Twice-Split Phosphorus Application Alleviates Low Temperature Stress by Improving Root Physiology and Phosphorus Accumulation, Translocation, and Partitioning in Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Sampling and Measurement
2.3.1. Root Physiology
2.3.2. Dry Matter Accumulation, Translocation, and Partitioning in Wheat Plants
2.3.3. Phosphorus Accumulation, Translocation, and Partitioning in Wheat Plants
- (1)
- Wheat plant phosphorus accumulation = Plant dry matter weight × phosphorus content.
- (2)
- Phosphorus translocation before flowering stage (PT) = Vegetative organ phosphorus accumulation at flowering stage − Vegetative organ phosphorus accumulation at maturity stage.
- (3)
- Phosphorus translocation rate before flowering stage (PTR) = PT ÷ Vegetative organ phosphorus accumulation at flowering stage × 100%.
- (4)
- Phosphorus translocation contribution rate before flowering stage to grains (PTCG) = PT ÷ Grain phosphorus accumulation at maturity × 100%.
- (5)
- Phosphorus accumulation after flowering stage (PAAF) = Phosphorus accumulation at maturity − Phosphorus accumulation at flowering stage.
- (6)
- Phosphorus accumulation contribution rate after flowering stage to grains (PACG) = 100% − PTCG.
- (7)
- Phosphorus harvest index (PHI) = Grains phosphorus accumulation at maturity ÷ wheat plant phosphorus accumulation at maturity × 100%.
2.3.4. Yield and Its Components
2.4. Statistical Analysis
3. Results
3.1. Antioxidant Enzyme Activities and MDA Content in Root
3.2. Acid Phosphatase Activity, Root Activity, Soluble Sugar, and Soluble Protein Content in Root
3.3. Dry Matter Accumulation and Partitioning at the Flowering and Maturity Stages of Wheat Plants
3.4. Phosphorus Accumulation and Partitioning at Flowering and Maturity Stages of Wheat Plants
3.5. Dry Matter Translocation at Flowering and Maturity Stages of Wheat Plants
3.6. Phosphorus Translocation at Flowering and Maturity Stages of Wheat Plants
3.7. Yield and Its Components in Wheat
4. Discussion
4.1. Effects of Optimizing Phosphorus Application on Root Physiology
4.2. Effects of Optimizing Phosphorus Application on Dry Matter and Phosphorus Accumulation, Translocation, and Partitioning
