Abstract
Allelopathy is a phenomenon that has both beneficial and deleterious influences among plants within the same ecosystem. The allelopathic activity of sunflower on cereals, one of the most popular crops in crop rotation, is still poorly studied and understood. This experiment was aimed at evaluating the allelopathic potential of aqueous extracts of different parts of the sunflower (Helianthus annuus L.) grown in the Boreal environmental zone on seedling morphological parameters of spring barley (Hordeum vulgare L.) and spring wheat (Triticum aestivum L.). The following three factors were studied: factor A—two growth stages: flowering sunflower (FS) and ripe sunflower (RS); factor B—three sunflower plant parts: leaves and stems (L + S), heads (H), and roots (R) for extract preparation; factor C—five concentrations (25%, 50%, 75%, and 100% (initial concentration 1:10, referred to as 100% solution)) of sunflower aqueous extracts and deionised water (0%) as a control. FS extract revealed an inhibitory effect on all parameters of spring barley and spring wheat in comparison to RS extract. Extracts from different plant parts differed in their allelopathic effects. Compared with L + S extract, R extract significantly stimulated SG and the morphological characteristics of wheat. H extract significantly inhibited barley RL and SL. With increasing concentrations of the extracts, the values of all investigated parameters were significantly inhibited for both receptor plants.
1. Introduction
Allelopathy is a phenomenon that has both useful and deleterious influences among plants within the same ecosystem [1]. Allelopathy refers to the influence of a plant on the germination and successive development of a neighbouring plant by emitting allelopathic compounds into the environment [2,3]. The abundance of allelopathic plants and allelopathic compounds in them is an unlimited reservoir of new and, so far, unrevealed mechanisms of action [4]. Plants with allelopathic properties cause allelopathic influence on plants through the evaporation and leaching of allelochemicals from leaves and stems, root exudates, or excretion into the surroundings during the decomposition of plant residues [5,6,7]. The allelochemicals may affect the growth and development of recipient plants, including growth stimulation or inhibition [1,8,9,10]. The mode of activity of allelochemicals depends on their concentration. Low concentrations of them promote germination and growth, and high concentrations inhibit germination of seed and seedling growth [9,11,12]. Cultivation of similar plant species for many years leads to loss of soil nutrients, exacerbation of pests and diseases, deterioration of soil quality, and ecological imbalance, which ultimately has a significant influence on crop yield and production quality [13,14,15]. In an agricultural field where the same crops are often grown in crop sequences, allelopathic competition can negatively affect crop yields [5]. Allelopathic compounds that appear in the soil during the disintegration of above-ground plant parts and roots can influence the germination and development of succeeding crops [16,17], formation of dry mass [18,19] and photosynthesis [4,20,21].
Sunflower is one of the most important allelopathic plants due to the bioactive compounds they secrete, including allelochemicals. Due to the sufficiently high allelopathic character of sunflower, the germination and growth of nearby plant species are inhibited [4,16,22].
The allelopathic attributes of plants can be used for the control of weeds and the suppression of their germination and growth [10,23,24,25,26,27]. However, there is a lack of data on the effects of allelopathic compounds of sunflower, grown in cool climate zones, on protected plants [28].
The allelopathic potential of sunflowers has been studied in their habitual growing regions for some crops: corn [19,29], rice [16], mung bean [30], wheat [19,20,31], lettuce [17], radish [9], sponge gourd (Luffa cylindrica) [32], and mustard [4,33]. The allelopathic effect of sunflowers on peas was studied by Polish researchers [34,35] and Lithuanian researchers [11,12]. However, the allelopathic activity of sunflower for other popular crop rotation crops in this region, such as cereals, is still poorly studied and understood.
Climate change unlocks opportunities to expand the area of sunflower cultivation to regions where sunflowers were not grown before and to the northern part of Europe, where the growing season has lengthened and winters have become milder. Until now, sunflowers were rarely grown in the Boreal environment, but as the climate changes, this all-purpose crop can make a great addition to crop rotations. This region is not the usual climate zone for sunflowers and heat-loving plants. Sunflowers growing under unusual, cool climate conditions may accumulate different amounts of allelopathic substances than those growing in warm climate zones. However, there is a lack of knowledge about the action of sunflowers grown in crop rotation in the Boreal environmental zone and their influence on soil properties, productivity dynamics, and chemical composition. We also know little about the concentration of allelochemical compounds accumulated in sunflowers grown in the mentioned climate zone and their influence on plant development in the crop sequence.
This study aimed to evaluate the allelopathic effect of aqueous extracts of different parts of sunflowers on seed germination and morphological parameters of juvenile seedlings of spring barley and spring wheat in the Boreal region.
2. Materials and Methods
2.1. Collection of Plant Materials
The investigation was carried out at the Institute of Agriculture, Lithuanian Research Centre for Agriculture and Forestry. The above-ground part of the plant and the roots of the donor plant (Helianthus annus L., cultivar ‘Peredovick’) were collected in the flowering stage and the full maturity stage from the experimental field of the Institute of Agriculture. The grains of target cereals as receptor plants—spring barley (Hordeum vulgare L., cultivar ‘Freedway’) and spring wheat (Triticum aestivum L., cultivar ‘Colada’)—were derived by the Department of Cereal Breeding, Institute of Agriculture, Lithuania.
