1. Introduction
In Central Europe’s plant life, certain species of the
Reynoutria Houtt. genus (also known as
Polygonum Sieb. and Zucc. or
Reynoutria Houtt.) have permanently established themselves in natural environments [
1]. In Poland, three types of knotweed exist: Japanese knotweed (
Reynoutria japonica (Houtt.) Ronse Decr. or
Reynoutria japonica Houtt.), Sakhalin knotweed (
Reynoutria sachalinensis F. Schmidt), and a hybrid of the two, Czech knotweed (
Reynoutria × bohemica) [
2,
3]. All three species are categorized as invasive alien species (IAS) and pose an extreme threat to the native ecosystem [
4]. Their introduction into the environment or movement in natural habitats is prohibited by applicable legal acts [
5,
6]. Since 2012, their import, possession, breeding, propagation, and sale have also required a special permit from the General Director for Environmental Protection. Since 2021, Poland has had a legal order to control invasive alien species [
5]. In addition, legal provisions oblige monitoring of the effectiveness of actions taken to eliminate them from the environment.
In Poland, the most common type of knotweed is the Japanese knotweed, as reported by Tokarska-Guzik et al. [
4]. It is also considered one of the top 100 invasive species by the Global Invasive Species Database (compiled in 2010) and one of the 18 most invasive species worldwide according to the Delivering Alien Species Inventories for Europe [
7]. The sites with the highest density of this plant are currently located in the southern region of Poland. The dispersion of rhizomes with water (especially during river floods) contributes to the spread of plants along river valleys. Rhizome fragments can often spread due to unintentional human actions [
8]. The invasion is mainly caused by fast-growing rhizomes and shoots that can regenerate quickly. Even a small piece of a rhizome weighing only 0.7 g and measuring 1 cm long or a section of a shoot with just one node can give rise to a new plant when placed in soil or water [
9,
10]. As a perennial plant, Japanese knotweed especially colonizes riverine habitats (e.g., riparian forests) [
11]. It hinders the growth and development of plants such as
Phalaris arundinacea,
Phragmites australis, species of the genus
Petasites, and
Alnus glutinosa [
8]. The species of Japanese knotweed has several characteristics that make it invasive, including its large size, which blocks light for other plants; its ability to regenerate quickly; and its allelopathic effect, which affects other plants [
12,
13,
14,
15,
16]. Studies have shown that it is additionally in ruderal areas but is also increasingly found in agricultural areas [
9,
11]. Unfortunately, the proliferation of Japanese knotweed can harm natural habitats, reducing biodiversity and posing a significant threat [
4,
17].
There are several methods to control the population of knotweed. These methods include mechanical treatments like mowing; cutting using special machines or by hand; digging up, removing, or burning the whole plant; and removing soil containing rhizomes [
18]. However, mowing alone can cause more intense and dense underground biomass growth [
19]. In Poland, removing entire soil layers with knotweed is not recommended [
20]. While Djeddour et al. [
21] recommend using biological methods to control Japanese knotweed, the possibility of losing control over the invasive species’ natural enemies means that safer, albeit more costly, methods are usually preferred. In controlling knotweed, selective methods are allowed, e.g., smearing with herbicide or application with special spray guns [
18]. These treatments are time-consuming, and their effectiveness could be higher. In addition, using chemicals in protected areas is questionable [
3], and it is not allowed near reservoirs and watercourses [
9].
Current methods for controlling invasive plants must be more effective, as they are costly and labor-intensive and fail to limit underground biomass. That allows the plants to regenerate and spread, displacing native flora. Combining novel methods of knotweed control could be a suitable solution. For example, a recent technique based on water steam pressure exposing rhizomes has been proposed [
22]. That method destroys rhizomes using hot steam and could be combined with another innovative treatment of bare rhizomes with microwave radiation. It was proven that microwave radiation can stimulate or inhibit plant growth, depending on the intensity and duration of exposure. Research has shown that a single 10 min treatment with microwaves at 2.45 GHz can prevent regrowth of invasive hogweed (
Heracleum sp.) for an entire season [
23].
A hyperspectral imaging method is a low-cost and fast technology to decipher even small changes in the biochemical structure of plants [
24,
25]. It can be employed in analysis to search for the biochemical background of Japanese knotweed’s reaction to microwaves. To further investigate the effectiveness of high-frequency microwaves in invasive weed
Reynoutria japonica control, this study analyzed young knotweed offshoots’ regeneration and physiological condition following microwave radiation of their rhizomes. We hypothesize that the microwave radiation applied to the
Reynoutria japonica rhizomes harms the growth and development of shoots, affecting the plants’ biometric parameters. Additionally, we hypothesize that the microwaving of rhizomes leads to a gradual decline in the vegetation indices of the plants.
2. Materials and Methods
2.1. Plant Material
For the study, a selected population of Japanese knotweed in the meadow community of the
Alopecuretum pratensis association, located near the Vistula embankments in Kraków (N: 50°02′34.31″ E: 19°52′43.31″,
Figure 1), was chosen. Plant specimens were botanically identified based on the work of Rutkowski [
26] and then archived in the herbarium resources of the Department of Agroecology and Plant Production, the University of Agriculture in Krakow. Japanese knotweed rhizome was collected on 8–9 November 2022, from the soil profile up to 7 cm deep.
2.2. Laboratory Assessment
The research was conducted in the Department of Forest Utilization, Engineering, and Technology laboratory at the University of Agriculture in Krakow.
The rhizomes were cut into sections of equal 6 cm length, but only those with 1.0–3.0 cm diameter were selected for further research. A total of 104 pieces of rhizomes were prepared, divided into 13 groups, each of 8 pieces.
Then, a total of eight pieces of previously cut rhizomes were weighed collectively on a laboratory scale (manufacturer: RADWAG, Radom, PL) (
Figure 2) and then treated with an open stream of microwave radiation from a specially constructed antenna at times of 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, or 60 s. The antenna was a microwave emitter consisting of a magnetron generating electromagnetic waves with a frequency of 2450 MHz and a power of 800 W. The average surface power density for the dimensions of the antenna was 30 kW·m
−2 [
23].