4.3. Effects of Optimizing Phosphorus Application on Yield and Its Components
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, C.; Dawson, T.; Macdiarmid, J.; Matthews, R.; Smith, P. The impact of population growth and climate change on food security in Africa: Looking ahead to 2050. Int. J. Agric. Sustain. 2017, 15, 124–135. [Google Scholar] [CrossRef]
- Godber, O.F.; Wall, R. Livestock and food security: Vulnerability to population growth and climate change. Glob. Chang. Biol. 2014, 20, 3092–3102. [Google Scholar] [CrossRef]
- Erenstein, O.; Chamberlin, J.; Sonder, K. Estimating the global number and distribution of maize and wheat farms. Glob. Food Secur. 2021, 30, 100558. [Google Scholar] [CrossRef]
- Xu, H.; Hassan, M.A.; Sun, D.; Wu, Z.; Jiang, G.; Liu, B.; Ni, Q.; Yang, W.; Fang, H.; Li, J. Effects of low temperature stress on source–sink organs in wheat and phosphorus mitigation strategies. Front. Plant Sci. 2022, 13, 807844. [Google Scholar] [CrossRef]
- Liu, L.; Ji, H.; An, J.; Shi, K.; Ma, J.; Liu, B.; Tang, L.; Cao, W.; Zhu, Y. Response of biomass accumulation in wheat to low-temperature stress at jointing and booting stages. Environ. Exp. Bot. 2019, 157, 46–57. [Google Scholar] [CrossRef]
- Hu, X.; Ma, J.; Qian, W.; Cao, Y.; Zhang, Y.; Liu, B.; Tang, L.; Cao, W.; Zhu, Y.; Liu, L. Effects of low temperature on the amino acid composition of wheat grains. Agronomy 2022, 12, 1171. [Google Scholar] [CrossRef]
- Su, H.; Tan, C.; Liu, Y.; Chen, X.; Li, X.; Jones, A.; Zhu, Y.; Song, Y. Physiology and molecular breeding in sustaining wheat grain setting and quality under spring cold stress. Int. J. Mol. Sci. 2022, 23, 14099. [Google Scholar] [CrossRef]
- Bandara, A.Y.; Weerasooriya, D.K.; Bell, T.H.; Esker, P.D. Prospects of alleviating early planting-associated cold susceptibility of soybean using microbes: New insights from microbiome analysis. J. Agron. Crop Sci. 2021, 207, 171–185. [Google Scholar] [CrossRef]
- Mohammadi, R.; Sadeghzadeh, B.; Ahmadi, H.; Bahrami, N.; Amri, A. Field evaluation of durum wheat landraces for prevailing abiotic and biotic stresses in highland rainfed regions of Iran. Crop J. 2015, 3, 423–433. [Google Scholar] [CrossRef]
