The Effect of Biofumigation on the Microbiome Composition in Replanted Soil in a Fruit Tree Nursery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Soil Analyses
2.2.1. Identification of Soil Microorganisms—DNA Extraction
2.2.2. PCR Amplification
2.3. Statistical and Bioinformatics Analyses
3. Results and Discussion
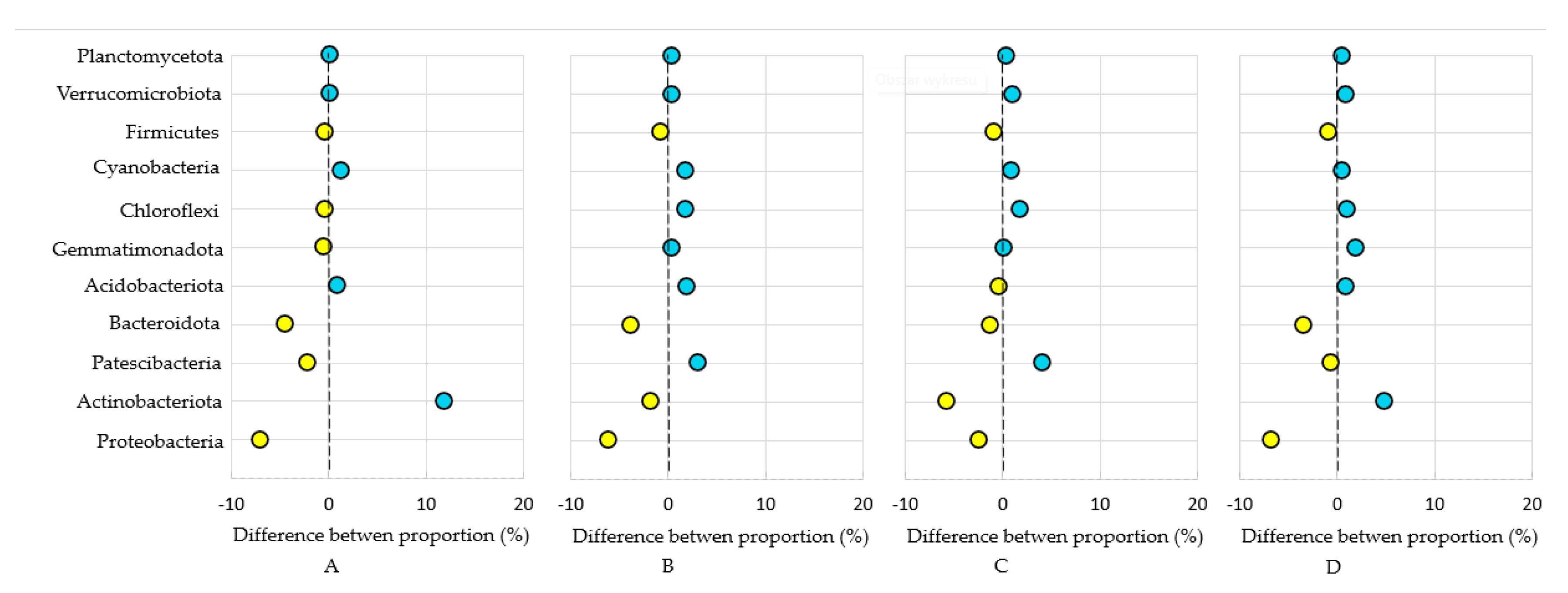
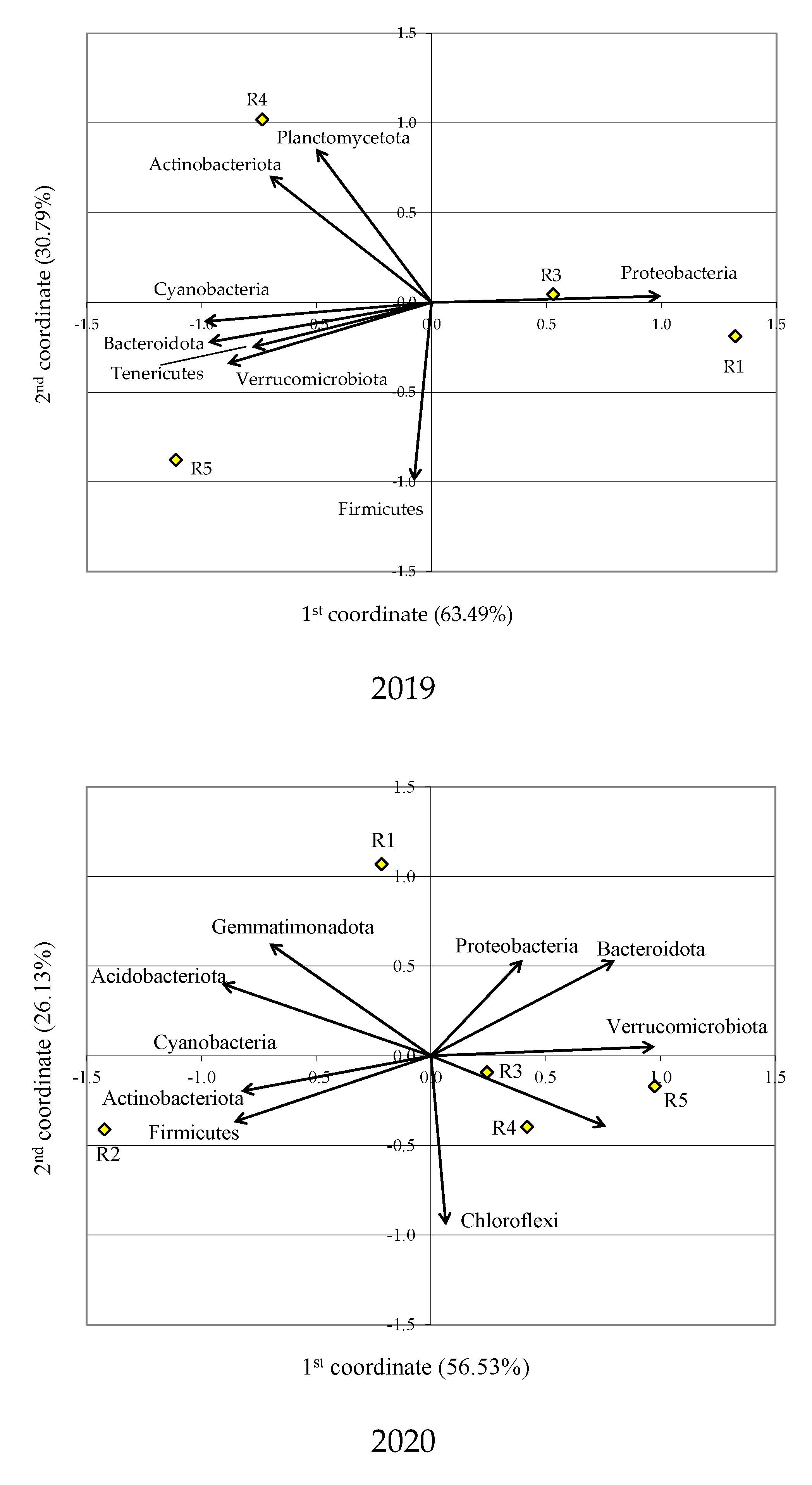
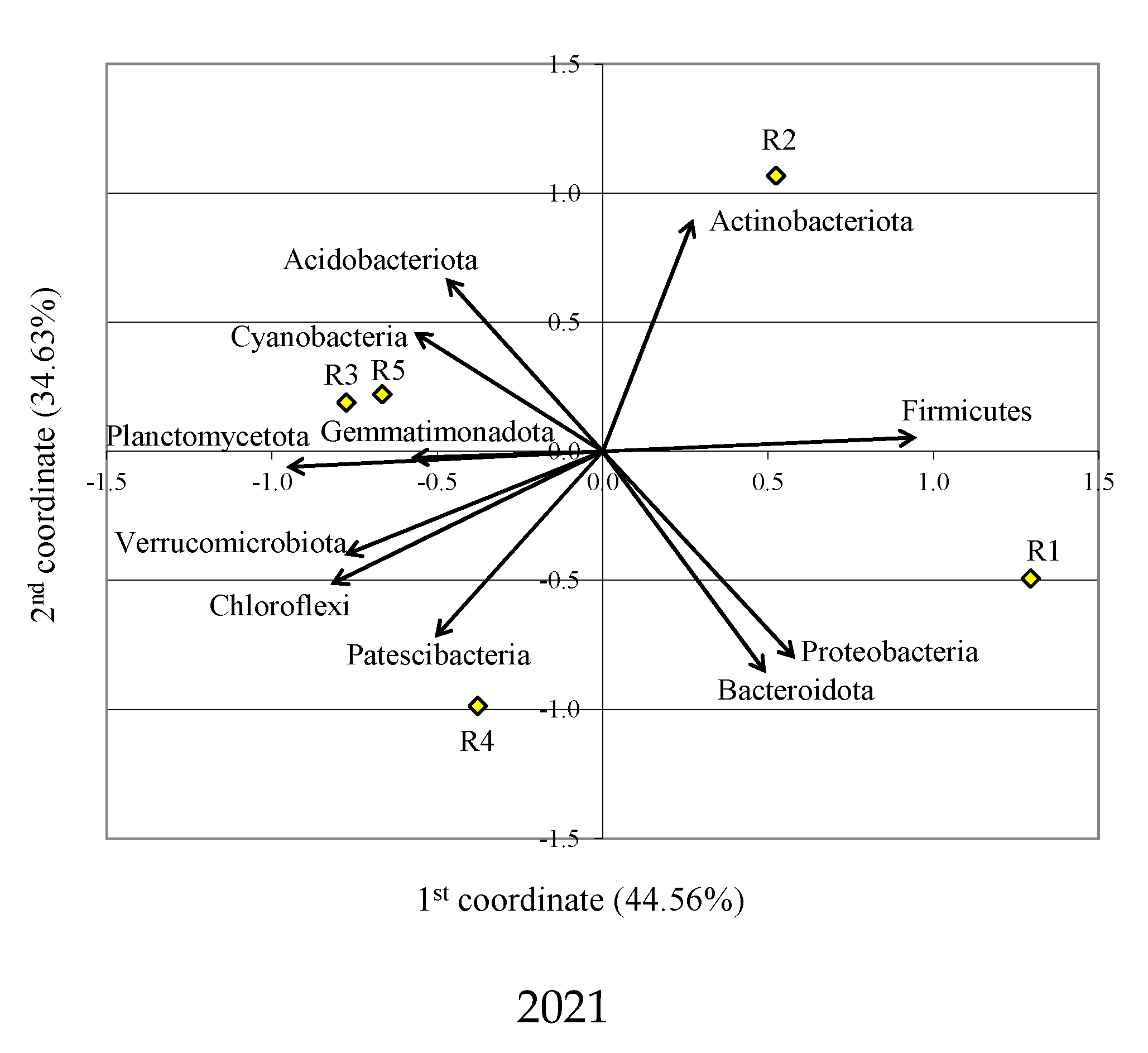
3.1. Bacterial Phyla
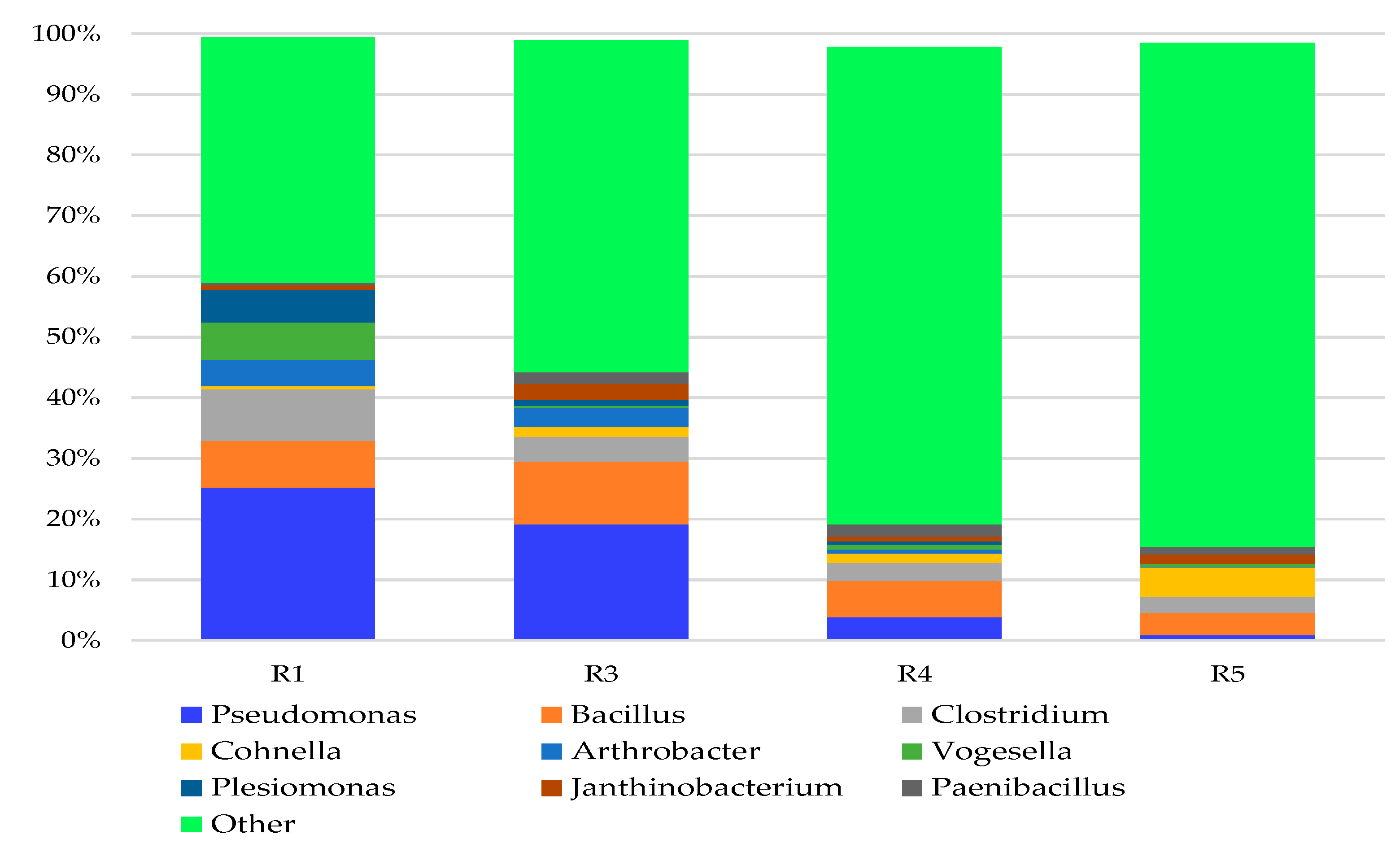
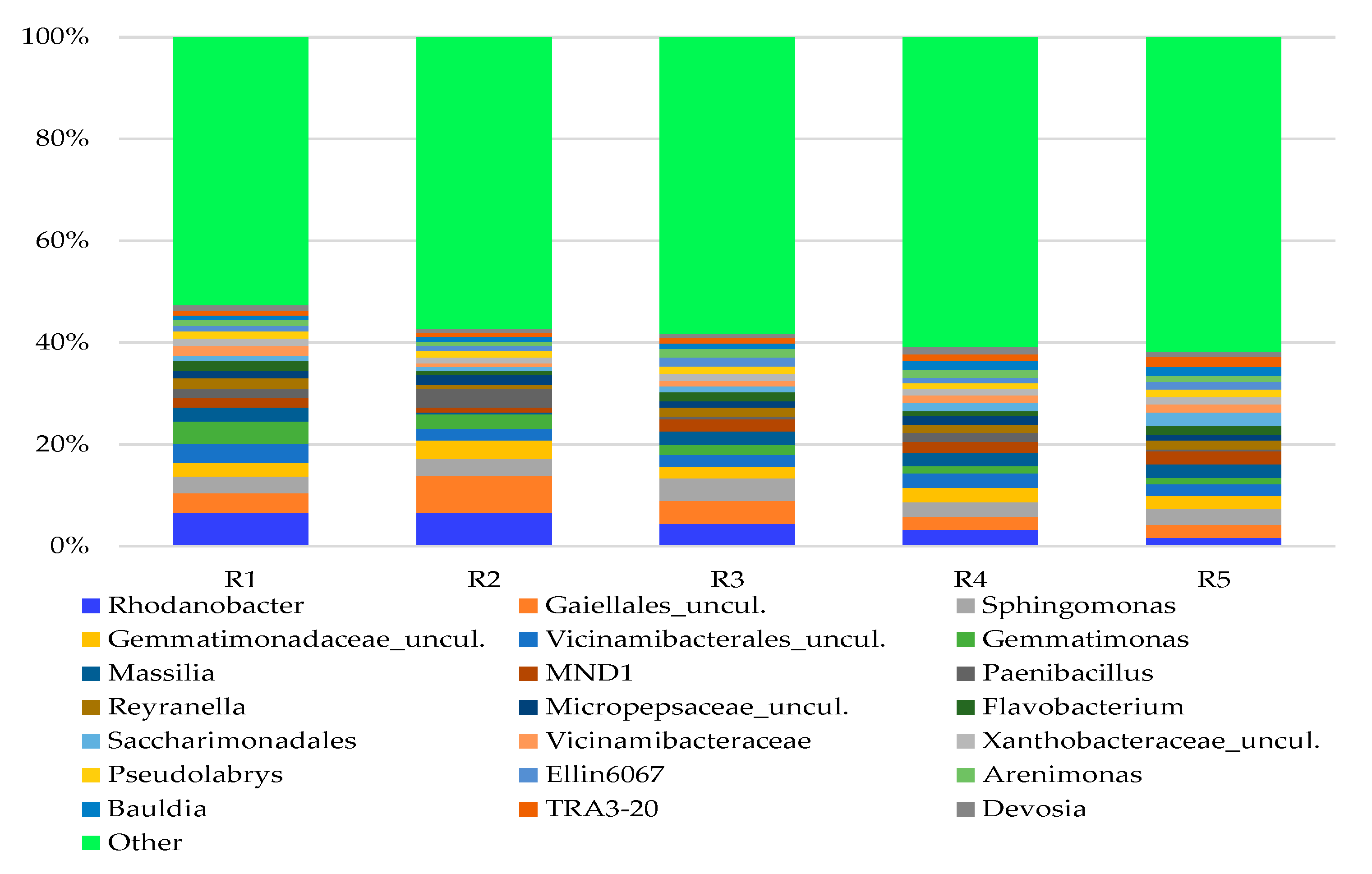
3.2. Bacterial Genera
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Winkelmann, T.; Smalla, K.; Amelung, W.; Baab, G.; Grunewaldt-Stöcker, G.; Kanfra, X.; Meyhöfer, R.; Reim, S.; Schmitz, M.; Vetterlein, D. Apple replant disease: Causes and Mitigation Strategies. Curr. Issues Mol. Biol. 2019, 30, 89–106. [Google Scholar] [CrossRef]
- Mazzola, M.; Manici, L.M. Apple replant disease: Role of microbial ecology in cause and control. Annu. Rev. Phytopathol. 2012, 50, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Volk, G.M.; Chao, C.T.; Norelli, J.; Brown, S.K.; Fazio, G.; Peace, C.; Mc Ferson, J.; Zhong, G.Y.; Bretting, P. The vulnerability of US apple (Malus) genetic resources. Genet. Resour. Crop Evol. 2015, 62, 765–794. [Google Scholar] [CrossRef]
- Israel, D.W.; Giddens, J.E.; Powell, W.W. The toxicity of peach tree roots. Plant Soil 1973, 39, 103–112. [Google Scholar] [CrossRef]
- Benizri, E.; Piutti, S.; Verger, S.; Pagès, L.; Vercambre, G.; Poessel, J.L.; Michelot, P. Replant diseases: Bacterial community structure and diversity in peach rhizosphere as determined by metabolic and genetic fingerprinting. Soil Biol. Biochem. 2005, 37, 1738–1746. [Google Scholar] [CrossRef]
- Mai, W.F.; Abawi, G.S. Determining the cause and extent of apple, cherry and pear replant diseases under controlled conditions. Phytopathology 1978, 68, 1540–1544. [Google Scholar] [CrossRef]
- Reginato, G.; Córdova, C.; Mauro, C. Evaluation of rootstock and management practices to avoid cherry replant diesease in Chile. Acta Hortic. 2008, 795, 357–362. [Google Scholar] [CrossRef]
- Spethmann, W.; Otto, G. Replant Problems and Soil Sickness. In Encyclopedia of Rose Science; Roberts, A.V., Debener, T., Gudin, S., Eds.; Elsevier: Oxford, UK, 2003; pp. 169–180. [Google Scholar] [CrossRef]
