [CrossRef]
- Craine, J.M.; Morrow, C.; Stock, W.D. Nutrient concentration ratios and co-limitation in South African grasslands. New Phytol. 2008, 179, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Olde Venterink, H.; Wassen, M.J.; Verkroost, A.W.M.; De Ruiter, P.C. Species Richness–Productivity Patterns Differ between N-, P-, and K-Limited Wetlands. Ecology 2003, 84, 2191–2199. [Google Scholar] [CrossRef]
- Liu, C.; Rubæk, G.H.; Liu, F.; Andersen, M.N. Effect of partial root zone drying and deficit irrigation on nitrogen and phosphorus uptake in potato. Agric. Water Manag. 2015, 159, 66–76. [Google Scholar] [CrossRef]
- Oliveira, E.M.M.; Ruiz, H.A.; Alvarez V., V.H.; Ferreira, P.A.; Costa, F.O.; Almeida, I.C.C. Nutrient supply by mass flow and diffusion to maize plants in response to soil aggregate size and water potential. Rev. Bras. Ciência Solo 2010, 34, 317–328. [Google Scholar] [CrossRef]

| CO2 | Irrigation Regimes | ∆DM (g Plant−1) | Biomass Allocation (%) | SDW (g Plant−1) | LDW (g Plant−1) | WU (L Plant−1) | WUE (g L−1) | ||
|---|---|---|---|---|---|---|---|---|---|
| Leaf | Stem | Root | |||||||
| C400 | FI | 25.9 ± 1.6 | 73.0 ± 1.5 | 21.6 ± 1.4 | 5.4 ± 0.2 | 26.6 ± 1.5 | 20.5 ± 1.0 | 7.0 ± 0.3 | 3.7 ± 0.3 |
| DI | 25.7 ± 0.5 | 68.5 ± 1.2 | 24.9 ± 1.2 | 6.7 ± 0.2 | 26.2 ± 1.8 | 19.2 ± 1.2 | 4.9 ± 0 | 5.2 ± 0.1 | |
| PRI | 25.8 ± 2.0 | 67.6 ± 1.2 | 25.6 ± 1.2 | 6.8 ± 0.1 | 26.1 ± 0.5 | 18.9 ± 0.3 | 4.9 ± 0 | 5.3 ± 0.4 | |
| C800 | FI | 53.8 ± 2.1 | 74.3 ± 1.3 | 19.2 ± 1.4 | 6.5 ± 0.2 | 52.6 ± 2.1 | 41.8 ± 1.9 | 9.7 ± 0.7 | 5.6 ± 0.3 |
| DI | 50.3 ± 1.6 | 70.7 ± 1.1 | 21.5 ± 1.0 | 7.9 ± 0.4 | 50.2 ± 2.4 | 38.4 ± 1.3 | 6.8 ± 0 | 7.4 ± 0.2 | |
| PRI | 52.1 ± 2.7 | 72.0 ± 0.7 | 21.8 ± 0.3 | 7.3 ± 0.4 | 48.8 ± 1.3 | 37.8 ± 0.8 | 6.8 ± 0 | 7.6 ± 0.4 | |
| Output of two-way ANOVA | |||||||||
| [CO2] (C) | *** | * | ** | ** | *** | *** | *** | *** | |
| Irrigation regimes (IR) | ns | ** | * | *** | ns | ** | *** | *** | |
| C × IR | ns | ns | ns | ns | ns | ns | ns | ns | |
| CO2 | Irrigation Regimes | RL (m) | RS (cm2) | RD (mm) | RV (cm3) | SRL (m g−1) | RTD (g cm−3) | R/S | RDW (g Plant−1) |
|---|---|---|---|---|---|---|---|---|---|
| C400 | FI | 12.9 ± 1.1 | 280.5 ± 28.6 | 0.54 ± 0.02 | 4.5 ± 0.6 | 9.9 ± 0.8c | 0.3 ± 0.02 | 0.06 ± 0.003 | 1.5 ± 0.1 |
| DI | 60.8 ± 4.6 | 718.0 ± 55.8 | 0.38 ± 0.01 | 7.1 ± 0.5 | 35.9 ± 2.7a | 0.2 ± 0.02 | 0.07 ± 0.003 | 1.9 ± 0.2 | |
| PRI | 43.1 ± 6.4 | 609.8 ± 62.7 | 0.45 ± 0.03 | 7.4 ± 0.6 | 25.8 ± 2.9ab | 0.2 ± 0.01 | 0.07 ± 0.003 | 1.9 ± 0.0 | |
| C800 | FI | 75.0 ± 19.7 | 1045.5 ± 132.7 | 0.47 ± 0.04 | 12.4 ± 0.6 | 21.9 ± 5.9b | 0.3 ± 0.01 | 0.07 ± 0.003 | 3.6 ± 0.1 |
| DI | 99.5 ± 24.5 | 1324.4 ± 240.7 | 0.47 ± 0.05 | 15.2 ± 1.6 | 26.3 ± 4.5ab | 0.2 ± 0.01 | 0.08 ± 0.005 | 4.3 ± 0.4 | |
| PRI | 123.0 ± 14.3 | 1569.4 ± 123.7 | 0.43 ± 0.03 | 17.0 ± 1.1 | 30.8 ± 4.6ab | 0.2 ± 0.01 | 0.09 ± 0.005 | 3.8 ± 0.3 | |
| Output of two-way ANOVA | |||||||||
| CO2 (C) | *** | *** | ns | *** | ns | ns | ** | *** | |
| Irrigation regimes (IR) | * | ** | ns | ** | ** | ** | ** | ns | |
| C × IR | ns | ns | ns | ns | * | ns | ns | ns | |
