The Differentiations in the Soil Nematode Community in an Agricultural Field after Soil Amendment Using Composted Coffee Waste in Various Concentrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coffee Waste Composting
2.2. Experimental Field Area
2.3. Experimental Design and Sampling
2.4. Laboratory Methods
2.4.1. Chemical Analysis of Composted Coffee Waste and Soil Samples
2.4.2. Nematode Extraction and Identification
2.5. Statistical Analyses
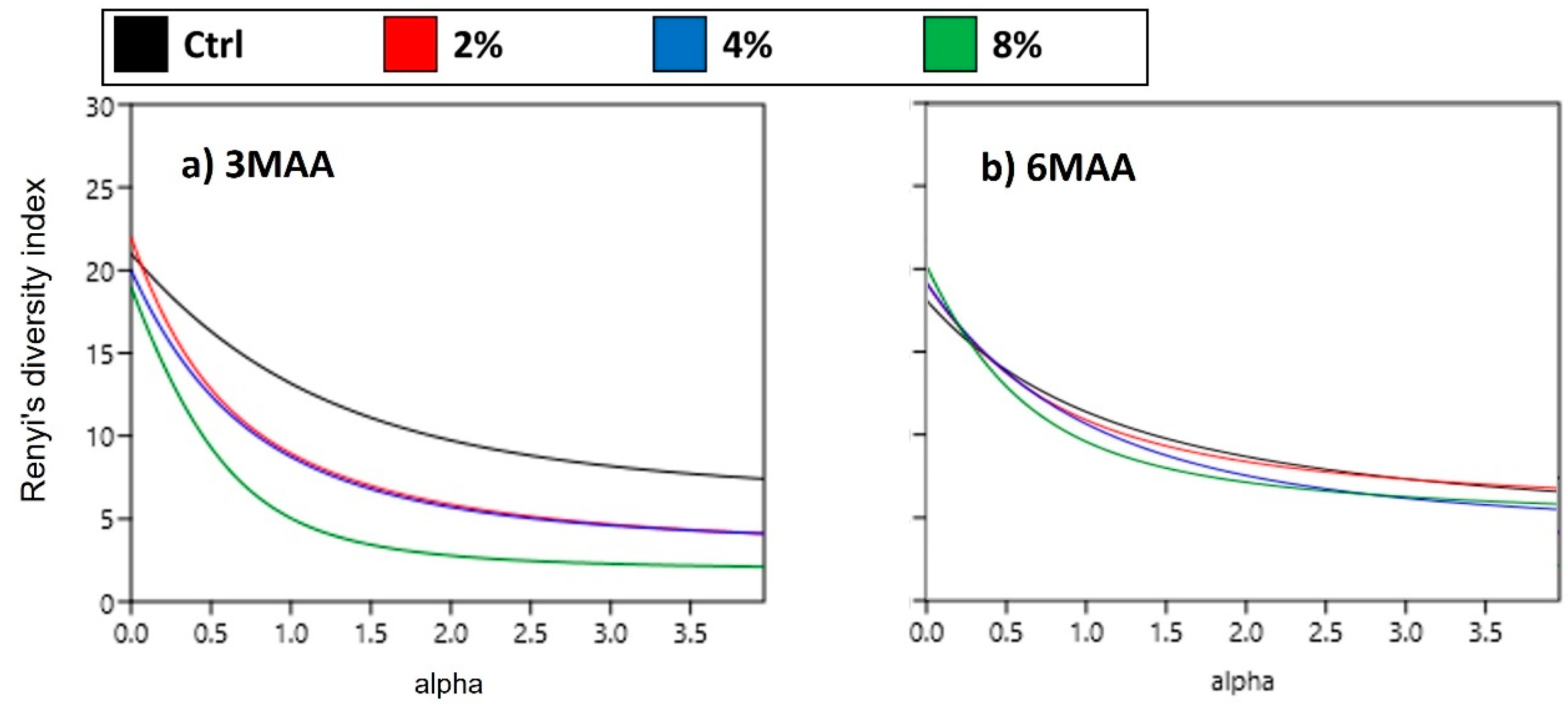
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandes, A.S.; Mello, F.V.C.; Thode Filho, S.; Carpes, R.M.; Honorio, J.G.; Marques, M.R.C.; Felzenszwalb, I.; Ferraz, E.R.A. Impacts of discarded coffee waste on human and environmental health. Ecotoxicol. Environ. Saf. 2017, 141, 30–36. [Google Scholar] [CrossRef] [PubMed]
- International Coffee Organization. World Coffee Consumption. 2022. Available online: http://www.ico.org/prices/newconsumption-table.pdf (accessed on 6 September 2023).