- Baumann, A.; Yim, B.; Grunewaldt-Stöcker, G.; Liu, B.; Beerhues, L.; Sapp, M.; Nesme, J.; Sørensen, S.J.; Smalla, K.; Winkelmann, T. Rose replant disease: Detailed analyses of plant reactions, root endophytes and rhizosphere microbial communities. Acta Hortic. 2020, 1283, 97–104. [Google Scholar] [CrossRef]
- Deal, D.; Mail, W.; Boothroyd, C. A survey of biotic relationships in grape replant situations. Phytopathology 1972, 62, 503–507. [Google Scholar] [CrossRef]
- Westphal, A.; Browne, G.T.; Schneider, S. Evidence for biological nature of the grape replant problem in California. Plant Soil 2002, 242, 197–203. Available online: https://www.jstor.org/stable/24122574 (accessed on 20 February 2010). [CrossRef]
- Elmer, W. Asparagus decline and replant problem: A look back and a look forward at strategies for mitigating losses. Acta Hortic. 2018, 1223, 195–204. [Google Scholar] [CrossRef]
- Wu, L.; Wang, J.; Huang, W.; Wu, H.; Chen, J.; Yang, Y.; Zhang, Z.; Lin, W. Plant-microbe rhizosphere interactions mediated by Rehmannia glutinosa root exudates under consecutive monoculture. Sci. Rep. 2015, 5, 15871. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Lin, Y.M.; Hong, T.; Wu, C.Z.; Li, J. Soil sickness in horticulture and forestry: A review. Allelopathy J. 2019, 47, 57–72. [Google Scholar] [CrossRef]
- Mai, W.F.; Merwin, I.A.; Abawi, G.S. Diagnosis, etiology and management of replant disorders in New York cherry and apple orchards. Acta Hortic. 1994, 363, 33–41. [Google Scholar] [CrossRef]
- Mai, W.F.; Abawi, G.S. Controlling replant diseases of pome and stone fruits in Northeastern United States by preplant fumigation. Plant Dis. 1981, 65, 859–864. [Google Scholar] [CrossRef]
- Weller, D.M.; Raaijmakers, J.M.; Gardener, B.B.M.; Thomashow, L.S. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 2002, 40, 309–348. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.S.; Merwin, I.A.; Thies, J.E. Long-term orchard groundcover management systems affect soil microbial communities and apple replant disease severity. Plant Soil 2008, 304, 209–225. [Google Scholar] [CrossRef]
- Manici, L.; Kelderer, M.; Franke-Whittle, I.; Rühmer, T.; Baab, G.; Nicoletti, F.; Caputo, F.; Topp, A.; Insam, H.; Naef, A. Relationship between root-endophytic microbial communities and replant disease in specialized apple growing areas in Europe. Appl. Soil Ecol. 2013, 72, 207–214. [Google Scholar] [CrossRef]