Immediately after microwaving, the knotweed rhizomes were weighed again on a laboratory scale with an accuracy of 0.1 g.
The treatment of knotweed rhizomes with microwaves was carried out in the morning on 10 November 2022, at the air temperature in the laboratory of 20–21 °C, air humidity of 88%, and average humidity of the rhizomes of 89%.
Both before and after microwave treatment, the temperature of the rhizomes was measured using a FLIR E60 thermal imaging camera equipped with Wi-Fi (manufacturer: FLIR Systems, Inc., Wilsonville, OR, USA), which measures temperature in the range from −20 to +120 °C (±2 °C or ±2%) at a thermal sensitivity of 0.05 °C. The camera was placed approximately 0.5–1.0 m above the rhizomes, and photos were taken with a resolution of MSX 320 × 240 pixels and IR 240 × 180 pixels. Each image was then analyzed using FLIR ResearchIR MAX (manufacturer: Teledyne FLIR LLC, Wilsonville, OR, USA) based on mathematical processing of the pixel color scale, and average temperatures were calculated for each rhizome treatment time.
2.3. Plant Growth Conditions
After treatment with microwaves, the rhizomes of knotweed were planted into pots with a capacity of 330 cm
3, which were placed in a greenhouse belonging to the Department of Botany, Physiology and Plant Protection, University of Agriculture in Krakow. The soil for the pots was delivered from the forest nursery of the Niepołomice Forest District, PL. Based on the granulometric composition of soil according to a soil grading diagram [
27] and PN 04032 [
28] with the Bouyoucos sieve and areometric method, modified by Casagrande and Prószyński [
29], the soil had the following fractions: sand (2–0.5 mm)—79%, silt (0.05–0.002 mm)—19%, clay (<0.002 mm)—2%. The studied soil was classified as loamy sand (
Figure S1). The chemical properties of the soil were as follows: pH in KCl—5.9, pH in H
2O—6.8; organic matter—1.14%; total nitrogen—9.6 g·kg
−1 d.m. soil; P
2O
5—7.5 mg and K
2O—16.2 mg·100 g
−1 soil. Then, the soil was sterilized for 20–30 min using steam at a temperature of 90–100 °C. The soil substrate prepared in this way was placed in plastic pots with a capacity of 0.33 L. The bulk density of the soil in the pots was 1.52 kg·dm
−3, and humidity was ~35%. Next, one 6 cm-long rhizome was placed in each pot to a 2–3 cm depth. Control pots contained a piece of rhizome that was not treated with microwaves. Each rhizome contained at least two buds. From then on, the air temperature in the greenhouse was regulated at 18–19 °C, and the photoperiod was 12/12 h. The plants were watered every other day by the so-called automatic irrigation system of 120 mL per pot up to a humidity of ca. 75%. Humidity was determined at the beginning of the study using readings from a WLAN data logger, testo Saveris 2-H1 (manufacturer: Testo SE & Co., KGaA, Titisee-Neustadt, DE, Germany).
2.4. Spectrometric Studies of Japanese Knotweed Offshoots
Offshoots, grown from the rhizomes, reached a height of 10.1–44.5 cm and had an average of up to 6 pairs of mature leaves. The leaves between the 3rd and 5th leaf pair were measured spectrometrically to collect 9 measurements from each object. The hyperspectral imaging technology and portable leaf spectrometer CI-710s SpectraVue (CID Bio-Science, Inc. (Hamburg, Germany) with software version 0.9.38.0) were employed to assess changes in the biochemical structure of tested offshoots. The device operates in absorbance, transmittance, and reflectance modes and has a wide spectral range from near ultraviolet to infrared (360–1100 nm), making it possible to calculate various vegetation indices. The measurements were made intravitally in the middle part of the leaf blade; therefore, the same offshoots were examined on three dates: I—60th day, II—74th day, and III—88th day of growth, i.e., each time one day before performing biometric analyses. The object consisted of nine offshoots treated with microwaves for times ranging from 5 to 60 s, but the offshoots emerged only within the objects treated for 0–25 s.
The following vegetation indices were measured: Lichtenthaler index 2 (Lic2) and Zarco–Tejada and Miller (ZMI)—relative nitrogen content in leaves; Water Band index (WBI)—leaf dehydration; Photochemical reflectance index (PRI)—assessment of the amount of light used in the photosynthesis process; Anthocyanin reflectance index 2 (ARI2)—relative anthocyanin content in leaves; Carotenoid reflectance index 2 (CRI2)—relative content of carotenoids in leaves; Plant senescence reflectance index (PSRI)—leaf senescence; and absolute content of chlorophylls, i.e., chlorophyll a (CPHLA) and b (CPHLB) (µg·cm
−3). The equation formulas of the indices are depicted in
Table S1.
2.5. Biometric Analyses of Japanese Knotweed Offshoots
The first biometric measurements of young offshoots were performed 61 days after planting (DAP) and simultaneous microwave treatment of the knotweed rhizomes. The offshoot’s height, the stem’s diameter at 0.3 cm above the soil surface, and the nodes on the stem and leaves were counted. The width and length were measured on the three best-developed leaves of each offshoot from the middle part of the shoot. This paper presents the results of three series of biometric measurements performed on the following dates: IA—11 January, IIB—25 January, and IIIC—8 February. However, five series of biometric measurements of offshoots were carried out, and the next two measurements were carried out on the dates IVD—30 March and VE—22 April. The first three series of measurements were made on the same offshoots, regularly every two weeks, and the remaining two on new offshoots formed after cutting the knotweed. Considering the days since the rhizomes were treated with microwaves, biometric measurements of the offshoots were performed on IA—61, IIB—75, and IIIC—89 days after microwave treatment.