- Tokimoto, T.; Kawasaki, N.; Nakamura, T.; Akutagawa, J.; Tanada, S. Removal of lead ions in drinking water by coffee grounds as vegetable biomass. J. Colloid Interface Sci. 2005, 281, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Randell, P.; Pickin, J.; Grant, B. Waste Generation and Resource Recovery in Australia: Reporting Period 2010/11; Final Report Prepared for DSEWPC; Blue Environment Pty Ltd.: Docklands, Australia, 2014; Volume 128. [Google Scholar]
- Pujol, D.; Liu, C.; Gominho, J.; Olivella, M.À.; Fiol, N.; Villaescusa, I.; Pereira, H. The chemical composition of exhausted coffee waste. Ind. Crops Prod. 2013, 50, 423–429. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Pastoriza, S.; Rufián-Henares, J.Á.; Párraga, J.; Martín-García, J.M.; Delgado, G. Impact of spent coffee grounds as organic amendment on soil fertility and lettuce growth in two Mediterranean agricultural soils. Arch. Agron. Soil Sci. 2018, 64, 790–804. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Martín-García, J.; Delgado, R.; Sánchez-Marañón, M.; Delgado, G. Short-term effects of spent coffee grounds on the physical properties of two Mediterranean agricultural soils. Int. Agrophysics 2019, 33, 205–216. [Google Scholar] [CrossRef]
- Afriliana, A.; Hidayat, E.; Yoshiharu, M.; Taizo, M.; Harada, H. Evaluation of Potency Spent Coffee Grounds for Make Black Compost. In E3S Web of Conferences, Proceedings of the 3rd International Conference on Agricultural and Life Sciences (ICALS 2019), Jember, Indonesia, 31 July–2 August 2019; EDP Sciences: Les Ulis, France, 2020; Volume 142, p. 04002. [Google Scholar]
- Morikawa, C.K.; Saigusa, M. Recycling coffee grounds and tea leaf wastes to improve the yield and mineral content of grains of paddy rice. J. Sci. Food Agric. 2011, 91, 2108–2111. [Google Scholar] [CrossRef]
- Yamane, K.; Kono, M.; Fukunaga, T.; Iwai, K.; Sekine, R.; Watanabe, Y.; Iijima, M. Field evaluation of coffee grounds application for crop growth enhancement, weed control, and soil improvement. Plant Prod. Sci. 2014, 17, 93–102. [Google Scholar] [CrossRef]
- Kasongo, R.K.; Verdoodt, A.; Kanyankagote, P.; Baert, G.; van Ranst, E. Coffee waste as an alternative fertilizer with soil improving properties for sandy soils in humid tropical environments. Soil Use Manag. 2010, 27, 94–102. [Google Scholar] [CrossRef]
- Kasongo, R.K.; Verdoodt, A.; Kanyankogote, P.; Baert, G.; Van Ranst, E. Response of Italian ryegrass (Lolium multiflorum Lam.) to coffee waste application on a humid tropical sandy soil. Soil Use Manag. 2013, 29, 22–29. [Google Scholar] [CrossRef]
- Cruz, R.; Baptista, P.; Cunha, S.; Pereira, J.A.; Casal, S. Carotenoids of lettuce (Lactuca sativa L.) grown on soil enriched with spent coffee grounds. Molecules 2012, 17, 1535–1547. [Google Scholar] [CrossRef]