- Atucha, A.; Emmett, B.; Bauerle, T.L. Growth rate of fine root systems influences rootstock tolerance to replant disease. Plant Soil 2014, 376, 337–346. [Google Scholar] [CrossRef]
- Yin, C.; Xiang, L.; Wang, G.; Wang, Y.; Shen, X.; Chen, X.; Mao, Z. How to plant apple trees to reduce replant disease in apple orchard: A study on the phenolic acid of the replanted apple orchard. PLoS ONE 2016, 11, e0167347. [Google Scholar] [CrossRef]
- Sobiczewski, P.; Treder, W.; Bryk, H.; Klamkowski, K.; Krzewinska, D.; Mikicinski, A.; Berczynski, S.; Tryngiel-Gac, A. The impact of phytosanitary treatments in the soil with signs of fatigue on the growth of apple seedlings and populations of bacteria and fungi. Pol. J. Agron. 2018, 34, 11–22. [Google Scholar] [CrossRef]
- Grunewaldt-Stöcker, G.; Mahnkopp, F.; Popp, C.; Maiss, E.; Winkelmann, T. Diagnosis of apple replant disease (ARD): Microscopic evidence of early symptoms in fine roots of different apple rootstock genotypes. Sci. Hortic. 2019, 243, 583–594. [Google Scholar] [CrossRef]
- Liu, E.T.; Wang, G.S.; Li, Y.Y.; Shen, X.; Chen, X.S.; Song, F.H.; Wu, S.J.; Che, Q.; Mao, Z.Q. Replanting affects the tree growth and fruit quality of Gala apple. J. Integr. Agric. 2014, 13, 1699–1706. [Google Scholar] [CrossRef]
- Henfrey, J.L.; Baab, G.; Schmitz, M. Physiological stress responses in apple under replant conditions. Sci. Hortic. 2015, 194, 111–117. [Google Scholar] [CrossRef]
- Lucas, M.; Balb´ın-Suarez, A.; Smalla, K.; Vetterlein, D. Root growth, function and rhizosphere microbiome analyses show local rather than systemic effects in apple plant response to replant disease soil. PLoS ONE 2018, 13, e0204922. [Google Scholar] [CrossRef]
- Nicola, L.; Insam, H.; Pertot, I.; Stres, B. Reanalysis of microbiomes in soils affected by apple replant disease (ARD): Old foes and novel suspects lead to the proposal of extended model of disease development. Appl. Soil Ecol. 2018, 129, 24–33. [Google Scholar] [CrossRef]
- Balb’ın-Suarez, A.; Lucas, M.; Vetterlein, D.; Sørensen, S.; Winkelmann, S.; Smalla, K.; Jacquiod, S. Exploring prokaryotic determinants of apple replant disease (ARD): A microhabitat approach under split-root design. FEMS Microbiol. Ecol. 2020, 96, fiaa211. [Google Scholar] [CrossRef]