2.2. Details of the Laboratory Experiment
Three factors have been studied: factor A—donor plant growth stage (GS): flowering sunflower (FS) and ripe sunflower (RS); factor B—aqueous extract from different parts of donor plants: leaves and stems (L + S), heads (H), and roots (R); and factor C—concentration: 0%, 25%, 50%, 75%, and 100% w/v.
Sunflowers were divided into parts (roots, stems and leaves, and heads). All these parts were chopped into pieces (10 mm) and air-dried. Aqueous sunflower extracts were prepared by adding 100 g of different air-dried sunflower parts to 1.0 L of deionised water and soaking for 24 h at room temperature (25 ± 2 °C). The extract was filtered using filter paper. The resulting solution is considered a standard 100% solution. Extracts were then diluted with deionised water to concentrations of 25%, 50%, 75% and 100% (by volume) and stored in a refrigerator at 4 °C until use. These extracts were used for bioassays. The deionised water was used as a no-extract control.
The grains of the target plants were washed with deionised water. Grain germination was determined by the Cigar Roll method as described by Watt et al. [36].
2.3. Germination and Biometric Parameters
To ascertain the allelopathic impact of sunflower part extracts on the grain germination of spring barley and spring wheat, the following indicators were determined: seed germination (SG) and germination index (GI). To evaluate the effect of extracts on seedlings’ development of target plants, we established root length (RL), shoot length (SL), fresh mass of roots (RFM), and fresh mass of shoots (SFM) of target plants in each roll. Five days after sowing (DAS), grain germination (SG) was recorded by counting the number of germinated grains in each roll. Grain with a 1 mm radicle was kept as germinated. Germination index (GI) was calculated [37]:
where Gt is the number of grains emerging on a given day and Dt is the time after setting the grains for germination.
At 8 DAS and 14 DAS, the root length (RL) and shoot length (SL) were measured by using a ruler. The fresh mass of seedling roots (RFM) and fresh mass of shoots (SFM) were estimated using an electronic balance with an accuracy of 0.001 g. RFM and SFM were estimated at 8 DAS and 14 DAS.
2.4. Biologically Active Compounds
The total polyphenolic compounds (TPC) content and total flavonoids (TF) content were determined according to spectrophotometric methods [38].
2.5. Allelopathic Potential Determination
The inhibitory rate (IR) was calculated using the following formulas:
where T is the treatment value and C is the control value [39]. IR > 0 represents a stimulatory effect, and IR < 0 represents an inhibitory effect.
IR = 1 − C/T, if T ≥ C
IR = T/C − 1, if T ˂ C
The synthetical allelopathic effect index (SE) was calculated as the arithmetic mean value of IR [39]. Here SE = (IRSG + IRRL + IRSL + IRRFM + IRSFM)/5, where SG is the seed germination; RL and SL are the root and shoot lengths, respectively; and RFM and SFM are the root and shoot fresh mass, respectively.
2.6. Statistical Analysis
A three-way ANOVA model was used to determine the effects of two growth stages of sunflower (factor A), three plant parts (factor B), and five concentrations of extracts (factor C) on the SG, RL, SL, RFM, and SFM of spring barley and spring wheat. Statistical significance was evaluated using the Fisher’s least significant difference (LSD) test at the probability levels of p ≤ 0.05 and p ≤ 0.01. The statistical analysis was done using the software ANOVA from the statistical software package Selekcija, version 4.0 [40].
3. Results
3.1. Effect of Sunflower Extracts on Germination of Spring Barley and Spring Wheat at 5 DAS
3.1.1. Spring Barley
A three-way ANOVA showed that seed germination (SG) and germination index (GI) of spring barley were significantly (p ≤ 0.01) influenced by sunflower growth stage (GS) (factor A), sunflower aqueous extract concentration (factor C), and their interaction (A × C) (Table 1). The extract concentration was the main factor responsible for 64.3% of the total variability of SG in spring barley. The influence of factor A and interaction A × C was lower, only 4.6% and 5.3%, respectively, but also significant (p ≤ 0.01).

Table 1.
Effects of donor plant development, plant parts, and aqueous extract concentration of sunflower plants on seed germination (SG) and germination index (GI) at 5 DAS of spring barley and spring wheat.
The data averaged across plant parts and extract concentrations showed that extract from flowering sunflower (FS) inhibited SG in spring barley by 34% compared with RS extract. The data averaged across donor plant GS and extract concentrations demonstrated that the highest SG of spring barley was for plants treated with R extract (+16%, compared with L + S); however, the difference was insignificant. The data averaged across donor plant GS and plant parts showed that the SG of spring barley significantly and progressively decreased as the concentration was increased. At 25%, 50%, 75%, and 100% concentrations, SG was reduced by 63%, 80%, 65%, and 92%, respectively, compared with the control (0%).
3.1.2. Spring Wheat
SG and GI of spring wheat were significantly (p < 0.01) influenced by all factors and their interactions (Table 1). Extract concentration was the main factor (factor C) that explained 35.7% of the total variability of SG. Donor plant GS (factor A) and plant parts (factor B) governed a similar percentage of variation—14.3 and 14.5%, respectively.
The data averaged across plant parts and extract concentrations showed that FS extract suppressed SG in the spring wheat by 34%. The data averaged across donor plant GS and extract concentrations revealed that the response of SG in spring wheat as affected by extracts from different parts of sunflower was varied. The lowest SG was for plants treated with the L + S extract. In comparison with L + S extract, H and R extracts had a stimulatory effect on the SG, significantly increasing the index by 17% and 60%, respectively. The data averaged across donor plant GS and plant parts showed a significant (p ≤ 0.05) decrease in spring wheat SG with increasing extract concentration: 0% < 25% < 50% < 75% < 100%. In comparison with the control treatment (0%), SG was suppressed by 22%, 38%, 48%, and 59% under 25%, 50%, 75%, and 100% extract concentrations, respectively.