- Cruz, R.; Gomes, T.; Ferreira, A.; Mendes, E.; Baptista, P.; Cunha, S.; Pereira, J.A.; Ramalhosa, E.; Casal, S. Antioxidant activity and bioactive compounds of lettuce improved by espresso coffee residues. Food Chem. 2014, 145, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.; Morais, S.; Mendes, E.; Pereira, J.A.; Baptista, P.; Casal, S. Improvement of vegetables elemental quality by espresso coffee residues. Food Chem. 2014, 148, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.; Mendes, E.; Torrinha, Á.; Morais, S.; Pereira, J.A.; Baptista, P.; Casal, S. Revalorization of spent coffee residues by a direct agronomic approach. Food Res. Int. 2015, 73, 190–196. [Google Scholar] [CrossRef]
- Hardgrove, S.J.; Livesley, S.J. Applying spent coffee grounds directly to urban agriculture soils greatly reduces plant growth. Urban For. Urban Green. 2016, 18, 1–8. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Navarro-Alarcón, M.; Rufián-Henares, J.Á.; Pastoriza, S.; Montilla-Gómez, J.; Delgado, G. Phytotoxicity and chelating capacity of spent coffee grounds: Two contrasting faces in its use as soil organic amendment. Sci. Total Environ. 2020, 717, 137247. [Google Scholar] [CrossRef]
- Santos, C.; Fonseca, J.; Aires, A.; Coutinho, J.; Trindade, H. Effect of different rates of spent coffee grounds (SCG) on composting process, gaseous emissions and quality of end-product. Waste Manag. 2017, 59, 37–47. [Google Scholar] [CrossRef]
- Dafouz, R.; Cáceres, N.; Rodríguez-Gil, J.L.; Mastroianni, N.; de Alda, M.L.; Barceló, D.; de Miguel, Á.G.; Valcárcel, Y. Does the presence of caffeine in the marine environment represent an environmental risk? A regional and global study. Sci. Total Environ. 2018, 615, 632–642. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Cervera-Mata, A.; Fernández-Arteaga, A.; Pastoriza, S.; Rufián-Henares, J.Á.; Delgado, G. Why Should We Be Concerned with the Use of Spent Coffee Grounds as an Organic Amendment of Soils? A Narrative Review. Agronomy 2022, 12, 2771. [Google Scholar] [CrossRef]
- de Bomfim, A.S.C.; de Oliveira, D.M.; Walling, E.; Babin, A.; Hersant, G.; Vaneeckhaute, C.; Dumont, M.-J.; Rodrigue, D. Spent Coffee Grounds Characterization and Reuse in Composting and Soil Amendment. Waste 2023, 1, 2–20. [Google Scholar] [CrossRef]
- Wakasawa, H.; Takahashi, K.; Mochizuki, K. Application and composting conditions of coffee grounds, 1: Application of coffee grounds in soil. Jpn. J. Soil Sci. Plant Nutr. 1998, 69, 1–6. [Google Scholar]
- Shoenberger, E. Effect of Coffee Grounds on the Soil Nematode Population of Meloidogyne Hapla under Greenhouse Conditions. ECHOcommunity, Resources, Research-Posters. 2018. Available online: http://edn.link/gxhdzj (accessed on 20 October 2023).