The present study considers only the results from the first three measurement series (IA–IIIC), performed at two-week intervals. However, the results from two subsequent series were not considered because the measurements were performed on new offshoots after the plants were cut down, and the results obtained were parallel to those presented.
2.6. Statistical Analysis
- (a)
Spectrometric measurements
Data obtained within each treatment (duration of exposure to microwaves) were statistically tested using the Friedman ANOVA procedure, with the Dunn-Bonferroni post-hoc test (α = 0.05) to demonstrate differences occurring over time within each object separately (between subsequent measurement dates). Analyzes were performed in the PQStat program. The results were depicted as heat maps using MS Excel 2019, and tables with homogeneous groups were denoted in
Supplementary Materials (Tables S2 and S3).
- (b)
Biometric analysis
The results were subjected to a two-way analysis of variance (ANOVA) and Tukey’s multiple comparison tests, with a significance level of 0.05. Tukey’s comparative tests allowed for a detailed analysis of mean values by distinguishing statistically homogeneous groups. A confidence interval was determined for the means of the examined features, assuming a confidence level of 0.05. Calculations were performed using the statistical software package Statistica 13.1 (StatSoft Inc., Tulsa, OK, USA).
The effective dose (ED50) value was estimated for the biometric characteristics of
Reynoutria japonica. The ED50 value is a value causing a 50% decrease in a particular trait [
30] and was estimated using the three-parameter log-logistic function, where the lower limit equals zero. The calculations were performed in the
drc package [
31] in the R ver. 4.3.1 software.
3. Results
3.1. Regeneration of Japanese Knotweed following the Microwave Treatments
The average temperature of control rhizomes, i.e., those not treated with microwaves, was 13.3 °C. The temperature of rhizomes treated with microwaves at a frequency of 2.45 GHz and a power of 800 W for times of 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, or 60 s reached the following values: 25.6, 37.5, 39.4, 54.8, 56.1, 56.9, 63.4, 66.5, 70.0, 79.8, 90.8, and 91.1 °C, respectively (
Table 1).
Based on the weight of all the rhizomes, the lowest loss of rhizome weight (by 0.5%) was recorded following microwave treatment at times of 5 and 10 s. A similar low loss of weights (by 0.9%, equal to 0.7 g) was observed following microwave treatment for 20 s. In turn, the highest loss of rhizome weight, above 4%, occurred following microwave treatment for 35, 50, and 60 s.