By comparing both receptor plants, it was found that the response of barley to the inhibitory effect of sunflower extracts was more pronounced than that of wheat. The seed germination of spring barley was on average 33–80% lower than that of spring wheat.
3.2. Effect of Sunflower Extracts on Morphological Parameters of Spring Barley and Spring Wheat at 8 DAS and 14 DAS
3.2.1. Root Length (RL) and Shoot Length (SL) of Spring Barley
A three-way ANOVA of the 8 DAS and 14 DAS data showed that donor plant GS (factor A), plant parts (factor B), sunflower aqueous extract concentration (factor C), and interactions A × C and B × C had a significant (p < 0.01) effect on root length (RL) of spring barley (Table 2). The extract concentration (factor C) was the main factor responsible for 89.4% and 47.6% of the total variability of RL at 8 DAS and 14 DAS, respectively.

Table 2.
Effects of donor plant development, plant parts, and aqueous extract concentration of sunflower plants on root length (RL, cm) at 8 DAS and 14 DAS of spring barley and spring wheat.
The data averaged across plant parts and extract concentrations showed that FS sunflower extract significantly (p < 0.05) inhibited RL of sunflower by 23% and 33% at 8 DAS and 14 DAS, respectively, in comparison with RS extract.
The data averaged across donor plant GS and extract concentrations revealed that, compared with L + S extract, H extract significantly (p ≤ 0.05) inhibited RL at 8 DAS and tended to decrease RL at 14 DAS. R extract had a stimulatory effect on RL at 14 DAS (by 71%).
The data averaged across donor plant GS and plant parts showed that RL of spring barley was highest in the control treatment (0%), and compared to the 0%, all concentrations revealed a significant (p ≤ 0.05) inhibitory effect on RL. In comparison with the control treatment (0%), RL was inhibited by 82%, 87%, 88%, and 97% at 8 DAS and by 30%, 70%, 86%, and 98% at 14 DAS, respectively, under 25%, 50%, 75%, and 100% extract concentration.
Donor plant GS (factor A), sunflower extract concentration (factor C), and interactions A × C and B × C had a significant (p < 0.01) effect on shoot length (SL) of spring barley at 8 DAS and 14 DAS (Table 3). The plant parts (factor C) had a significant (p < 0.01) effect on SL only at 14 DAS. The extract concentration was the main factor responsible for 87.7 and 72.0% of the total variability of SL at 8 DAS and 14 DAS, respectively.

Table 3.
Effects of donor plant development, plant part, and aqueous extract concentration of sunflower plant shoot length (SL, cm) at 8 DAS and 14 DAS of spring barley and spring wheat.
The data averaged across plant parts and extract concentrations demonstrated that FS sunflower extract had a stronger inhibitory effect than RS extract, and compared with RS extract, it significantly (p < 0.05) inhibited SL of sunflower by 21% and 31% at 8 DAS and 14 DAS, respectively.
The data averaged across donor plant GS and extract concentrations revealed that, compared with L + S extract, H extract significantly (p ≤ 0.05) suppressed the SL of spring barley at 8 DAS and 14 DAS. R extract had an inhibitory effect on SL at 8 DAS (by 7%) and a stimulatory effect at 14 DAS (by 15%), compared with L + S extract.
The data averaged across donor plant GS and plant parts showed that, compared to 0%, the inhibitory effect on SL significantly increased with the application of an increasing extract concentration from 25% to 100% by 86–96% and 62–95%, respectively, at 8 DAS and 14 DAS.
3.2.2. Root Length (RL) and Shoot Length (SL) of Spring Wheat
A three-way ANOVA showed that all factors and their interactions had a significant (p < 0.05 and p < 0.01) effect on root length (RL) of spring wheat at 8 DAS and 14 DAS (Table 2). The extract concentration (factor C) was responsible for the largest part of the total variability of spring wheat RL: 83.1% and 56.8% at 8 DAS and 14 DAS, respectively.
The data averaged across plant parts and extract concentrations showed that, compared with RS extract, FS extract inhibited RL of spring wheat by 37% and 48%, respectively, at 8 DAS and 14 DAS.
The data averaged across donor plant GS and extract concentrations revealed that the lowest RL of spring wheat was for plants treated with L + S extracts. H and R extracts, compared with L + S extracts, in most cases significantly (p ≤ 0.05) stimulated RL by 10% and 50% at 8 DAS and by 7% and 81% at 14 DAS, respectively.
The data averaged across donor plant GS and plant parts demonstrated that the RL of spring wheat progressively and significantly decreased as the concentration of extracts was increased. At 25%, 50%, 75%, and 100% concentrations, RL was reduced by 71%, 85%, 93%, and 96%, respectively, at 8 DAS and by 24%, 69%, 83%, and 96%, respectively, at 14 DAS, compared to the control (0%).
A three-way ANOVA showed that all factors and their interactions had a significant (p < 0.01) effect on the SL of spring wheat at 8 DAS and 14 DAS (Table 3). The extract concentration (factor C) was the main factor that explained 73.5% and 52.1% of the total variability of SL at 8 DAS and 14 DAS, respectively.