- Xu, H.; Hou, K.; Fang, H.; Liu, Q.; Wu, Q.; Lin, F.; Deng, R.; Zhang, L.; Chen, X.; Li, J. Twice-split application of phosphorus alleviates low temperature impacts on wheat by greater spikelet development and setting. J. Integr. Agric. [CrossRef]
- Jiang, G.; Hassan, M.A.; Muhammad, N.; Arshad, M.; Chen, X.; Xu, Y.; Xu, H.; Ni, Q.; Liu, B.; Yang, W. Comparative physiology and transcriptome analysis of young spikes in response to late spring coldness in wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 13, 811884. [Google Scholar] [CrossRef] [PubMed]
- Equiza, M.A.; Miravé, J.P.; Tognetti, J.A. Morphological, anatomical and physiological responses related to differential shoot vs. root growth inhibition at low temperature in spring and winter wheat. Ann. Bot. 2001, 87, 67–76. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Rasheed, R.; Hussain, I.; Iqbal, M.; Farooq, M.U.; Saleem, M.H.; Ali, S. Taurine modulates dynamics of oxidative defense, secondary metabolism, and nutrient relation to mitigate boron and chromium toxicity in Triticum aestivum L. plants. Environ. Sci. Pollut. R. 2022, 29, 45527–45548. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef]
- Hadid, M.L.; Ramadan, K.M.; El-Beltagi, H.S.; Ramadan, A.A.; El-Metwally, I.M.; Shalaby, T.A.; Bendary, E.S.; Alwutayd, K.M.; Saudy, H.S. Modulating the antioxidant defense systems and nutrients content by proline for higher yielding of wheat under water deficit. Not. Bot. Horti Agrobo. 2023, 51, 13291. [Google Scholar] [CrossRef]
- Antoniou, C.; Savvides, A.; Christou, A.; Fotopoulos, V. Unravelling chemical priming machinery in plants: The role of reactive oxygen–nitrogen–sulfur species in abiotic stress tolerance enhancement. Curr. Opin. Plant. Biol. 2016, 33, 101–107. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant. Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Iqbal, M.; Tang, Y.; Khan, S.; Guan, D.; Li, G. Phosphorus mobilization in plant–soil environments and inspired strategies for managing phosphorus: A review. Agronomy 2022, 12, 2539. [Google Scholar] [CrossRef]
- Nedelciu, C.E.; Ragnarsdottir, K.V.; Schlyter, P.; Stjernquist, I. Global phosphorus supply chain dynamics: Assessing regional impact to 2050. Glob. Food Secur. 2020, 26, 100426. [Google Scholar] [CrossRef]