- Thligene, N.; Mezzapesa, G.N.; Mondelli, D.; Trani, A.; Veronico, P.; Melillo, M.T.; Dumontet, S.; Miano, T.; Sasanelli, N. Effect of Coffee Silver Skin and Brewers’ Spent Grain in the Control of Root-Knot Nematodes. Helminthologia 2019, 56, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Vela-Cano, M.; Cervera-Mata, A.; Purswani, J.; Pozo, C.; Delgado, G.; González-López, J. Bacterial community structure of two Mediterranean agricultural soils amended with spent coffee grounds. Appl. Soil Ecol. 2019, 137, 12–20. [Google Scholar] [CrossRef]
- Du Preez, G.; Daneel, M.; De Goede, R.; Du Toit, M.J.; Ferris, H.; Fourie, H.; Geisen, S.; Kakouli-Duarte, T.; Korthals, G.; Sánchez-Moreno, S.; et al. Nematode-based indices in soil ecology: Application, utility, and future directions. Soil Biol. Biochem. 2022, 169, 108640. [Google Scholar] [CrossRef]
- Kekelis, P.; Papatheodorou, E.M.; Terpsidou, E.; Dimou, M.; Aschonitis, V.; Monokrousos, N. The Free-Living Nematodes as Indicators of the Soil Quality in Relation to the Clay Content, When Coffee Waste Is Applied. Agronomy 2022, 12, 2702. [Google Scholar] [CrossRef]
- Available online: https://meteosearch.meteo.gr/data/list-station-files720.cfm (accessed on 7 November 2023).
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis; Klute, A., Ed.; SSSA: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Watanabe, F.S.; Olsen, S.R. Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Sci. Soc. Am. J. 1965, 29, 677–678. [Google Scholar] [CrossRef]
- Thomas, G.W. Exchangeable Cations. In Methods of Soil Analysis Part-2 Chemical and Mineralogical Properties; Page, A.L., Ed.; ASA: Schaumburg, IL, USA; SSSA: Madison, WI, USA, 1982; pp. 159–165. [Google Scholar]
- S’Jacob, J.J.; Van Bezooijen, J. Manual for Practical Work in Nematology; Landbouwhogeschool: Wageningen, The Netherlands, 1984; pp. 40–70. [Google Scholar]
- Bongers, T. Systematisch gedeelte. In De Nematoden van Nederland: Vormgeving en Technische Realisatie, 2nd ed.; Uitgeverij Pirola: Schoorl, The Netherlands, 1994; pp. 67–383. [Google Scholar]
- Yeates, G.W.; Bongers, T.D.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera-an outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; de Goede, R.G. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Ferris, H. Form and function: Metabolic footprints of nematodes in the soil food web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Sieriebriennikov, B.; Ferris, H.; de Goede, R.G. NINJA: An automated calculation system for nematode-based biological monitoring. Eur. J. Soil Biol. 2014, 61, 90–93. [Google Scholar] [CrossRef]
- Taylor, R.A.J. Taylor’s Power Law: Order and Pattern in Nature; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Abd-Elgawad, M.M.M. Optimizing sampling and extraction methods for plant-parasitic and entomopathogenic nematodes. Plants 2021, 10, 629. [Google Scholar] [CrossRef]
- Patil, G.; Taillie, C. An overview of diversity. In Ecological Diversity in Theory and Practice; Grassle, J., Patil, G., Smith, W., Taillie, C., Eds.; International Cooperative Publishing House: Fairland, ML, USA, 1979. [Google Scholar]
- Rényi, A. On measures of entropy and information. In Proceedings of the Fourth Berkeley Symposium on Mathematical Statistics and Probability, Volume 1: Contributions to the Theory of Statistics, Berkley, CA, USA, 20 June–30 July 1961; pp. 547–562. [Google Scholar]
- Ricotta, C. From theoretical ecology to statistical physics and back: Self-similar landscape metrics as a synthesis of ecological diversity and geometrical complexity. Ecol. Modell. 2000, 125, 245–253. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, A.; Ryan, P. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. Available online: https://palaeo-electronica.org/2001_1/past/past.pdf (accessed on 3 August 2023).