The biomass loss of microwaved rhizomes corresponded with the growth of young offshoots. On the 61st day after planting, microwave-treated knotweed rhizomes were found in each pot where microwaves were used for 5, 10, or 15 s. In addition, young knotweed offshoots were also found in the objects microwaved for times of 20, 25, or 30 s. Out of eight repetitions, in the objects exposed to microwaves for 20 s, only three offshoots were found (which constituted 38% of the total), while in the objects microwaved for 25 or 30 s, there was only one offshoot (which accounted for 13% for each treatment).
The rhizomes exposed to microwaves for 35, 40, 45, 50, 55, or 60 s did not produce any offshoots, and, for those rhizomes, a weight loss of more than 3% was observed. In contrast, each control rhizome not subjected to microwaves produced 100% offshoots.
3.2. Spectrochemical Analysis of Leaves
Depending on the time of exposure to microwave radiation, changes in the condition of knotweed leaves were recorded in subsequent measurement series. Most rhizomes exposed to microwave radiation for over 20 s did not emerge. In turn, the dose of microwave radiation of 25 s delayed the emergence of shoots. The first (I) measurement series of spectrometric measurements was 60 days after planting the rhizomes. For the control, no statistically significant differences were found between subsequent measurement series for the tested vegetation indices (
Table S2).
The values of vegetation indices relating to the content of chlorophyll (a and b), anthocyanins, and carotenoids are shown in
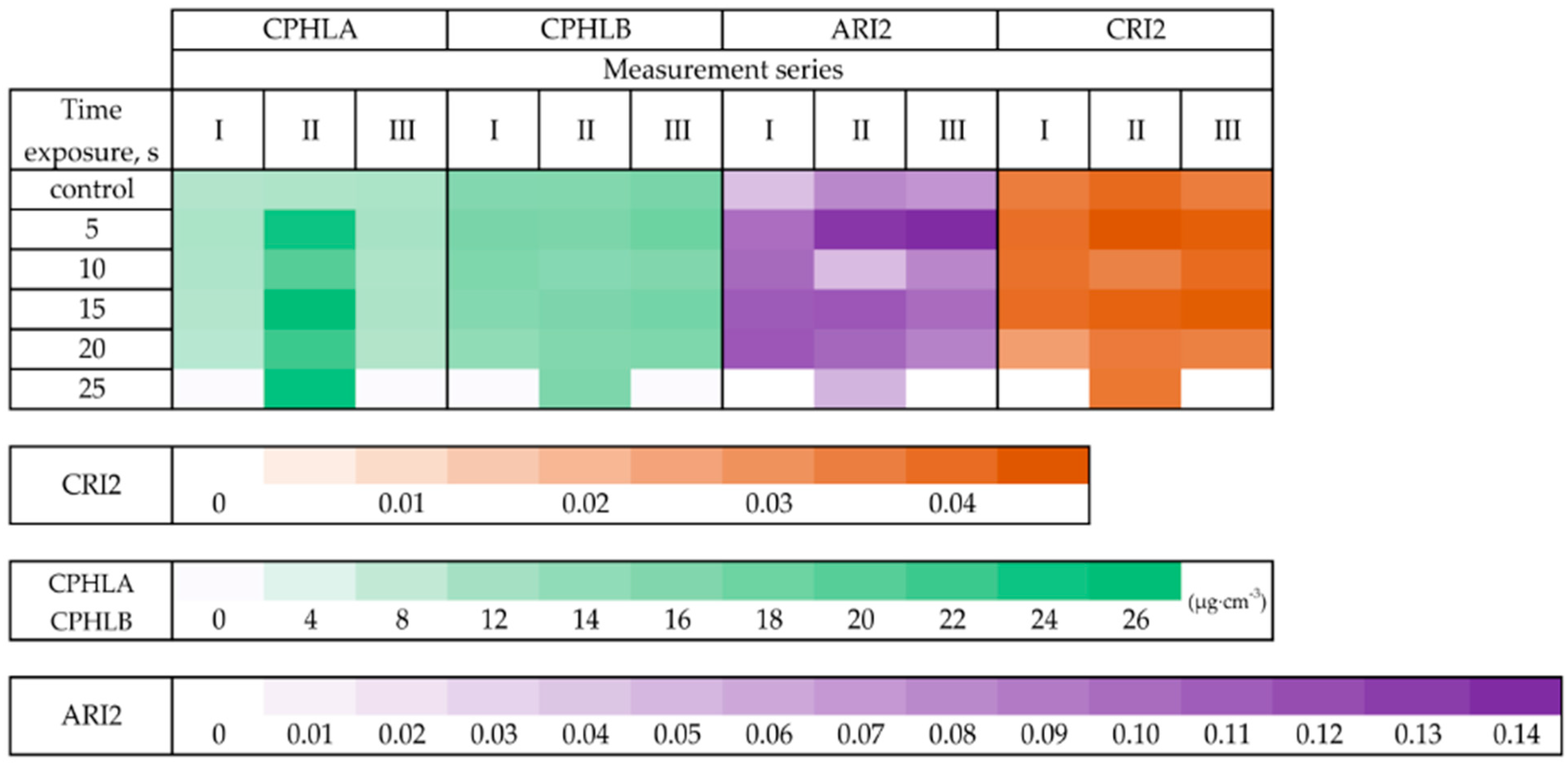
Figure 3. The highest indices indicating chlorophyll
a content in Japanese knotweed leaves were recorded on the 74th day after planting the rhizomes (II measurement series). In the first measurements series, the Anthocyanins index (ARI2) had higher values (0.10–0.11) for knotweed shoots grown from rhizomes treated with microwaves for 10 to 20 s.
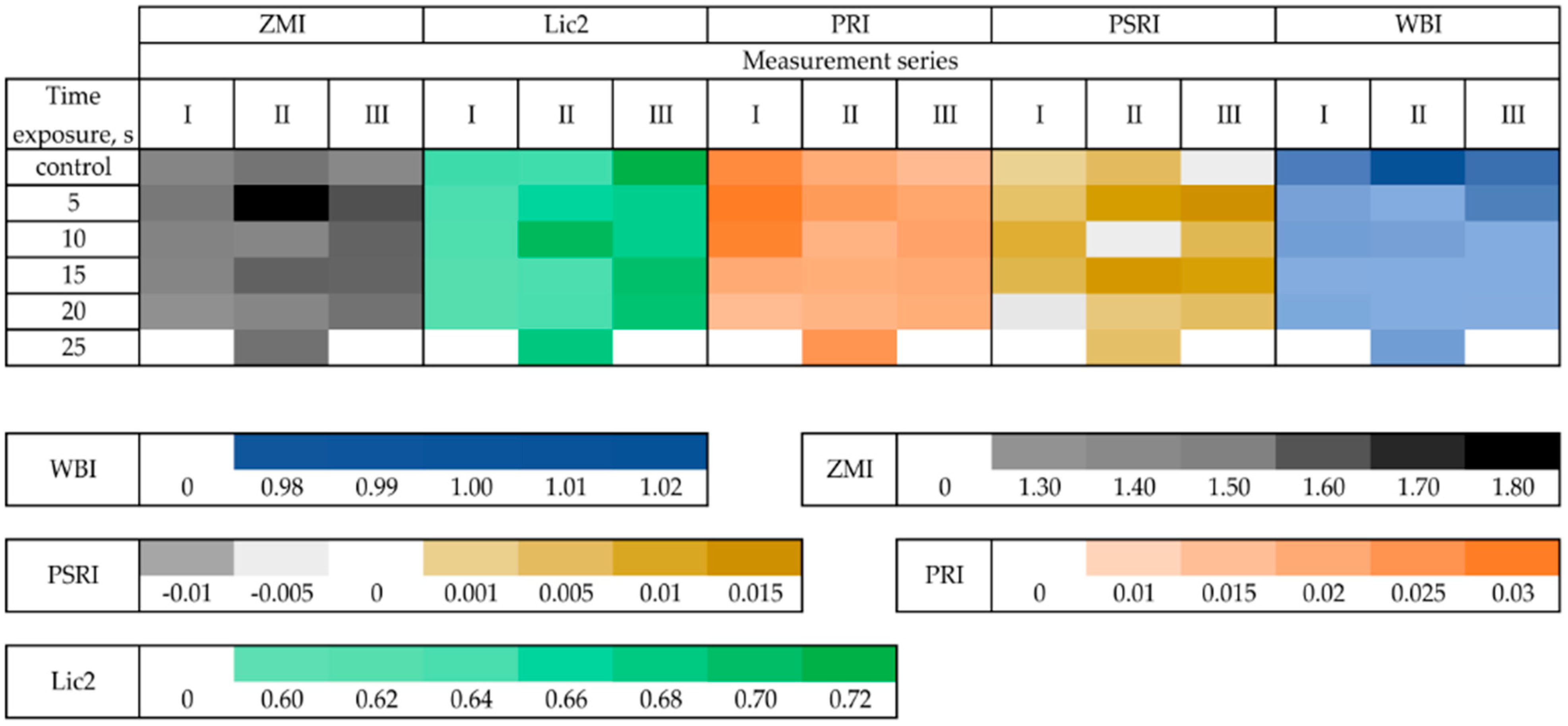
The relative nitrogen content in leaves measured by the ZMI and Lic2 index increased with time in the subsequent measurement series (
Figure 4), with minor exceptions (ZMI for control). Rhizomes treated for 25 s produced a few offshoots in the first and the third measurements. Therefore, a “0” value occurred. All the tested offshoots were ready for the measurements on the second series date. Results did not indicate disturbances in nitrogen content in the leaves, except for the 20 s treatment, where the recorded values were increasing. The values for the Lic2 index were statistically higher in the second and third measurement series (treatments for 10 and 15 s) (
Figure 4,
Table S3). The PRI index reflects the amount of light used in the photosynthesis process. It is related to the changes in xanthophyll composition [
32]. The higher the index values, the better the use of light in the photosynthesis process (PSII), thus indicating better productivity and no damage to plant cells [
33]. This study recorded no significant changes in the PRI index (
Figure 4,
Table S3).