- Soetan, K.; Olaiya, C.; Oyewole, O. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Gahoonia, T.S.; Nielsen, N.E.; Lyshede, O.B. Phosphorus (P) acquisition of cereal cultivars in the field at three levels of P fertilization. Plant Soil 1999, 211, 269–281. [Google Scholar] [CrossRef]
- Shaaban, A.; El-Mageed, T.A.A.; El-Momen, W.R.A.; Saudy, H.S.; Al-Elwany, O.A. The integrated application of phosphorous and zinc affects the physiological status, yield and quality of canola grown in phosphorus-suffered deficiency saline soil. Gesunde Pflanz. 2023, 75, 1813–1821. [Google Scholar] [CrossRef]
- Saudy, H.; Noureldin, N.; Mubarak, M.; Fares, W.; Elsayed, M. Cultivar selection as a tool for managing soil phosphorus and faba bean yield sustainability. Arch. Agron. Soil. Sci. 2019, 66, 414–425. [Google Scholar] [CrossRef]
- El Mazlouzi, M.; Morel, C.; Robert, T.; Yan, B.; Mollier, A. Phosphorus uptake and partitioning in two durum wheat cultivars with contrasting biomass allocation as affected by different P supply during grain filling. Plant Soil 2020, 449, 179–192. [Google Scholar] [CrossRef]
- Rafiullah, M.J.; Dost, M.; Shah, F. Phosphorus nutrient management through synchronization of application methods and rates in wheat and maize crops. Plants 2020, 9, 1389. [Google Scholar] [CrossRef]
- Xu, H.; Wu, Z.; Xu, B.; Sun, D.; Hassan, M.A.; Cai, H.; Wu, Y.; Yu, M.; Chen, A.; Li, J. Optimized phosphorus application alleviated adverse effects of short-term low-temperature stress in winter wheat by enhancing photosynthesis and improved accumulation and partitioning of dry matter. Agronomy 2022, 12, 1700. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Ashraf, M. Role of mineral nutrition in alleviation of drought stress in plants. Aust. J. Crop Sci. 2011, 5, 764–777. [Google Scholar]
- Li, J.; Li, Z.; Li, X.; Tang, X.; Liu, H.; Li, J.; Song, Y. Effects of spraying KH2PO4 on flag leaf physiological characteristics and grain yield and quality under heat stress during the filling period in winter wheat. Plants 2023, 12, 1801. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, Z.; Man, J.; Ma, S.; Gao, Z.; Zhang, Y. Tillage practices affect dry matter accumulation and grain yield in winter wheat in the North China Plain. Soil Tillage Res. 2016, 160, 73–81. [Google Scholar] [CrossRef]