| CO2 | Irrigation Regimes | [C] (mg g−1) | [N] (mg g−1) | [P] (mg g−1) | [K] (mg g−1) | [Ca] (mg g−1) | [Mg] (mg g−1) | [S] (mg g−1) | [15N] (mg g−1) |
|---|---|---|---|---|---|---|---|---|---|
| C400 | FI | 412.2 ± 4.1 | 50.9 ± 0.9 | 4.2 ± 0.09 | 25.0 ± 1.9 | 51.2 ± 1.1 | 4.1 ± 0.2 | 7.2 ± 0.5 | 1.5 ± 0.1 |
| DI | 421.4 ± 2.9 | 54.8 ± 1.1 | 3.8 ± 0.1 | 24.6 ± 2.0 | 49.5 ± 1.5 | 3.9 ± 0.2 | 5.7 ± 0.3 | 2.2 ± 0.3 | |
| PRI | 420.4 ± 1.1 | 54.0 ± 1.8 | 3.8 ± 0.05 | 22.5 ± 1.4 | 47.1 ± 1.7 | 3.6 ± 0.1 | 5.9 ± 0.4 | 1.4 ± 0.2 | |
| C800 | FI | 417.6 ± 1.4 | 45.4 ± 2.2 | 4.2 ± 0.16 | 25.0 ± 0.7 | 55.2 ± 1.7 | 3.7 ± 0.2 | 7.8 ± 0.1 | 1.1 ± 0.1 |
| DI | 418.4 ± 3.1 | 43.7 ± 1.6 | 3.7 ± 0.27 | 24.5 ± 1.8 | 54.2 ± 1.9 | 3.7 ± 0.1 | 7.6 ± 0.4 | 1.6 ± 0.1 | |
| PRI | 423.9 ± 1.7 | 49.6 ± 3.3 | 3.0 ± 0.39 | 22.7 ± 1.2 | 50.5 ± 1.6 | 3.4 ± 0.1 | 7.2 ± 0.7 | 1.5 ± 0.1 | |
| Output of two-way ANOVA | |||||||||
| CO2 (C) | ns | *** | ns | ns | ** | * | ** | 0.052 | |
| Irrigation regimes (IR) | * | ns | ** | ns | * | * | ns | ** | |
| C × IR | ns | ns | ns | ns | ns | ns | ns | ns | |
| CO2 | Irrigation Regimes | C (g Plant−1) | N (g Plant−1) | P (mg Plant−1) | K (mg Plant−1) | Ca (g Plant−1) | Mg (mg Plant−1) | S (mg Plant−1) | 15N (mg Plant−1) |
|---|---|---|---|---|---|---|---|---|---|
| C400 | FI | 8.5 ± 0.5 | 1.0 ± 0.05 | 86.4 ± 5.1d | 506.8 ± 17.2 | 1.1 ± 0.1 | 82.5 ± 3.1 | 147.7 ± 13.2 | 31.0 ± 2.2 |
| DI | 8 ± 0.1 | 1.0 ± 0.02 | 71.2 ± 1.5d | 463.6 ± 31.8 | 0.9 ± 0.0 | 74.3 ± 2.4 | 107.5 ± 6.6 | 41.0 ± 5.2 | |
| PRI | 8.1 ± 0.5 | 1.0 ± 0.06 | 73.2 ± 3.9d | 431.9 ± 41.5 | 0.9 ± 0.1 | 68.7 ± 5.5 | 112.4 ± 8.2 | 26.9 ± 4.7 | |
| C800 | FI | 17.5 ± 0.8 | 1.9 ± 0.05 | 175.8 ± 11.2a | 1041.3 ± 43.5 | 2.3 ± 0.1 | 155.2 ± 7.9 | 328.0 ±17.6 | 45.7 ± 5.8 |
| DI | 15.8 ± 0.4 | 1.7 ± 0.04 | 141.4 ± 13.3b | 929.5 ± 89.3 | 2.0 ± 0.0 | 138.7 ± 6.3 | 287.9 ± 21.9 | 61.7 ± 3.5 | |
| PRI | 16.3 ± 0.6 | 1.9 ± 0.11 | 113.3 ± 11.1c | 867.4 ± 18.5 | 1.9 ± 0.1 | 129.5 ± 5.8 | 273.9 ± 21.1 | 58.2 ± 4.2 | |
| Output of two-way ANOVA | |||||||||
| CO2 (C) | *** | *** | *** | *** | *** | *** | *** | *** | |
| Irrigation regimes (IR) | ns | ns | ** | * | ** | ** | * | * | |
| C × IR | ns | ns | * | ns | ns | ns | ns | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, G.; Xu, X.; Cui, B.; Zhang, M.; Liu, J.; Wei, Z.; Liu, F. Effect of Partial Root-Zone Irrigation on Plant Growth, Root Morphological Traits and Leaf Elemental Stoichiometry of Tomato under Elevated CO2. Agronomy 2023, 13, 3069. https://doi.org/10.3390/agronomy13123069
Wei G, Xu X, Cui B, Zhang M, Liu J, Wei Z, Liu F. Effect of Partial Root-Zone Irrigation on Plant Growth, Root Morphological Traits and Leaf Elemental Stoichiometry of Tomato under Elevated CO2. Agronomy. 2023; 13(12):3069. https://doi.org/10.3390/agronomy13123069
Chicago/Turabian StyleWei, Guiyu, Xiangnan Xu, Bingjing Cui, Manyi Zhang, Jie Liu, Zhenhua Wei, and Fulai Liu. 2023. "Effect of Partial Root-Zone Irrigation on Plant Growth, Root Morphological Traits and Leaf Elemental Stoichiometry of Tomato under Elevated CO2" Agronomy 13, no. 12: 3069. https://doi.org/10.3390/agronomy13123069