The higher values of the PSRI index indicate an increased carotenoid/chlorophyll ratio, and this index is a good approximator of the stress in plants [
34]. In the tested offshoots, statistically significant higher values occurred for the treatments for 5 or 20 s; the values increased with time, indicating stress in plants despite increased chlorophyll content (
Figure 4). The WBI index is a ratio of reflectance (R900/R970), where the wavelengths correspond to the water absorption band set at 970–980 nm [
35]. No significant fluctuations in the WBI index were noted, except for the 25 s treatment.
3.3. Biometrical Characteristic of Japanese Knotweed Offshoots following Their Rhizome Treatment with Microwaves
The time of microwave treatment of knotweed rhizomes was a factor that significantly differentiated the biometric characteristics of young offshoots grown from the treated rhizomes (
Table 2). There was no significant differentiation in the biometric characteristics of knotweed offshoots depending on the measurement series (IA–IIIC). Also, no significant interaction was found between the microwave time and the measurement series.
The value of the effective dose (ED50), i.e., the time of exposure of rhizomes to microwave radiation, which caused a 50% decrease in the analyzed biometric features of
Reynoutria japonica, at each of the three dates, is presented in
Table 3. The data show that the effective time of exposure of rhizomes to microwaves (the so-called ED50 dose) for the following features: values for plant length, stem diameter, number of nodes on the stem, length, and width of the leaf blade were similar and ranged from 16.8 to 19.0 s. However, for the number of leaves on the stem, ED50 ranged from 14.7 to 18.9 s.
The homogenous groups of the six analyzed biometric features of young knotweed offshoots depending on the time of exposure of their rhizomes to microwaves are presented in
Table S4. No significant differences were found considering the length and diameter of the stems, the number of nodes, and leaves on the stem between the control and rhizomes exposed to microwave treatment for times of 5, 10, or 15 s. Considering the length and width of the leaf blade, significant differences were shown between the control and the microwaved plants. However, microwave treatment for 20, 25, or 30 s did not significantly affect the biometric characteristics among the treatments. Moreover, plants whose rhizomes were subjected to microwaves for 20, 25, or 30 s differed significantly only in terms of the diameter of the stem and the number of nodes per offshoot.
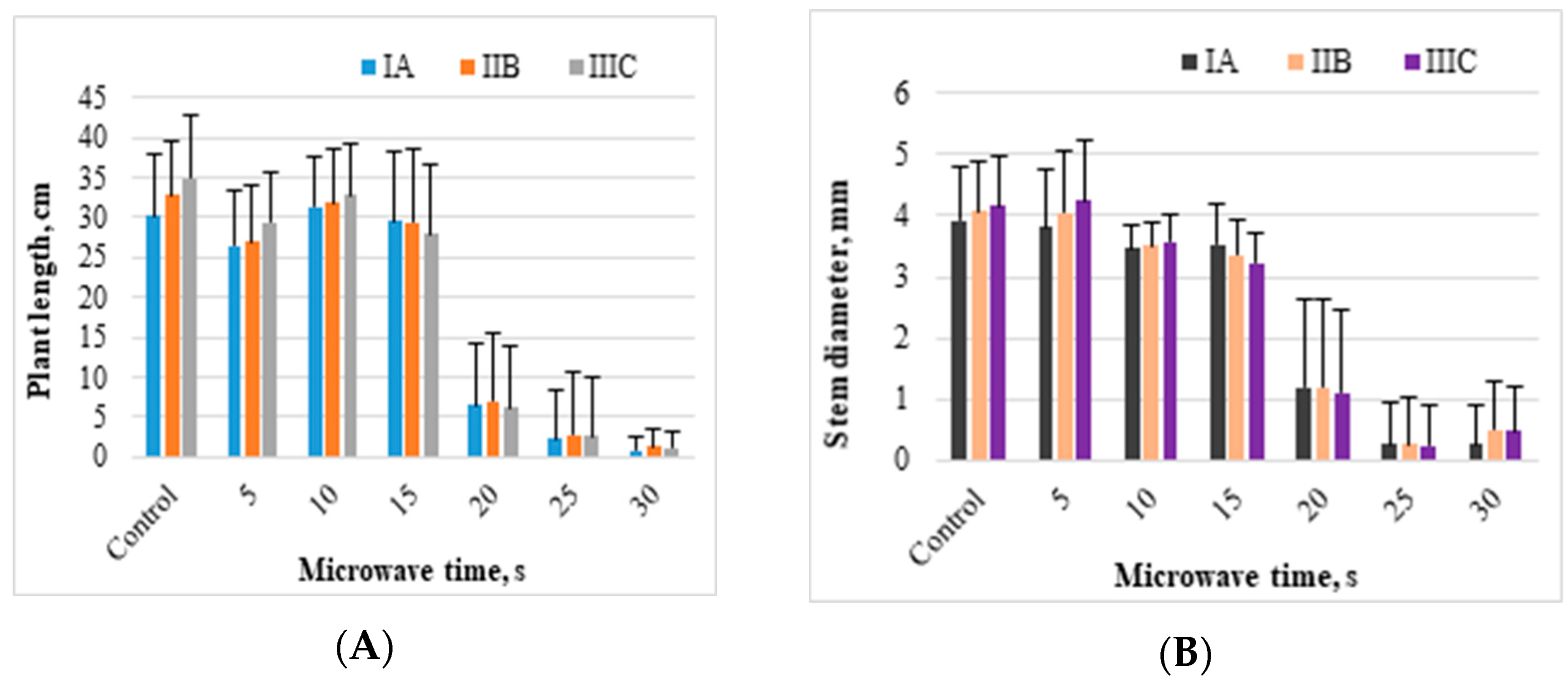
The length of the Japanese knotweed offshoots and the diameters of their stems varied and depended on the time of exposure to microwaves (
Figure 5A,B). Eight control offshoots from the three measurement series (IA–IIIC) had an average length of 32.6 cm. Control offshoots and those microwaved for 5, 10, or 15 s increased their length from 1.5 to 4.8 cm in subsequent measurement series. Microwaving rhizomes for 15 s with the associated temperature of almost 40 °C resulted in shorter offshoots, by approximately 11%, compared to control. For comparison, the average length of the knotweed microwaved for 30 s, with a temperature oscillating around 60 °C, was 1.2 cm. That means the microwaves limited knotweed growth by 96% compared to the control. Microwaving the rhizomes for 25 s, generating a temperature of 56.1 °C, resulted in a 2.6 cm average length of the knotweed offshoots from three measurement series (a 92% reduction in offshoot length compared to control).