- Jiang, B.; Shen, J.; Sun, M.; Hu, Y.; Jiang, W.; Wang, J.; Li, Y.; Wu, J. Soil phosphorus availability and rice phosphorus uptake in paddy fields under various agronomic practices. Pedosphere 2021, 31, 103–115. [Google Scholar] [CrossRef]
- Tian, F.; Nie, J.; Zhou, Z.; Gu, A.; Zheng, Z.; Yang, T.; Huang, Y.; Yin, D.; Wang, Z. Effects of combined application of biochar and relatively low-level chemical fertilizer on dry matter, yield, accumulation and transport of nitrogen, phosphorus and potassium in maize. J. Maize Sci. 2021, 29, 158–165. (In Chinese) [Google Scholar]
- Maqbool, S.; Hassan, M.A.; Xia, X.; York, L.M.; Rasheed, A.; He, Z. Root system architecture in cereals: Progress, challenges and perspective. Plant J. 2022, 110, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Lu, L.; Li, J.; Du, Z.; Liu, T.; Li, W.; Xu, F.; Shi, L.; Shou, H.; Wang, C. Purple acid phosphatase 10c encodes a major acid phosphatase that regulates plant growth under phosphate-deficient conditions in rice. J. Exp. Bot. 2020, 71, 4321–4332. [Google Scholar] [CrossRef] [PubMed]
- Dodor, D.E.; Tabatabai, M.A. Effect of cropping systems on phosphatases in soils. J. Plant Nutr. Soil Sci. 2003, 166, 7–13. [Google Scholar] [CrossRef]
- Li, G.; Fu, P.; Cheng, G.; Lu, W.; Lu, D. Delaying application time of slow-release fertilizer increases soil rhizosphere nitrogen content, root activity, and grain yield of spring maize. Crop J. 2022, 10, 1798–1806. [Google Scholar] [CrossRef]
- Kazemi-Shahandashti, S.-S.; Maali-Amiri, R.; Zeinali, H.; Khazaei, M.; Talei, A.; Ramezanpour, S.-S. Effect of short-term cold stress on oxidative damage and transcript accumulation of defense-related genes in chickpea seedlings. J. Plant Physiol. 2014, 171, 1106–1116. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.; Arora, S. Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J. Plant Growth Regul. 2013, 32, 216–232. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Liu, B.; Liu, L.; Asseng, S.; Zou, X.; Li, J.; Cao, W.; Zhu, Y. Modelling the effects of heat stress on post-heading durations in wheat: A comparison of temperature response routines. Agric. For. Meteorol. 2016, 222, 45–58. [Google Scholar] [CrossRef]