- Manici, L.M.; Ciavatta, C.; Kelderer, M.; Erschbaumer, G. Replant problems in South Tyrol: Role of fungal pathogens and microbial population in conventional and organic apple orchards. J. Plant Soil 2003, 256, 315–324. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, W.; Chen, R.; Wang, H.; Duan, Y.; Chen, X.; Shen, X.; Yin, C.; Mao, Z. Quicklime and Superphosphate Alleviating Apple Replant Disease by Improving Acidified Soil. ACS Omega 2022, 7, 7920–7930. [Google Scholar] [CrossRef]
- Spath, M.; Insam, H.; Peintner, U.; Kelderer, M.; Kuhnert, R.; Franke-Whittle, I.H. Linking soil biotic and abiotic factors to apple replant disease: A greenhouse approach. J. Phytopathol. 2015, 163, 287–299. [Google Scholar] [CrossRef]
- Tilston, E.L.; Deakin, G.; Bennett, J.; Passey, T.; Harrison, N.B.; O’Brien, F.; Fernandez, F.; Xu, X.M. Candidate causal organisms for apple replant disease in the UK. Phytobiomes J. 2018, 2, 261–274. [Google Scholar] [CrossRef]
- Yin, C.; Duan, Y.N.; Xiang, L.; Wang, G.; Zhang, X.; Shen, X.; Chen, X.; Zhang, M.; Mao, Z. Effects of phloridzin, phloretin and benzoic acid at the concentrations measured in soil on the root proteome of Malus hupehensis Rehd Seedlings. Scientia Hort. 2018, 228, 10–17. [Google Scholar] [CrossRef]
- Cabos, R.Y.M.; Hara, A.H.; Tsang, M.M.C. Hot water drench treatment for control of reniform nematodes in potted dracaena. Nematropica 2012, 42, 72–79. [Google Scholar]
- Yim, B.; Winkelmann, T.; Ding, G.-C.; Smalla, K. Different bacterial communities in heat and gamma irradiation treated replant disease soils revealed by 16S rRNA gene analysis—Contribution to improved aboveground apple plant growth? Front. Microbiol. 2015, 6, 1224. [Google Scholar] [CrossRef] [PubMed]
- Nicola, L.; Turco, E.; Albanese, D.; Donati, C.; Thalheimer, M.; Pindo, M.; Insam, H.; Cavalieri, D.; Pertot, I. Fumigation with dazomet modifies soil microbiota in apple orchards affected by replant disease. Appl. Soil Ecol. 2017, 113, 71–79. [Google Scholar] [CrossRef]
- Nyoni, M.; Mazzola, M.; Wessels, J.P.B.; McLeod, A. The efficacy of semiselective chemicals and chloropicrin/1,3-dichloropropene–containing fumigants in managing apple replant disease in South Africa. Plant Dis. 2019, 103, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, R.E.; Zasada, I.A.; Hesse, C.; DeVetter, L.W. Brassicaceous seed meal, root removal, and chemical fumigation vary in their effects on soil quality parameters and Pratylenchus penetrans in a replanted floricane raspberry production system. Appl. Soil Ecol. 2019, 133, 44–51. [Google Scholar] [CrossRef]