The microwave treatment of the rhizomes for 5 s (at a temperature of 25.6 °C) did not reduce the diameter of the knotweed stem compared to the control. Following the microwave treatment for 10 s (temperature of 37.5 °C), the diameter of the knotweed stem (3.5 mm) was smaller by 13% compared to the control. Higher microwave times of 25 and 30 s caused a reduction in stem diameter by an average of 0.3 and 0.4 mm compared to the control.
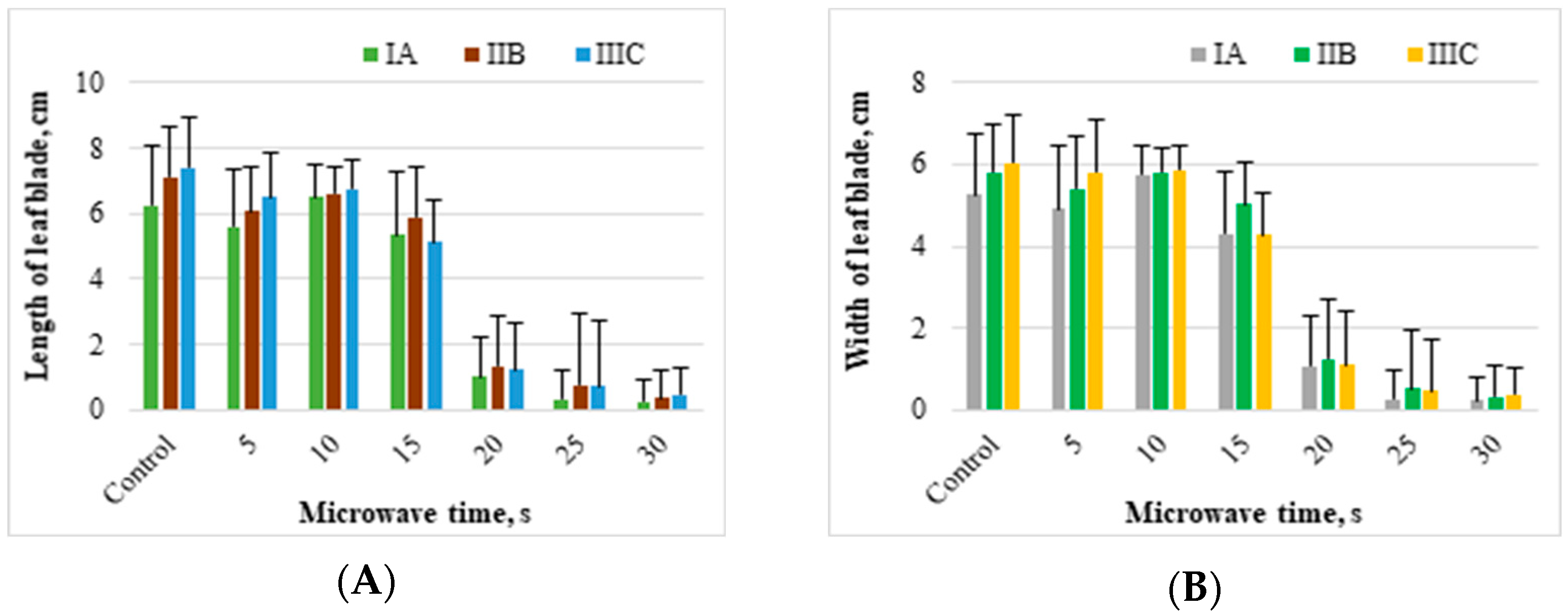
Biometrical characteristics of knotweed, such as the length and width of leaf blades, also differed under the influence of microwaves (
Figure 6A,B). The differences in these parameters were significant even following the shortest microwave exposure time. The leaf blades of the control offshoots were the longest and widest among all the analyzed treatments. Their average length from the three measurement series was 6.9 cm, and their width was around 5.7 cm. In subsequent measurement series, the parameters mentioned above increased. The same relationship was found for offshoots exposed to microwaves for 5 or 10 s. However, in objects microwaved for 10 s, the increase in the length and width of the leaf blade in subsequent measurement series was small and amounted to 0.1–0.2 cm. Microwaving for 20 s with the temperature generated during this process (55 °C) significantly reduced the length and width of leaf blades by 83 and 81%, respectively, to the length and width of the leaves of the control. Offshoots grown from the rhizomes treated for 25 or 30 s had a leaf blade average length of 0.6 and 0.4 cm, respectively. In turn, the width of the leaf blades of these offshoots was, on average, 0.4 and 0.3 cm, respectively. Compared to the length and width of the control leaves, the leaf blades of offshoots from the rhizome microwaved for 30 s were approximately 95% shorter and narrower.