The data averaged across plant parts and extract concentrations showed that FS extract suppressed the SL of spring wheat by 42% and 56% at 8 DAS and 14 DAS, respectively, in comparison with RS extract.
The data averaged across donor plant GS and extract concentration demonstrated that H and R extracts had a stimulating effect on the SL of spring wheat. Compared with L + S, H extract significantly (p ≤ 0.05) increased SL by 10% at 14 DAS, whereas R extract stimulated SL by 44% at 8 DAS and by 85% at 14 DAS.
The data averaged across donor plant GS and plant parts revealed that the RL of spring wheat significantly (p ≤ 0.05) decreased with increasing concentrations of extracts. At 25%, 50%, 75%, and 100% concentrations, RL was reduced by 69%, 86%, 91%, and 98%, respectively, at 8 DAS and by 37%, 69%, 80%, and 97%, respectively, at 14 DAS, compared with 0%.
Spring barley was more sensitive to the applied extracts in terms of RL and SL. Comparing the responses of both receptor plants to the applied extracts, it was found that the RL of barley was on average 26–71% and 42–65% lower than that of spring wheat, at 8 DAS and 14 DAS, respectively. The SL of spring barley, compared with spring wheat, was on average 2–44% and 13–45% lower at 8 DAS and 14 DAS, respectively, in 50% of cases.
3.2.3. Root Fresh Mass (RFM) and Shoot Fresh Mass (SFM) of Spring Barley and Spring Wheat
In comparison to the control treatment (0%), all extracts had an inhibitory effect on the RFM and SFM of both plants: both indices of spring barley were suppressed by 7–74% and 47–98% and of spring wheat by 8–97% and 37–99%, respectively (Figure 1).
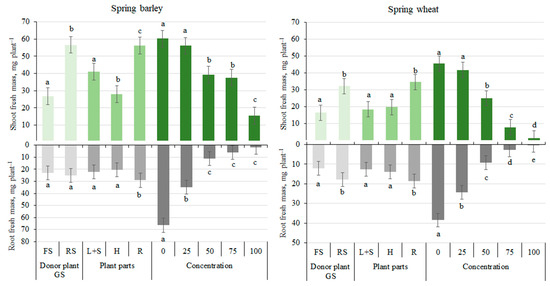
Figure 1.
Effects of donor plant growth stage (GS), plant parts, and aqueous extract concentrations of sunflower plants on the fresh mass of the shoot and root of the target plant species of spring barley and spring wheat. Different letters denote a statistically significant difference (p ≤ 0.05 according to LSD) among treatments. The error bars show SE. FS—flowering sunflowers; RS—ripe sunflowers; L + S—leaves and steams; H—heads; R—roots.
The data revealed that FS significantly (p ≤ 0.05) inhibited RFM and SFM of both target plants (Figure 1). In comparison to the RS treatment, RFM and SFM of spring barley decreased by 53% and 7%, respectively, whereas indices of spring wheat were suppressed by 49% and 32%, respectively.
The significant (p ≤ 0.05) stimulating effect of R extract on RFM and SFM was found for spring barley (+37% and +32%, respectively) and spring wheat (+88% and +48%, respectively), compared to L + S extract. Meanwhile, H extract had an inhibiting effect on both indices, but a significant difference was only observed for spring barley SFM (−32%).
All extracts and concentrations of extracts showed an inhibitory effect on the root/shoot fresh mass ratio (Figure 2).
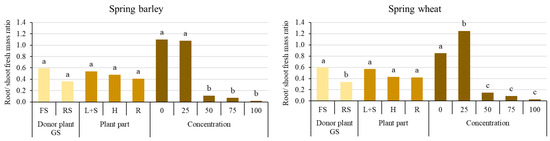
Figure 2.
Effects of donor plant growth stage (GS), plant parts, and aqueous extract concentrations on root/shoot fresh weight ratios of spring barley and spring wheat. Different letters denote a statistically significant difference (p ≤ 0.05 according to LSD) among treatments. The error bars show SE. FS—flowering sunflowers; RS—ripe sunflowers; L + S—leaves and steams; H—heads; R—roots.
3.3. Allelopathic Effects of Sunflower Aqueous Extract on Spring Barley and Spring Wheat
The inhibitory rate (IR) demonstrated the allelopathic effect of tested factors on seed germination and morphological parameters (Table 4). It was found that different concentrations of extract had an inhibiting effect on the parameters of spring barley in 94% of the cases and spring wheat in 95% of the cases.

Table 4.
The inhibitory rate (IR) for seed germination and morphological parameters of spring barley and spring wheat at 14 DAS.
Figure 3 shows the relationship between the synthetical effect (SE) for seed germination and all tested morphological parameters of receptor plants and concentrations of sunflower aqueous extract. The correlation coefficient of spring barley was 0.4418–0.9823 and of spring wheat was 0.8399–0.9931. According to the SE values, the allelopathic effect of aqueous extracts of sunflower parts on spring barley and spring wheat was as follows: H < L + S < R and L + S < H < R, respectively.