- Liu, L.; Miao, Q.; Wang, H.; Xue, Y.; Qi, S.; Zhang, J.; Li, J.; Meng, Q.; Cui, Z. Optimizing phosphorus application for winter wheat production in the coastal saline area. Agronomy 2022, 12, 2966. [Google Scholar] [CrossRef]
- Li, C.; Liu, M.; Dai, C.; Zhu, Y.; Zhu, M.; Ding, J.; Zhu, X.; Zhou, G.; Guo, W. Morphology and nitrogen uptake and distribution of wheat plants as influenced by applying remedial urea prior to or post low-temperature stress at seedling stage. Agronomy 2022, 12, 2338. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.F.; Wu, W.H. Potassium and phosphorus transport and signaling in plants. J. Integr. Plant Biol. 2021, 63, 34–52. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.R.; Shane, M.W.; White, P.J. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef] [PubMed]
- El Mazlouzi, M.; Morel, C.; Chesseron, C.; Robert, T.; Mollier, A. Contribution of external and internal phosphorus sources to grain P loading in durum wheat (Triticum durum L.) grown under contrasting P levels. Front. Plant Sci. 2020, 11, 870. [Google Scholar] [CrossRef]
- Han, Y.; White, P.J.; Cheng, L. Mechanisms for improving phosphorus utilization efficiency in plants. Ann. Bot. 2022, 129, 247–258. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef]
- Saudy, H.S.; El–Metwally, I.M. Nutrient utilization indices of NPK and drought management in groundnut under sandy soil conditions. Commun. Soil Sci. Plan. 2019, 50, 1821–1828. [Google Scholar] [CrossRef]
- Roberts, T.L.; Johnston, A.E. Phosphorus use efficiency and management in agriculture. Resour. Conserv. Recycl. 2015, 105, 275–281. [Google Scholar] [CrossRef]
- Schröder, J.; Smit, A.; Cordell, D.; Rosemarin, A. Improved phosphorus use efficiency in agriculture: A key requirement for its sustainable use. Chemosphere 2011, 84, 822–831. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, W.; Chen, D.; Cai, A.; Ao, J.; Huang, Z. Optimizing soil and fertilizer phosphorus management according to the yield response and phosphorus use efficiency of sugarcane in southern China. J. Soil Sci. Plant Nutr. 2020, 20, 1655–1664. [Google Scholar] [CrossRef]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold stress effects on reproductive development in grain crops: An overview. Environ. Exp. Bot. 2010, 67, 429–443. [Google Scholar] [CrossRef]