- Hewavitharana, S.S.; Mazzola, M. Carbon source-dependent efects of aaerobic soil disinfestation on soil microbiome and suppression of Rhizoctonia solani AG-5 and Pratylenchus penetrans. Phytopathology 2016, 106, 1015–1028. [Google Scholar] [CrossRef]
- Browne, G.; Ott, N.; Poret-Peterson, A.; Gouran, H.; Lampinen, B. Efficacy of anaerobic soil disinfestation for control of Prunus replant disease. Plant Dis. 2018, 102, 209–219. [Google Scholar] [CrossRef]
- Forge, T.; Neilsen, G.; Neilsen, D. Organically acceptable practices to improve replant success of temperate tree-fruit crops. Sci. Hort. 2016, 200, 205–214. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Juárez, M.F.-D.; Insam, H.; Schweizer, S.; Naef, A.; Topp, A.-R.; Kelderer, M.; Rühmer, T.; Baab, G.; Henfrey, J.; et al. Performance evaluation of locally available composts to reduce replant disease in apple orchards of central Europe. Renew. Agric. Food Syst. 2018, 34, 543–557. [Google Scholar] [CrossRef]
- De Corato, U. Disease-suppressive compost enhances natural soil suppressiveness against soil-borne plant pathogens: A critical review. Rhizosphere 2020, 13, 100192. [Google Scholar] [CrossRef]
- Gimsing, A.L.; Kirkegaard, J.A. Glucosinolates and biofumigation: Fate of glucosinolates and their hydrolysis products in soil. Phytochem. Rev. 2009, 8, 299–310. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Sato, K.; Vicente, C.S.L.; Hasegawa, K. Nematicidal actions of the marigold exudate -terthienyl: Oxidative stress-inducing compound penetrates nematode hypodermis. Biol. Open 2019, 8, bio038646. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.N.K.; Jayasudha, S.M.; Kirankumar, K.C. Disease management by Biofumigation in organic farming system. J. Pharmacogn. Phytochem. 2018, 7, 676–679. Available online: http://www.phytojournal.com/archives/2018/vol7issue4/PartK/7-3-654-578.pdf (accessed on 11 April 2022).
- Gupta, P.; Vasudeva, N. In vitro antiplasmodial and antimicrobial potential of Tagetes erecta roots. Pharm. Biol. 2010, 48, 1218–1223. [Google Scholar] [CrossRef]