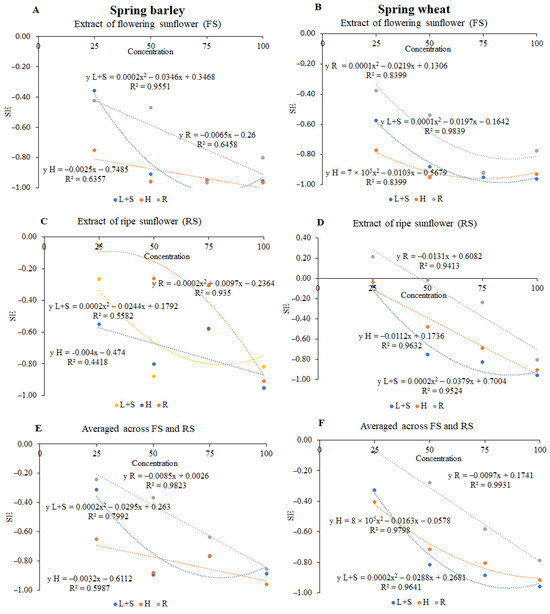
Figure 3.
The influences of the aqueous extracts of flowering sunflower (A,B), ripe sunflower (C,D), and averaged across extracts of flowering and ripe sunflower (E,F) on the synthetical effect (SE) for seed germination and all tested morphological parameters of spring barley and spring wheat under extract concentrations of 25%, 50%, 75%, and 100%. L + S—leaves and steams; H—heads; R—roots.
3.4. The Amount of Biologically Active Compounds
The highest amount of biologically active substances was determined in the H of FS: TPC was 32.5 mg g−1 RUE and TF was 39.3 mg g−1 RUE (Figure 4). In RS, most of these substances were found in the R: TPC was 17.2 mg g−1 RUE and TF was 19.0 mg g−1 RUE. About the plant parts, in FS material, the trend of H < L + S < R was found for the content of both TPC and TF, and in RS material, the trend was R < L + S < H.
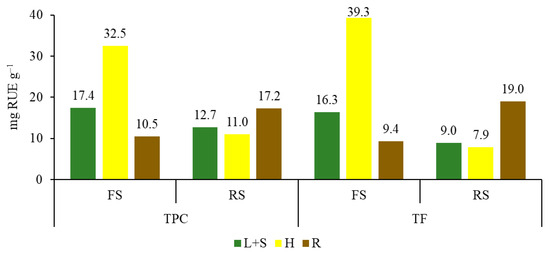
Figure 4.
Total polyphenolic compounds (TPC) content and total flavonoids (TF) content in leaves and stems (L + S), heads (H), and roots (R) of sunflower extracts. FS—flowering sunflowers; RS—ripe sunflowers.
4. Discussion
Substances released by plants with allelopathic properties can suppress the germination and development of nearby or succeeding plants [7,8]. Sunflowers are one of the most widely known allelopathic plants, so new data on the behaviour of these plants among other plants are sought [3,4,9,22,27,28]. Sunflowers accumulate water-soluble allelochemicals in their above-ground organs and roots [9,11,19]. Allelochemicals are mainly secondary metabolites that can have an inhibitory effect or stimulate the germination and growth of other plants [2,16,35]. The results of the present study revealed that sunflower water extracts possess significant allelopathic potential for spring barley and spring wheat germination and juvenile seedling development. By comparing both tested plants, it was found that barley was more sensitive to the allelopathic effect of sunflower extracts than spring wheat in terms of SG. Researchers who studied the influence of sunflower extracts on wheat and maize also found different sensitivities of these plants: the germination inhibition of wheat was twice as high as that of maize [19]. We found that, compared to RS extract, FS extract had an inhibitory effect on both tested plants, reducing SG by more than 30%, thereby confirming similar findings in previous studies. Buckwheat extracts collected in the flowering stage inhibited weed germination and growth to a greater extent compared to buckwheat collected in the ripening stage [41]. The phytotoxicity of some Brassica species extracts at the full flowering stage was higher than at the ripe stage [42].
Different allelopathic potentials of different parts of sunflower (root, shoot, and leaf) on seedlings of tested plants are associated with the concentration of allelopathic compounds in different organs of plants [4,16,28]. We found that the most allelopathic compounds were in the heads of flowering sunflowers (Figure 4). On the contrary, other researchers found that, in the study of sunflower allelopathy in weeds, the shoot of the sunflower had higher phytotoxic activity than the root [43]. In the experiment, it was found that the significant influence on SG of different parts of the donor plant was only for spring wheat, while no significant differences were found in spring barley. These results agreed with those of previous studies that found that different parts of sunflowers affected the tested plant differently [20]. Our findings revealed that the strength of the allelopathic effect of the three plant parts on the SG of two tested plants was as follows: L + S < H < R. There are partially conflicting data that indicate that the allelopathic effects of sunflower extracts vary in the order: root < stem < leaf [9]. In a study that investigated the allelopathy of sunflower root, stem, and leaf extracts for wheat and corn, it was also found that leaves have more allelochemicals than other parts, thus making them more phytotoxic [19].
The results of this experiment indicated that the concentration of extract was the most important factor governing the total variability of the SG, RL, SL, SFM, and RFM data. The values of these parameters for spring barley and spring wheat significantly decreased with increasing extract concentration: 0% < 25% < 50% < 75% < 100%. Our findings agree with those of previous studies [9,18,31,33], which reported the inhibitory effects of sunflower extracts on the germination of mustard, wild barley, wheat, and radish, and the magnitude of inhibition depended on the extract concentration. There are conflicting data demonstrating that donor plant extracts significantly acted on shoot and root growth, wherein the higher concentration revealed the strongest inhibitory effect and the lower concentration had stimulatory effects on some occasions [44].