- Perța-Crișan, S.; Ursachi, C.; Munteanu, F.D. Trends in valorisation of spent cofee grounds: A review. Sci. Tech. Bull. Ser. Chem. Food Sci. Eng. 2019, 16, 31–42. [Google Scholar]
- Horgan, F.G.; Floyd, D.; Mundaca, E.A.; Crisol-Martínez, E. Spent Coffee Grounds Applied as a Top-Dressing or Incorporated into the Soil Can Improve Plant Growth While Reducing Slug Herbivory. Agriculture 2023, 13, 257. [Google Scholar] [CrossRef]
- Zhi, D.; Li, H.; Nan, W. Nematode communities in the artificially vegetated belt with or without irrigation in the Tengger Desert, China. Eur. J. Soil Biol. 2008, 44, 238–246. [Google Scholar] [CrossRef]
- Sylvain, Z.A.; Wall, D.H. Linking soil biodiversity and vegetation: Implications for a changing planet. Am. J. Bot. 2011, 98, 517–527. [Google Scholar] [CrossRef]
- Gammoudi, N.; Nagaz, K.; Ferchichi, A. Potential Use of Spent Coffee Grounds and Spent Tea Leaves Extracts in Priming Treatment to Promote In Vitro Early Growth of Salt-and Drought-Stressed Seedlings of Capsicum annuum L. Waste Biomass Valorization 2020, 12, 3341–3353. [Google Scholar] [CrossRef]
- Akhtar, M.; Malik, A. Roles of organic soil amendments and soil organisms in the biological control of plant-parasitic nematodes: A review. Bioresour. Technol. 2000, 74, 35–47. [Google Scholar] [CrossRef]
- Boutsis, G.; Stamou, G.; Argyropoulou, M. Short term effects of soil disinfection with metham sodium and organic alternatives on nematode communities. Community Ecol. 2011, 12, 161–170. [Google Scholar] [CrossRef]
- Monokrousos, N.; Argyropoulou, M.D.; Tzani, K.; Menkissoglou-Spiroudi, U.; Boutsis, G.; D’Addabbo, T.; Ntalli, N. The Effect of Botanicals with Nematicidal Activity on the Structural and Functional Characteristics of the Soil Nematode Community. Agriculture 2021, 11, 326. [Google Scholar] [CrossRef]
- Argyropoulou, M.D.; Karmezi, M.; Tsiafouli, M.; Chalkos, D.; Bountla, A.; Vokou, D. Soil Amendments with Spearmint, Peppermint and Rosemary Enhance the Community of Free-Living Nematodes and Improve Soil Quality, While Having Strikingly Different Effects on Plant Growth. Life 2022, 12, 1121. [Google Scholar] [CrossRef] [PubMed]
- Theofilidou, A.; Argyropoulou, M.D.; Ntalli, N.; Kekelis, P.; Mourouzidou, S.; Zafeiriou, I.; Tsiropoulos, N.G.; Monokrousos, N. Assessing the Role of Melia azedarach Botanical Nematicide in Enhancing the Structure of the Free-Living Nematode Community. Soil Syst. 2023, 7, 80. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T. Nematode indicators of organic enrichment. J. Nematol. 2006, 38, 3. [Google Scholar]
- Neher, D.A.; Olson, R.K. Nematode communities in soils of four farm cropping management systems. Pedobiologia 1999, 43, 430–438. [Google Scholar]