- Padalia, H.; Chanda, S. Antimicrobial efficacy of different solvent extracts of Tagetes erecta L. flower, alone and in combination with antibiotics. Appl. Microbiol. 2015, 1, 106. [Google Scholar] [CrossRef]
- Wang, X.; Li, K.; Xu, S.; Duan, Y.; Wang, H.; Yin, C.; Chen, X.; Mao, Z.; Xiang, K. Effects of Different Forms of Tagetes erecta Biofumigation on the Growth of Apple Seedlings and Replanted Soil Microbial Environment. Horticulturae 2022, 8, 633. [Google Scholar] [CrossRef]
- Gałązka, A.; Grządziel, J.; Gałązka, R.; Ukalska-Jaruga, A.; Strzelecka, J.; Smreczak, B. Genetic and functional diversity of bacterial microbiome in soils with long term impacts of petroleum hydrocarbons. Front. Microbiol. 2018, 9, 1923. [Google Scholar] [CrossRef]
- Rawat, N.; Joshi, G.K. Bacterial community structure analysis of a hot spring soil by next generation sequencing of ribosomal RNA. Genomics 2019, 111, 1053–1058. [Google Scholar] [CrossRef]
- Finley, S.J.; Benbow, M.E.; Javan, G.T. Potential applications of soil microbial ecology and next-generation sequencing in criminal investigations. Appl. Soil Ecol. 2015, 88, 69–78. [Google Scholar] [CrossRef]
- Furtak, K.; Grządziel, J.; Gałązka, A.; Niedźwiecki, J. Prevalence of unclassified bacteria in the soil bacterial community from floodplain meadows (fluvisols) under simulated flood conditions revealed by a metataxonomic approachss. Catena 2020, 188, 104448. [Google Scholar] [CrossRef]
- Mahnkopp-Dirks, F.; Radl, V.; Kublik, S.; Gschwendtner, S.; Schloter, M.; Winkelmann, T. Molecular barcoding reveals the genus Streptomyces as associated root endophytes of apple (Malus domestica) plants grown in soils affected by apple replant disease. Phytobiomes J. 2021, 5, 177–189. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Swędrzyńska, D.; Małecka-Jankowiak, I. The Impact of Tillaging Spring Barley on Selected Chemical, Microbiological, and Enzymatic Soil Properties. Pol. J. Environ. Stud. 2017, 26, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomska, A.; Majchrzak, L.; Borowiak, K.; Wolna-Maruwka, A.; Waraczewska, Z.; Budka, A.; Gaj, R. The Influence of Tillage and Cover Cropping on Soil Microbial Parameters and Spring Wheat Physiology. Agronomy 2020, 10, 200. [Google Scholar] [CrossRef]
- Wang, M.; Pendall, E.; Fang, C.; Li, B.; Nie, M. A global perspective on agroecosystem nitrogen cycles after returning crop residue. Agric. Ecosyst. Environ. 2018, 266, 49–54. [Google Scholar] [CrossRef]