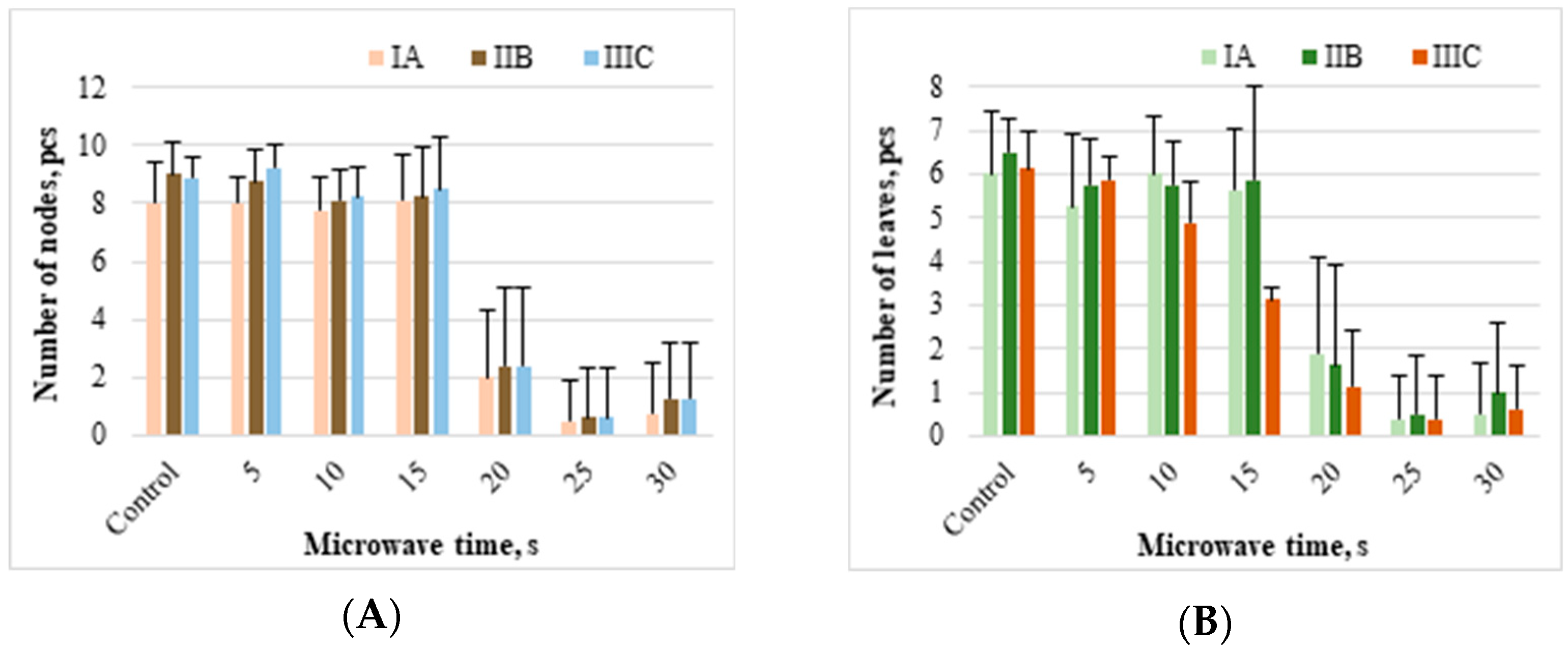
The average number of nodes and leaves on the stems of control and offshoots microwaved for 5 s, calculated from the three measurement series, were identical and equal to nine and six, respectively (
Figure 7A,B). Microwaving for 10 or 15 s reduced the mentioned parameters compared to the control by one unit; they were eight and five, respectively, and were identical for both given times. Only in offshoots whose rhizomes were microwaved for 5 s was there a tendency to increase the number of leaves on the stem in the subsequent measurement series. In other offshoots, including the control, such regularity was not observed, related to falling down of the lowest (oldest) leaves.
The condition of the Japanese knotweed offshoots on the 89th day after the microwave treatment is shown in
Figure 8.
4. Discussion
The results presented in this study regarding the influence of microwave radiation on spectrometric and biometric features of young Japanese knotweed offshoots are pioneering. So far, the microwave technique has been used to destroy another invasive species—Sosnowski’s hogweed (
Heracleum sosnowskyi Manden.). Using a horn antenna, microwaves with a frequency of 2.45 GHz and a power density of 30.0 kW·m
−2 were emitted onto the small part of the stem after cutting the plant, thus destroying it [
23]. It was found that the longer the exposure time of plants to microwaves, the less their regeneration. However, during field tests for Sosnowski hogweed, the effective plant elimination time was 10 min. The effective elimination time using microwaves is not yet known for Japanese knotweed. Based on preliminary research, it is assumed that longer time ranges should be used in which the plant is exposed to microwaves due to its highly developed root system.
The limited research on the exposure of plants to microwaves shows that species show different sensitivity to radiation frequencies, exposure time, and power intensity. For example, a stimulating effect was observed with a low-power microwave of redwood plants (
Sequoia Endl.) in in vitro culture [
36]. However, in the case of alfalfa (
Medicago L.), no differences in growth and biomass accumulation were found between plants irradiated with microwaves at a frequency of 2.45 GHz and a power density of 0.5–1.2 mW·cm
−2 and plants that were not subjected to such treatment [
37]. A similar relationship was demonstrated by Senavirathna and Asaeda [
38] for an aquatic invasive plant of the genus
Myriophyllum aquaticum [(Vell.) Verdc.], which was exposed to horizontally and vertically polarized continuous microwaves with a frequency of 2 GHz and a power density of 1.8 W·m
−2—there was no effect of microwaves on growth parameters, e.g., shoot length, stem diameter, and internode length. The opposite relationship was observed by Surducan et al. [
39], where long-term treatment at a frequency of 915 MHz and a maximum power density of 10 mW·m
−2 resulted in significant morphological modifications in common beans (
Phaseolus vulgaris L.).