The diversity of allelopathic materials and their concentration and allelopathic activity vary with the growth stage of the donor plant [45,46,47]. The results of the present investigation showed that the FS extract had a higher inhibitory effect on the RL and SL of spring barley and spring wheat than the RS extract. Our experimental data are in agreement with a previous study [47], where differences in the allelopathic effect were found among donor plant growth stages. This was also confirmed in the study with lettuce [17], where the shoot length of seedlings was more inhibited with sunflower leaves collected in the flowering stage than in the butonisation stage.
The influence of the sunflower aqueous extract, which has been reported to ameliorate crop productivity [23,48], could be due to the stimulation of various physiological processes in plants. In comparison with the control, sunflower extracts had a significant and negative influence on the dry mass of wheat, maize, and weed [19,49,50]. The results of the current experiment showed that SFM and RFM were significantly increased when R extract was applied to both barley and wheat. Regarding RFM and SFM data, depending on the extracts used, the trend observed was H < L + S < R for barley and L + S < H < R for wheat. Our findings are in line with previous results, conducted in habitual sunflower cultivation zones, indicating that the L extract had a stronger stimulating effect on the fresh mass of wheat in comparison with the R extract [20].
5. Conclusions
It was found that the highest and most significant inhibitory effect on SG and all morphological parameters of seedlings was revealed by FS extract in both spring barley and spring wheat, compared with RS extract.
In most cases, the H and R extracts were found to have a significant stimulatory effect on the SG, RL, SL, RFM, and SFM of spring wheat in comparison with the L + S extracts. The H and R extracts revealed a stimulating effect on barley parameters only in sparse cases, and it was not always significant. H extract significantly inhibited barley RL and SL, while R extract, 14 DAS, showed a stimulating effect on these parameters. Extract concentration was the main factor that explained the largest part of the total variability of all tested parameters. In both receptor plants, the values of all investigated parameters were significantly inhibited with increasing concentrations of the extracts.
According to the SE values, the allelopathic effect of aqueous extracts of sunflower parts on spring barley and spring wheat was as follows: H < L + S < R and L + S < H < R, respectively. According to the values of all parameters, the inhibitory effect of sunflower extracts was more pronounced on spring barley than on spring wheat.
A comprehensive investigation of the allelopathic effect of sunflowers grown in the Boreal environmental zone can potentially increase their proper use and improve crop sequence productivity in both conventional and sustainable agricultural practices.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The author declares no conflict of interest.
References
- Tanase, C.; Bujor, O.C.; Popa, V.I. Phenolic natural compounds and their influence on physiological processes in plants. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 45–58. [Google Scholar]
- Amena, D.; Azra, B.H.; Nazneen, S. Evaluation of allelopathic effect of aqueous leaf extract of parthenium (Pathenium hysterophorus L.) on seed germination and seedling growth in sunflower (Helianthus annus L.), soybean (Glycine max L.) and green gram (Phaseolus mungo L.). Int. J. Appl. Sci. Eng. Tech. 2019, 7, 429–438. [Google Scholar] [CrossRef]
- Sahoo, T.R.; Behera, B.; Paikaray, R.K.; Garnayak, L.M.; Sethi, D.; Jena, S.; Raza, B.; Panda, R.K.; Song, B.; Lal, M.K.; et al. Effects of sunflower residue management options on productivity and profitability of succeeding rice under different crop establishment methods. Field Crops Res. 2023, 290, 108763. [Google Scholar] [CrossRef]
- Pula, J.; Zandi, P.; Stachurska-Swakori, A.; Barabasz-Krasny, B.; Mozdžen, K.; Wang, Y. Influence of alcoholic extracts from Helianthus annnus L. roots on the photosynthetic activity of Sinapis alba L. cv. Barka plants. Acta Agr. Scand. B Soil Plant Sci. 2020, 70, 8–13. [Google Scholar]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Sicurezza, G.M.; Caporaso, S.; Esposito, A.; Mazzoleni, S. Phytotoxicity dynamics of decaying plant materials. New Phytol. 2006, 169, 571–578. [Google Scholar] [CrossRef]
- Iqbal, A.; Hamayun, M.; Shah, F.; Hussain, A. Role of plant bioactives in sustainable agriculture. In Environment, Climate, Plant and Vegetation Growth, 1st ed.; Fahad, S., Hasanuzzaman, M., Alam, M., Ullah, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 591–605. [Google Scholar]
- Gniazdowska, A.; Bogatek, R. Allelopathic interactions between plants. Multi-site action of allelochemicals. Acta Physiol. Plant. 2005, 27, 395–407. [Google Scholar] [CrossRef]