- Ji, H.; Xiao, L.; Xia, Y.; Song, H.; Liu, B.; Tang, L.; Cao, W.; Zhu, Y.; Liu, L. Effects of jointing and booting low temperature stresses on grain yield and yield components in wheat. Agric. For. Meteorol. 2017, 243, 33–42. [Google Scholar] [CrossRef]
- Liu, L.; Song, H.; Shi, K.; Liu, B.; Zhang, Y.; Tang, L.; Cao, W.; Zhu, Y. Response of wheat grain quality to low temperature during jointing and booting stages—On the importance of considering canopy temperature. Agric. For. Meteorol. 2019, 278, 107658. [Google Scholar] [CrossRef]
- Li, X.; Pu, H.; Liu, F.; Zhou, Q.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Winter wheat photosynthesis and grain yield responses to spring freeze. Agron. J. 2015, 107, 1002–1010. [Google Scholar] [CrossRef]
- Han, Q.; Kang, G.; Guo, T. Proteomic analysis of spring freeze-stress responsive proteins in leaves of bread wheat (Triticum aestivum L.). Plant Physiol. Bioch. 2013, 63, 236–244. [Google Scholar] [CrossRef]
- Li, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Jiang, D. Cold priming drives the sub-cellular antioxidant systems to protect photosynthetic electron transport against subsequent low temperature stress in winter wheat. Plant Physiol. Biochem. 2014, 82, 34–43. [Google Scholar] [CrossRef]
- Liu, L.; Xia, Y.; Liu, B.; Chang, C.; Xiao, L.; Shen, J.; Tang, L.; Cao, W.; Zhu, Y. Individual and combined effects of jointing and booting low-temperature stress on wheat yield. Eur. J. Agron. 2020, 113, 125989. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Liu, L.; Li, S.; Xie, J.; Xue, X.; Jiang, Y. Screening of phosphate-solubilizing bacteria and their abilities of phosphorus solubilization and wheat growth promotion. BMC Microbiol. 2022, 22, 296. [Google Scholar] [CrossRef]
- Rezakhani, L.; Motesharezadeh, B.; Tehrani, M.M.; Etesami, H.; Hosseini, H.M. Phosphate–solubilizing bacteria and silicon synergistically augment phosphorus (P) uptake by wheat (Triticum aestivum L.) plant fertilized with soluble or insoluble P source. Ecotox. Environ. Saf. 2019, 173, 504–513. [Google Scholar] [CrossRef] [PubMed]