In our research carried out in the greenhouse, the microwave radiation applied directly to the bare rhizomes of Japanese knotweed generated high temperatures. In turn, the high tissue temperature during rhizome treatment probably caused protein denaturation and tissue decomposition of the knotweed, which further resulted in the excretion and accumulation of sap in the rhizomes. According to Korablev et al. [
40], plant tissue membranes become hyperfluid at high temperatures, leading to the outflow of ions from cells, which reduces the activity of many enzymes. High temperature also causes water loss in cells. The research shows that the temperature limit beyond which plant growth and development no longer occurred was generated during microwaving of rhizomes for 35 s at 63 °C.
Additionally, under such conditions, the mass of rhizomes lost was over 3%. The strategy of plants is generally known to prevent loss and maintain the water content in cells, as well as to optimize the water supply of important organs [
41]. Under the influence of stress caused by microwave radiation in plants, physiological processes at the cellular level were probably inhibited. Water deficiency is considered one of the most significant factors that affect cell growth because it reduces turgor pressure [
42].
Premachandra et al. [
43], in studies with maize, and Wang and Huang [
44], in
Poa pratensis, observed significant lability of the cell membrane under the influence of water deficiency. Consequently, the mitosis process was impaired, cell elongation occurred, and, ultimately, the growth and yield of plants were limited [
45,
46]. The effects of water deficiency are reflected in reduced accumulation of aboveground biomass, early aging, and, finally, plant death. Water deficiency affects the morphological characteristics of plants, such as height, stem diameter, and leaf area. During times of limited water availability, a reduction in the height and diameter of the stem was observed in maize [
47,
48], sorghum [
49], soybean [
50], sunflower [
51], and potato [
52].
As a result of the microwave treatments, the emergence of new offshoots from the microwave-treated rhizomes was disrupted. Most rhizomes exposed to microwave radiation for over 20 s did not produce offshoots. The offshoots from the rhizomes microwaved for 25 s appeared 60 days after the treatment. Moreover, the length and width of leaf blades of knotweed offshoots whose rhizomes were microwaved for 30 s decreased by approximately 95% compared to the control. Plant height limitation may be due to impaired cell elongation because of the low availability of water [
51].
Interestingly, the biometrical results for offshoots did not correlate well with spectrophotometric analyses of their leaves. Based on UV-VIS spectrometry, the higher content of chlorophyll
a in knotweed leaves, which were grown from the microwave-treated rhizomes, may indicate effective biochemical processes leading to maintaining or increasing the efficiency of photosynthesis. Similar remarks were obtained for emitting UV-C radiation on grape leaves, i.e., with increasing exposure time, the chlorophyll
a content increased in the leaves [
53]. The higher values of chlorophyll
a content prove the invasive character of the tested species, i.e., its viability. The higher level of anthocyanins for the microwave-treated plants (higher ARI2 values), irrespective to the microwave duration time, indicate repair mechanisms occurring in the plant. This group of substances helps the plant to deal with several types of stresses by, e.g., ROS molecules scavenging, photosynthesis or transcription regulation, and reduced lipid peroxidation [
54]. The PSRI index is the indicator of leaf senescence [
55] or drought stress [
56]. This study registered higher PSRI values for the microwave treatment times of 5 and 20 s. Zelazny and Lukas [
57] have proven a correlation between PSRI values and hydric regime for plants regenerating after drought. It shows that there may exist a relationship between regeneration processes and monitored PSRI values over time. However, in our study, the WBI values remained the same. It could mean that, generally, the water content in the knotweed leaves did not change significantly during the experiment, taking the first measurement as a basis. It is worth mentioning that the control leaves were better hydrated over time, which is indicated by higher subsequent values in WBI but not statistically confirmed (
Figure 7,
Table S3).
5. Conclusions
This research could encourage further investigation into the effects of microwave radiation on Japanese knotweed offshoots and the development of a safe and efficient way to control the Japanese knotweed population in natural habitats. It has been shown that microwave radiation for 35 s (which generates a temperature of 63 °C) is sufficient to destroy directly knotweed rhizomes of a diameter of 1–3 cm. Microwave radiation used for 20–30 s significantly reduced the analyzed biometric characteristics of offshoots or destroyed the knotweed plants by 62–87%.
The first leaves where vegetation indices could be assessed spectrophotometrically appeared on the 60th day after planting the rhizomes treated with microwaves. The highest levels of pigments in leaves were recorded on the 74th day after planting the rhizomes based on the measurements of the CPHLA, CPHLB, ARI2, and CRI2 indices; however, only the chlorophyll a (CPHLA) content was statistically significantly higher regardless of the treatment. The highest CPHLA value was recorded for offshoots whose rhizomes were treated with microwaves for 15 s (median 26.2 µg·cm−3). It was also observed that the ARI2 (relative anthocyanin content in leaves) values in the first measurement period were much higher for the 10 s, 15 s, and 20 s treatments than those measured for the control and 5 s treatments. That suggests that the plant initiated the reaction mechanisms to microwave stress after 10 s of exposure. However, this effect was no longer visible after two weeks (in the 2nd measurement series). The ARI2 index could probably be considered a decision-supporting tool regarding the effectiveness of the applied microwave radiation. Statistically higher PSRI values occurred for the 5 or 20 s treatments, indicating stress in knotweed offshoots despite increased chlorophyll a content.
Microwaves used for 5–15 s did not significantly affect the biometric features such as offshoot height, stem diameter, and the number of nodes and leaves on the stem. However, the leaf blades’ length and width were significantly differentiated by microwaves applied for 5 s. In turn, microwaves applied for 20 s (55 °C), 25 s (56 °C), and 30 s (57 °C) significantly differentiated all the analyzed biometric features. The value of the effective dose (ED50), i.e., the time of exposure of rhizomes to microwave radiation, which caused a 50% decrease in the analyzed biometric features of Reynoutria japonica on each of the three dates, ranged from 14.7 to 19.0 s.