- Ferris, H.; Griffiths, B.S.; Porazinska, D.L.; Powers, T.O.; Wang, K.H.; Tenuta, M. Reflections on plant and soil nematode ecology: Past, present and future. J. Nematol. 2012, 44, 115–126. [Google Scholar]
- Gebremikael, M.T.; Steel, H.; Buchan, D.; Bert, W.; De Neve, S. Nematodes enhance plant growth and nutrient uptake under C and N-rich conditions. Sci. Rep. 2016, 6, 32862. [Google Scholar] [CrossRef]




| Variable | Variable | ||
|---|---|---|---|
| pH | 7.20 ± 0.9 | Mg (mg/g) | 1.60 ± 0.1 |
| C (%) | 45.00 ± 2.1 | Ca (mg/g) | 2.00 ± 0.2 |
| N (%) | 2.98 ± 0.2 | Na (mg/g) | 0.80 ± 0.0 |
| C/N | 15.10 ± 1.1 | Mn (ppm) | 0.06 ± 0.0 |
| K (mg/g) | 3.80 ± 0.4 | Cu (ppm) | 0.03 ± 0.0 |
| P (mg/g) | 11.10 ± 1.9 | Zn (ppm) | 0.02 ± 0.0 |
| Time | Treatment | MI | CI | EI | SI | PPI | MF |
|---|---|---|---|---|---|---|---|
| 3MAA | Control | 2.0 ± 0.0 b | 67.7 ± 11.3 ab | 51.4 ± 3.7 c | 16.0 ± 6.1 b | 3.0 ± 0.2 | 23.0 ± 5.5 c |
| CW-2% | 1.7 ± 0.1 c | 30.1 ± 8.1 cd | 71.7 ± 6.3 b | 11.8 ± 4.1 b | 2.9 ± 0.1 | 749.5 ± 109.1 bc | |
| CW-4% | 1.5 ± 0.1 d | 9.6 ± 1.4 d | 88.7 ± 1.7 a | 25.0 ± 8.7 b | 2.0 ± 1.2 | 1206.1 ± 48.4 ab | |
| CW-8% | 1.4 ± 0.1 d | 8.1 ± 2.4 d | 89.0 ± 3.6 a | 13.8 ± 4.5 b | 2.5 ± 1.4 | 2185.8 ± 1020.8 a | |
| 6MAA | Control | 1.9 ± 0.1 bc | 36.8 ± 6.1 c | 63.3 ± 4.2 bc | 23.5 ± 6.2 b | 2.9 ± 0.1 | 68.9 ± 5.3 c |
| CW-2% | 2.3 ± 0.0 a | 73.4 ± 4.5 a | 37.0 ± 2.7 d | 41.4 ± 2.9 a | 2.9 ± 0.1 | 72.8 ± 3.5 c | |
| CW-4% | 1.8 ± 0.0 bc | 28.6 ± 5.7 cd | 69.3 ± 3.5 b | 26.1 ± 5.4 b | 3.0 ± 1.1 | 55.3 ± 4.9 c | |
| CW-8% | 1.9 ± 0.1 bc | 46.4 ± 15.2 bc | 59.7 ± 7.0 bc | 17.7 ± 4.0 b | 3.0 ± 0.1 | 126.1 ± 30.0 c | |
| effect | Treatment | *** | *** | *** | ns | ns | * |
| Time | *** | ** | *** | * | ns | ** | |
| Tr*Time | ** | ** | *** | * | ns | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kekelis, P.; Argyropoulou, M.D.; Theofilidou, A.; Papatheodorou, E.M.; Aschonitis, V.; Monokrousos, N. The Differentiations in the Soil Nematode Community in an Agricultural Field after Soil Amendment Using Composted Coffee Waste in Various Concentrations. Agronomy 2023, 13, 2831. https://doi.org/10.3390/agronomy13112831
Kekelis P, Argyropoulou MD, Theofilidou A, Papatheodorou EM, Aschonitis V, Monokrousos N. The Differentiations in the Soil Nematode Community in an Agricultural Field after Soil Amendment Using Composted Coffee Waste in Various Concentrations. Agronomy. 2023; 13(11):2831. https://doi.org/10.3390/agronomy13112831
Chicago/Turabian StyleKekelis, Panagiotis, Maria D. Argyropoulou, Aphrodite Theofilidou, Effimia M. Papatheodorou, Vassilis Aschonitis, and Nikolaos Monokrousos. 2023. "The Differentiations in the Soil Nematode Community in an Agricultural Field after Soil Amendment Using Composted Coffee Waste in Various Concentrations" Agronomy 13, no. 11: 2831. https://doi.org/10.3390/agronomy13112831