- Rigon, C.A.G.; Salamoni, A.T.; Aguiar, A.C.M.; Cutti, L. Allelopathic effect of aqueous extracts of different organs of three sunflower cultivars on germination of radish. Biosci. J. 2018, 34, 577–586. [Google Scholar] [CrossRef]
- Okrushko, S.E. Allelopathic effect of couch grass (Elymus repens L.) on germination of common wheat seeds. Zemdirb. Agric. 2022, 109, 323–328. [Google Scholar] [CrossRef]
- Janušauskaitė, D. Allelopathic effect of aqueous extracts of common sunflower on seed germination and growth of field pea. Zemdirb. Agric. 2023, 110, 17–26. [Google Scholar] [CrossRef]
- Janusauskaite, D. The allelopathic activity of aqueous extracts of Helianthus annuus L., grown in boreal conditions, on germination, development, and physiological indices of Pisum sativum L. Plants 2023, 12, 1920. [Google Scholar] [CrossRef]
- Sainju, U.M.; Lenssen, A.W.; Barsotti, J.L. Dryland malt barley yield and quality affected by tillage, cropping sequence, and nitrogen fertilization. Agron. J. 2013, 105, 329–340. [Google Scholar] [CrossRef]
- Arlauskiene, A.; Ceseviciene, J.; Slepetiene, A. Effect of catch crop, straw management and fertilisation on the productivity of field pea and winter wheat crop sequence. Zemdirb. Agric. 2020, 107, 217–226. [Google Scholar] [CrossRef]
- Bogužas, V.; Skinulienė, L.; Butkevičienė, L.M.; Steponavičienė, V.; Petrauskas, E.; Maršalkienė, N. The Effect of Monoculture, Crop Rotation Combinations, and Continuous Bare Fallow on Soil CO2 Emissions, Earthworms, and Productivity of Winter Rye after a 50-Year Period. Plants 2022, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Bashir, U.; Javaid, A.; Bajwa, R. Effects of aquous extracts of sunflower (Helianthus annuus L.) on germination of seedling growth on the selected wheat (Triticum aestivum L.) varieties. Bangladesh J. Bot. 2017, 46, 1323–1332. [Google Scholar]
- Ravlić, M.; Markulj Kulundžić, A.; Baličević, R.; Marković, M.; Viljevac Vuletić, M.; Kranjac, D.; Sarajlić, A. Allelopathic potential of sunflower genotypes at different growth stages on lettuce. Appl. Sci. 2022, 12, 12568. [Google Scholar] [CrossRef]
- Ashrafi, Y.Z.; Sagehgi, S.; Mashhadi, R.H.; Hassan, A.M. Allelopathic effects of sunflower (Helianthus annuus) on germination and growth of wild barley (Hordeum spontaneum). J. Agric. Tech. 2008, 4, 219–229. [Google Scholar]
- Muhammad, Z.; Majeed, A. Allelopathic effects of aqueous extracts of sunflower on wheat (Triticum aestivum L.) and maize (Zea mays L.). Pak. J. Bot. 2014, 46, 1715–1718. [Google Scholar]
- Kamal, J.; Bano, A. Effects of sunflower (Helianthus annuus L.) extracts on wheat (Triticum aestivum L.) and physicochemical characteristics of soil. Afr. J. Biotechnol. 2008, 7, 4130–4135. [Google Scholar]
- Dadkhah, A. Phytotoxic effects of aqueous extract of eucalyptus, sunflower and sugar beet on seed germination, growth and photosynthesis of Amaranthus retroflexus. Allelopathy J. 2012, 29, 287–296. [Google Scholar]
- Flayyih, T.M.; Almarie, A.A. Allelopathic effect of sunflower residues on some soil properties and growth parameters of wheat, bean and flax crops. Rev. Bionat. 2022, 7, 38. [Google Scholar] [CrossRef]
- Alsaadawi, I.S.; Sarbout, A.K.; Al-Shamma, L.M. Differential allelopathic potential of sunflower (Helianthus annuus L.) genotypes on weeds and wheat (Triticum aestivum L.) crop. Arch. Agron. Soil Sci. 2012, 58, 1139–1148. [Google Scholar] [CrossRef]
- Batool, A.; Riaz, M.A.; Hassan, F.; Irum, S.; Iqbal, S.; Anwar, F.; Saadia, M. Screening of active formulation from combined sunflower (Helianthus annuus) and neem (Azadirachta indica) aqueous extract to control growth of lesser canary grass (Phalaris minor). Pakistan J. Bot. 2019, 51, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Marinov-Serafimov, P.; Enchev, S.; Golubinova, I. Allelopathic soil activity in the rotation of some forage and technical crops. Bulgarian J. Agric. Sci. 2019, 25, 980–985. [Google Scholar]
- AL-Behadili, A.A.; Fadhel, L.Z. Integration of Sunflower and Sorghum Water Extracts Applied Alone or in Combination with Reduced Doses of Chevalier for Weed Control in Wheat. Iraqi J. Sci. 2023, 64, 3330–3339. [Google Scholar]
- Flayyih, T.M.; Almarie, A.A. Total Phenolic Exudation and Allelopathic Potential of Sunflower Residues as Sustainable Weed Management. Iraqi J. Sci. 2023, 64, 2215–2222. [Google Scholar] [CrossRef]
- Rys, M.; Saja-Garbarz, D.; Skoczowski, A. Phytotoxic effects of selected herbal extracts on the germination, growth and metabolism of mustard and oilseed rape. Agronomy 2022, 12, 110. [Google Scholar] [CrossRef]
- Nafees, A.; Abbas, A.; Iram-us-Salam; Hussain, F. Bioassay test of allelopathic potential of sunflower (Helianthus annuus L.) against mung bean (Vigna radiata (L.) R. Wilczek). Ghazi Univ. J. Phytosci. 2021, 1, 70–79. [Google Scholar]