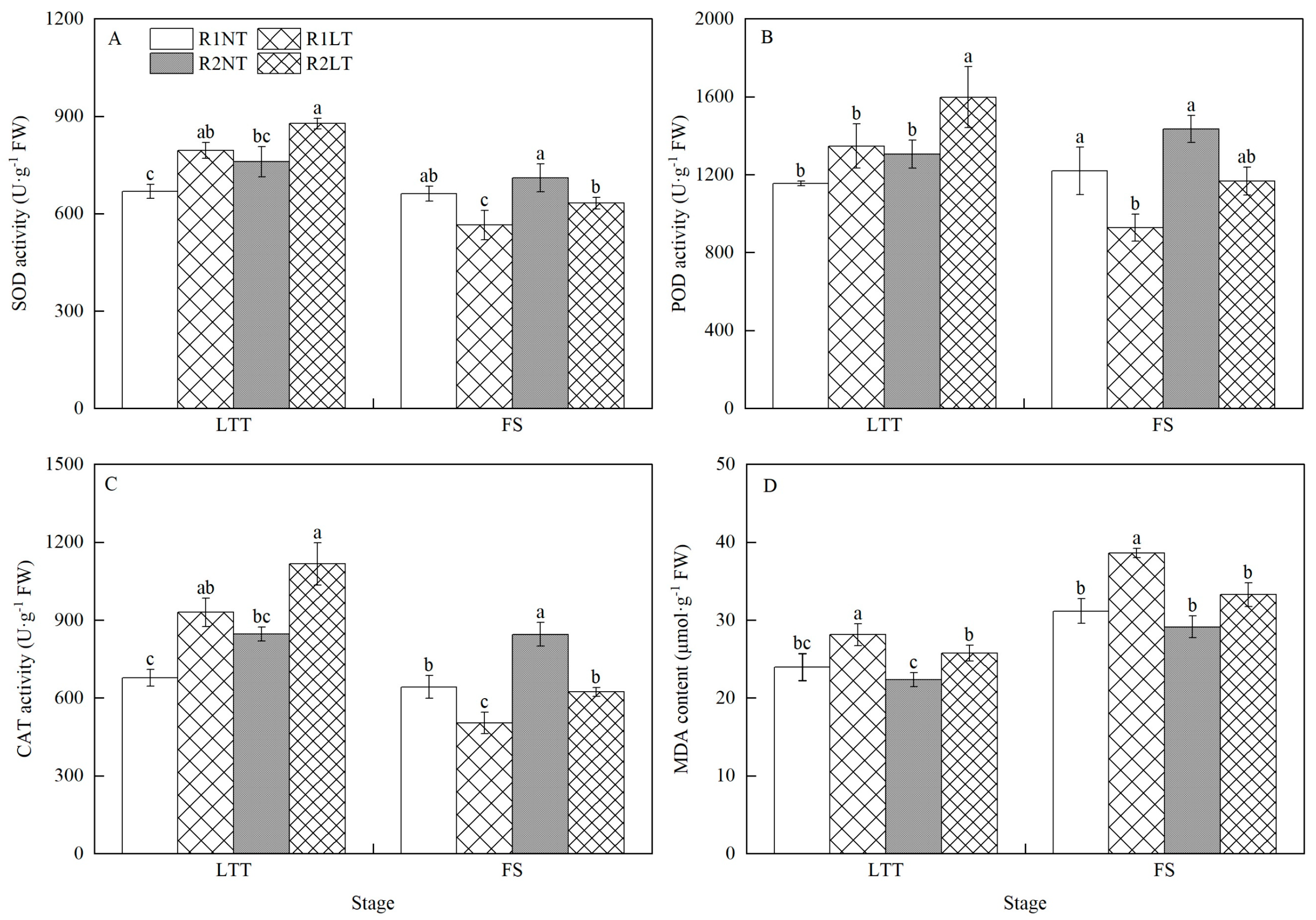



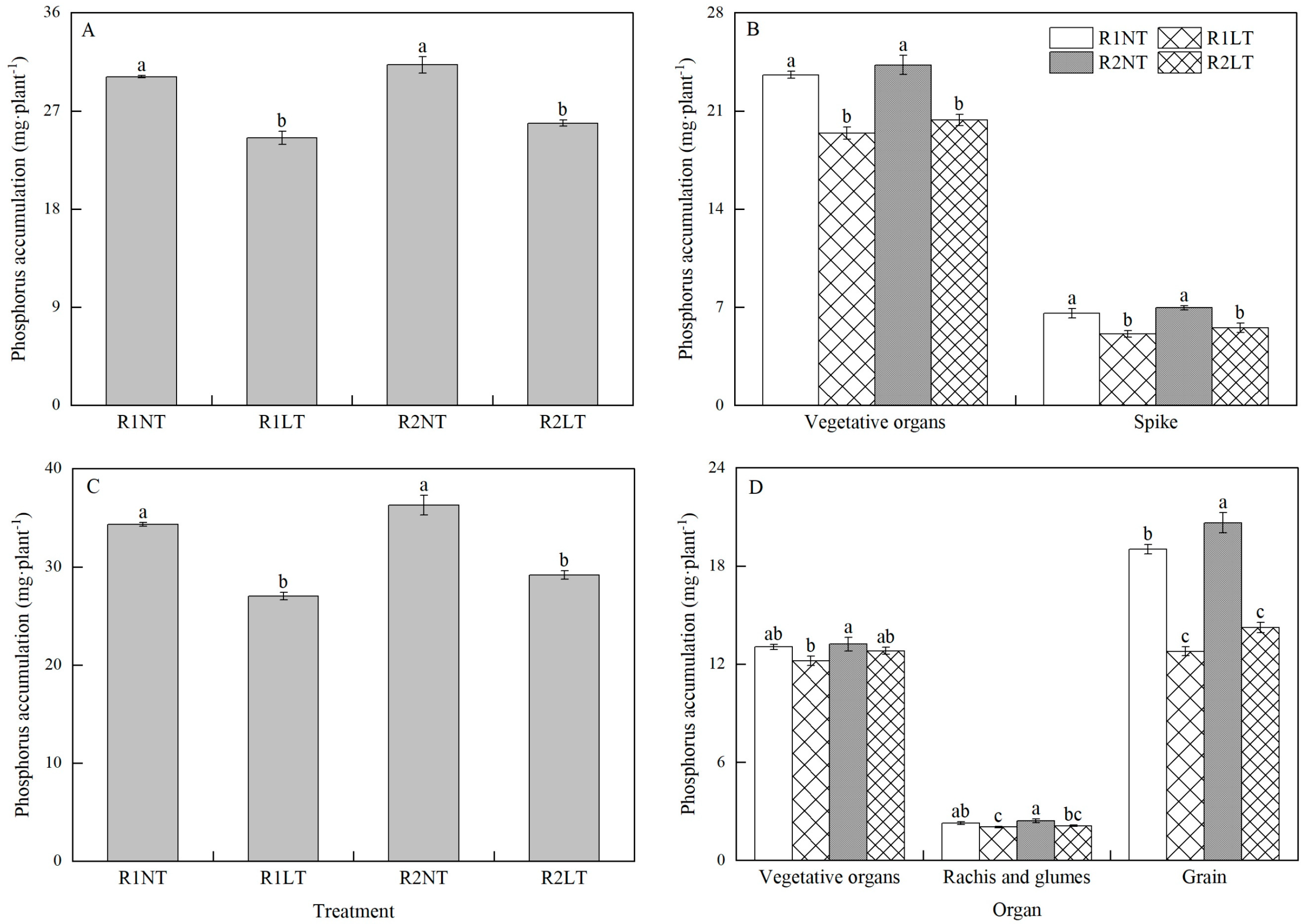
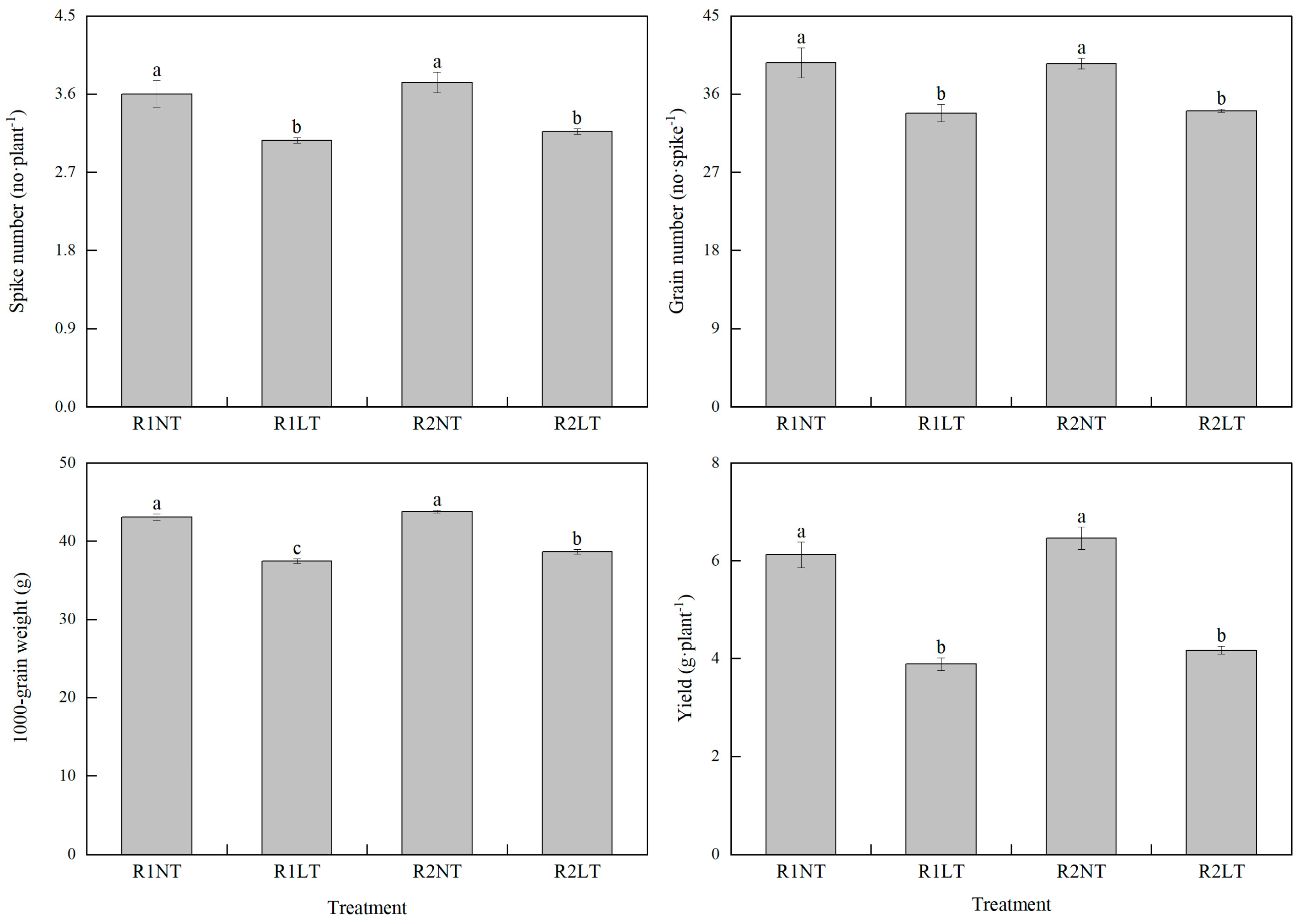
| Treatment | Dry Matter before Flowering Stage | Dry Matter after Flowering Stage | |||
|---|---|---|---|---|---|
| DMT (g·Plant−1) | PDMT (%) | CDMT (%) | DMAF (g·plant−1) | CDMAF (%) | |
| R1NT | 2.16 ± 0.11 a | 27.0 ± 0.9 a | 34.6 ± 1.5 a | 4.07 ± 0.07 a | 65.4 ± 1.5 a |
| R1LT | 1.42 ± 0.06 b | 25.5 ± 0.4 a | 37.4 ± 1.6 a | 2.37 ± 0.09 b | 62.6 ± 1.6 a |
| R2NT | 2.04 ± 0.09 a | 25.4 ± 1.0 a | 32.1 ± 1.6 a | 4.32 ± 0.15 a | 67.9 ± 1.6 a |
| R2LT | 1.41 ± 0.05 b | 24.3 ± 0.7 a | 35.5 ± 1.5 a | 2.56 ± 0.10 b | 64.5 ± 1.5 a |
| Treatment | Phosphorus before Flowering Stage | Phosphorus after Flowering Stage | PHI (%) | |||
|---|---|---|---|---|---|---|
| PT (mg·Plant−1) | PTR (%) | PTCG (%) | PAAF (mg·Plant−1) | PACG (%) | ||
| R1NT | 10.53 ± 0.32 a | 44.7 ± 1.0 a | 55.4 ± 1.1 a | 4.20 ± 0.30 ab | 44.6 ± 1.1 a | 55.4 ± 0.7 a |
| R1LT | 7.23 ± 0.54 b | 37.2 ± 2.1 b | 56.5 ± 3.2 a | 2.49 ± 0.32 c | 43.5 ± 3.2 a | 47.3 ± 0.8 b |
| R2NT | 11.05 ± 0.51 a | 45.5 ± 1.3 a | 53.5 ± 1.2 a | 5.07 ± 0.41 a | 46.5 ± 1.2 a | 56.9 ± 0.8 a |
| R2LT | 7.53 ± 0.20 b | 37.0 ± 0.3 b | 52.9 ± 1.2 a | 3.28 ± 0.22 bc | 47.1 ± 1.2 a | 48.8 ± 0.5 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Hassan, M.A.; Li, J. Twice-Split Phosphorus Application Alleviates Low Temperature Stress by Improving Root Physiology and Phosphorus Accumulation, Translocation, and Partitioning in Wheat. Agronomy 2023, 13, 2643. https://doi.org/10.3390/agronomy13102643
Xu H, Hassan MA, Li J. Twice-Split Phosphorus Application Alleviates Low Temperature Stress by Improving Root Physiology and Phosphorus Accumulation, Translocation, and Partitioning in Wheat. Agronomy. 2023; 13(10):2643. https://doi.org/10.3390/agronomy13102643
Chicago/Turabian StyleXu, Hui, Muhammad Ahmad Hassan, and Jincai Li. 2023. "Twice-Split Phosphorus Application Alleviates Low Temperature Stress by Improving Root Physiology and Phosphorus Accumulation, Translocation, and Partitioning in Wheat" Agronomy 13, no. 10: 2643. https://doi.org/10.3390/agronomy13102643