- Vishwajith; Halagalimath, S.P.; Ganajaxi, M. Allelopathic effects of sunflower on succeeding mungbean (Vigna radiata L. Wilczek) crop. Allelopath. J. 2017, 42, 37–48. [Google Scholar] [CrossRef]
- Kamal, J. Impact of allelopathy of sunflower (Helianthus annuus L.) roots extract on physiology of wheat (Triticum aestivum L.). Afr. J. Biotechnol. 2011, 10, 14465–14477. [Google Scholar]
- Nafees, A.; Abbas, A.; Iram-us-Salam; Hussain, F. Allelopathic effects of sunflower (Helianthus annuus L.) against Luffa cylindrica (L.) Roem. Ghazi Univ. J. Phytosci. 2021, 1, 124–132. [Google Scholar]
- Kupidłowska, E.; Gniazdowska, A.; Stepien, J.; Corbineau, F.; Vinel, D.; Skoczowski, A.; Janeczko, A.; Bogatek, R. Impact of sunflower (Helianthus annus L.) extracts upon reserve mobilization and energy metabolism in germinating mustard (Sinapis alba L.) seeds. J. Chem. Ecol. 2006, 32, 2569–2583. [Google Scholar] [CrossRef]
- Gawronska, H.; Ciarka, D.; Bernat, W.; Gawronski, S.W. Sunflower-desired allelopathic crop for sustainable and organic agriculture? In Allelopathy: New Concepts and Methodology, 1st ed.; Fujii, Y., Hiradate, S., Eds.; Science Publishers: Enfield, CT, USA, 2007; pp. 185–210. [Google Scholar]
- Oliwa, J.; Możdżeń, K.; Rut, G.; Rzepka, A. The influence of alcoholic extract from leaves of Helianthus annuus L. on germination and growth of Sinapis alba L. Mod. Phytomorphology 2017, 11, 91–97. [Google Scholar]
- Watt, M.; Moosavi, S.; Cunningham, S.C.; Kirkegaard, J.A.; Rebetzke, G.J.; Richards, R.A. A rapid, controlled-environment seedling roots creen for wheat correlates well with rooting depths at vegetative, but not reproductive, stages at two field sites. Ann. Bot. 2013, 112, 447–455. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Zhang, R.; Huang, Y.; Feng, S.; Ma, X.; Zhang, Y.; Sikdar, A.; Roy, R. Allelopathic effects of aqueous leaf extracts from four shrub species on seed germination and initial growth of Amygdalus pedunculata Pall. Forests 2018, 9, 711. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Scavo, A.; Pandino, G.; Restuccia, A.; Caruso, P.; Lombardo, S.; Mauromicale, G. Allelopathy in durum wheat landraces as affected by genotype and plant part. Plants 2022, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Raudonius, S. Application of statistics in plant and crop research: Important issues. Zemdirb. Agric. 2017, 104, 377–382. [Google Scholar] [CrossRef]
- Ðikič, M.; Gadžo, D.; Šarič, T.; Gavrič, T.; Muminovič, Š. Investigation of allelopathic potential of buckwheat. Herbologia 2008, 9, 59–71. [Google Scholar]
- Jafariehyazdi, E.; Javidfar, F. Comparison of allelopathic effects of some brassica species in two growth stages on germination and growth of sunflower. Plant Soil Environ. 2011, 57, 52–56. [Google Scholar] [CrossRef]
- Kandhro, M.N.; Tunio, S.D.; Rajpar, I.; Chachar, Q.D.; Gandahi, A.W. Allelopathic impact of sorghum and sunflower on germination and seedling growth of summer broadleaf weeds. Pak. J. Agric. Agril. Eng. Vet. Sci. 2015, 31, 229–239. [Google Scholar]
- Sarma, D.; Basumatary, P.; Datta, B.K. Allelopathic impact of Melastoma malabathricum L. on the seed germination and seedling growth of three agricultural crops. J. Indian Bot. Soc. 2019, 98, 183–193. [Google Scholar] [CrossRef]
- Patra, B.; Schluttenhofer, C.; Wu, Y.; Pattanaik, S.; Yuan, L. Transcriptional regulation of secondary metabolite biosynthesis in plants. Biochim. Biophys. Acta Gene Regul. Mech. 2013, 1829, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, M.V.; de Farias, S.G.G.; de Castro, D.P.; Silva, R.B.; de Oliveira Silva, D.Y.B.O.; Souto Dias, B.A.S.; da Silva, A.F.; dos Santos, G.N.L.; de Matos, D.C.P.; de Almada Oliveira, C.V.A. Allelopathy of the Leaf Extract of Eucalyptus Genetic Material on the Physiological Performance of Millet Seeds. Am. J. Plant Sci. 2018, 9, 34–45. [Google Scholar] [CrossRef][Green Version]
- Pannacci, E.; Masi, M.; Farneselli, M.; Tei, F. Evaluation of Mugwort (Artemisia vulgaris L.) Aqueous Extract as a Potential Bioherbicide to Control Amaranthus retroflexus L. in Maize. Agriculture 2020, 10, 642. [Google Scholar] [CrossRef]
- Farooq, M.; Nadeem, F.; Arfat, M.Y.; Nabeel, M.; Musadaq, S.; Cheema, S.A.; Nawaz, A. Exogenous application of allelopathic water extracts helps improving tolerance against terminal heat and drought stresses in bread wheat (Triticum aestivum L. Em. Thell.). J. Agron. Crop Sci. 2018, 204, 298–312. [Google Scholar] [CrossRef]
- Javaid, A.; Shafique, S.; Bajwa, R.; Shafique, S. Effect of aqueous extracts of allelopathic crops on germination and growth of Parthenium hysterophorus L. S. Afr. J. Bot. 2006, 72, 609–612. [Google Scholar] [CrossRef][Green Version]
- Naeem, M.; Cheema, Z.A.; Ihsan, M.Z.; Hussain, Y.; Mazari, A.; Abbas, H.T. Allelopathic effects of different plant water extracts on yield and weeds of wheat. Planta Daninha 2018, 36, e018177840. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).