- Liang, B.; Ma, C.; Fan, L.; Wang, Y.; Yuan, Y. Soil amendment alters soil physicochemical properties and bacterial community structure of a replanted apple orchard. Microbiol. Res. 2018, 216, 1–11. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, T.; Niu, M.; Chen, X.; Liu, C.; Wang, Y.; Chen, T. Biodegradation of Nonylphenol during Aerobic Composting of Sewage Sludge under Two Intermittent Aeration Treatments in a Full-Scale Plant. Environ. Pollut. 2018, 238, 783–791. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, L.; He, N.; Gong, D.; Gao, H.; Ma, Z.; Fu, L.; Zhao, M.; Wang, H.; Wang, C.; et al. Soil bacterial community as impacted by addition of rice straw and biochar. Sci. Rep. 2021, 11, 22185. [Google Scholar] [CrossRef]
- Chamizo, S.; Mugnai, G.; Rossi, F.; Certini, G.; De Philippis, R. Cyanobacteria inoculation improves soil stability and fertility on different textured soils: Gaining insights for applicability in soil restoration. Front. Environ. Sci. 2018, 6, 49. [Google Scholar] [CrossRef]
- Fazi, S.; Amalfitano, S.; Pernthaler, J.; Puddu, A. Bacterial communities associated with benthic organic matter in headwater stream microhabitats. Environ. Microbiol. Niem 2005, 7, 1633–1640. [Google Scholar] [CrossRef]
- Wolińska, A.; Kuźniar, A.; Zielenkiewicz, U.; Banach, A.; Błaszczyk, M. Indicators of arable soils fatigue–Bacterial families and genera: A metagenomic approach. Ecol. Indic. 2018, 93, 490–500. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Y.; Lin, Z.; Tuo, Y.; Li, H.; Wang, Y. Differences in soil microbial community composition between suppressive and root rot-conducive in tobacco fields. Curr. Microbiol. 2021, 78, 624–633. [Google Scholar] [CrossRef]
- Wu, S.; Xue, S.; Iqbal, Y.; Xing, H.; Jie, Y. Seasonal nutrient cycling and enrichment of nutrient-related soil microbes aid in the adaptation of ramie (Boehmeria nivea L.) to nutrient-deficient conditions. Front. Plant Sci. 2021, 12, 644904. [Google Scholar] [CrossRef] [PubMed]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.C. Current knowledge and perspectives of Paenibacillus: A review. Microb Cell Fact. 2016, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Gao, K.; Guo, Z.; Liu, Y.; Jiang, L.; Liu, C.; Wang, G. Different responses of soil bacterial and fungal communities to 3 years of biochar amendment in an alkaline soybean soil. Front. Microbiol. 2021, 12, 630418. [Google Scholar] [CrossRef]
- Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef]
- Talwar, C.; Nagar, S.; Kumar, R.; Scaria, J.; Lal, R.; Negi, R.K. Defining the environmental adaptations of genus Devosia: Insights into its expansive short peptide transport system and positively selected genes. Sci. Rep. 2020, 10, 1151. [Google Scholar] [CrossRef]
- Semenov, M.V.; Krasnov, G.S.; Semenov, V.M.; van Bruggen, A. H.C. Long-term fertilization rather than plant species shapes rhizosphere and bulk soil prokaryotic communities in agroecosystems. Appl. Soil Ecol. 2020, 154, 103641. [Google Scholar] [CrossRef]
- Lee, H.; Oh, S.Y.; Lee, Y.M.; Jang, Y.; Jang, S.; Kim, C.; Kim, J.J. Successional variation in the soil microbial community in Odaesan National Park, Korea. Sustainability 2020, 12, 4795. [Google Scholar] [CrossRef]
- Kraut-Cohen, J.; Shapiro, O.H.; Dror, B.; Cytryn, E. Pectin Induced Colony Expansion of Soil-Derived Flavobacterium Strains. Front. Microbiol. 2021, 12, 651891. [Google Scholar] [CrossRef] [PubMed]
- Kanfra, X.; Wrede, A.; Mahnkopp-Dirks, F.; Winkelmann, T.; Heuer, H. Networks of free-living nematodes and co-extracted fungi, associated with symptoms of apple replant disease. Appl. Soil Ecol. 2022, 172, 104368. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Winkelmann, T. Biofumigation for fighting replant disease-A Review. Agronomy 2020, 10, 425. [Google Scholar] [CrossRef]
- Yi, S.; Li, F.; Wu, C.; Ge, F.; Feng, C.; Zhang, M.; Lu, H. Co-transformation of HMs-PAHs in rhizosphere soils and adaptive responses of rhizobacteria during whole growth period of rice (Oryza sativa L.). J. Environ. Sci. 2023, 132, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.Y.A.; Ward, A.C.; Goodfellow, M. Exploration of Amycolatopsis diversity in soil using genus-specific primers and novel selective media. Syst. Appl. Microbiol. 2006, 29, 557–569. [Google Scholar] [CrossRef]
- Aparicio, J.D.; Raimondo, E.E.; Gil, R.A.; Benimeli, C.S.; Polti, M.A. Actinobacteria consortium as an efficient biotechnological tool for mixed polluted soil reclamation: Experimental factorial design for bioremediation process optimization. J. Hazard. Mater. 2018, 342, 408–417. [Google Scholar] [CrossRef]

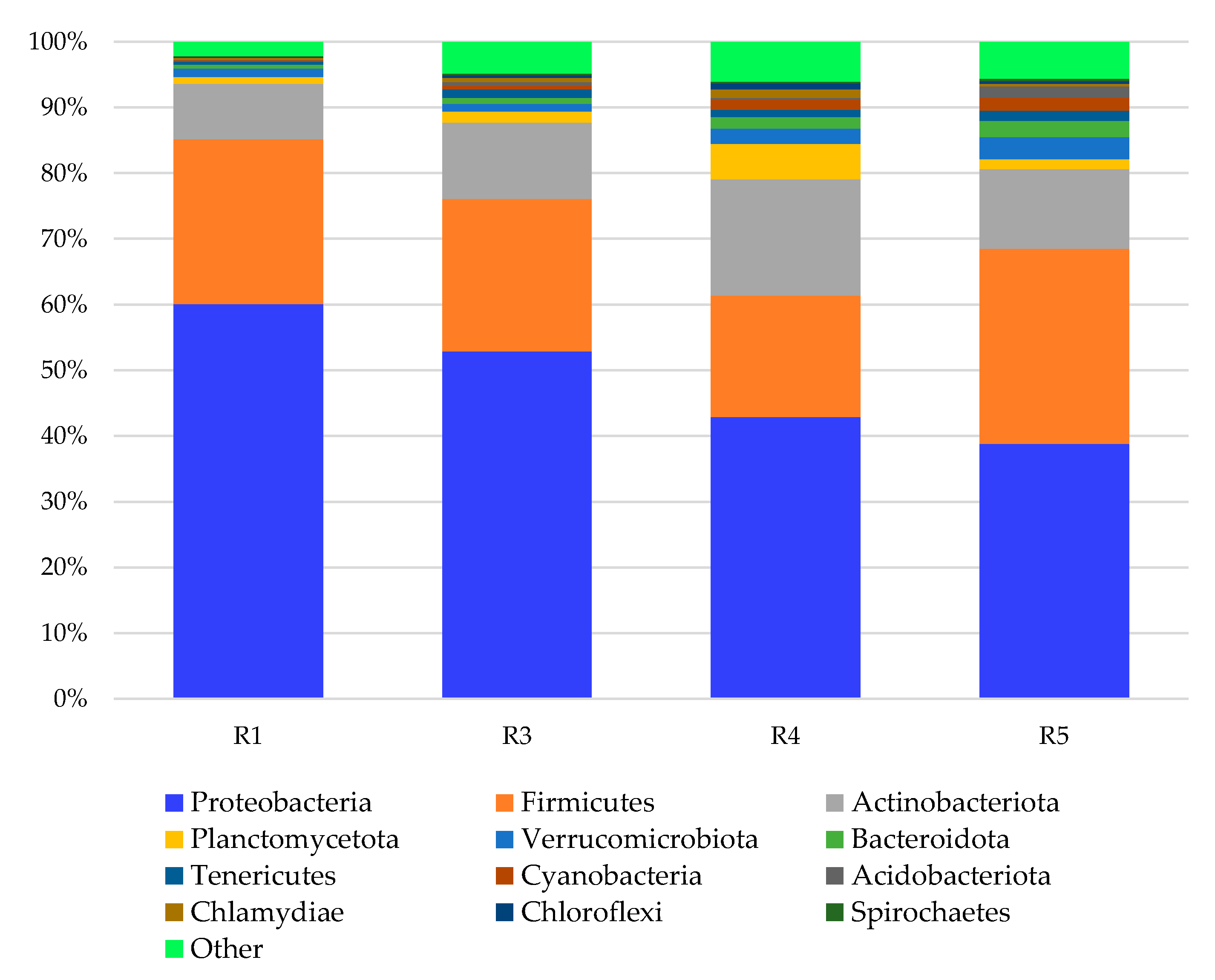
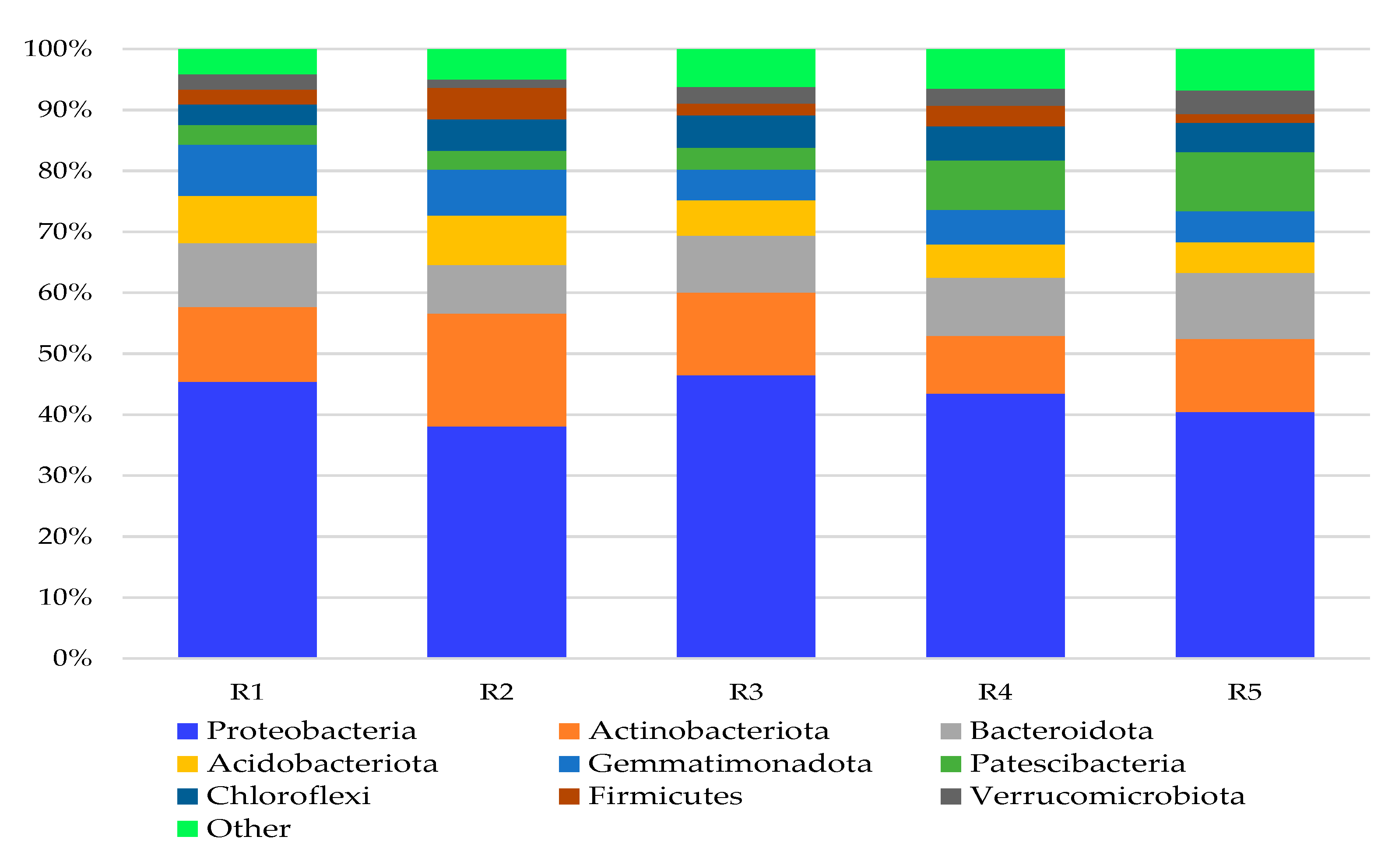
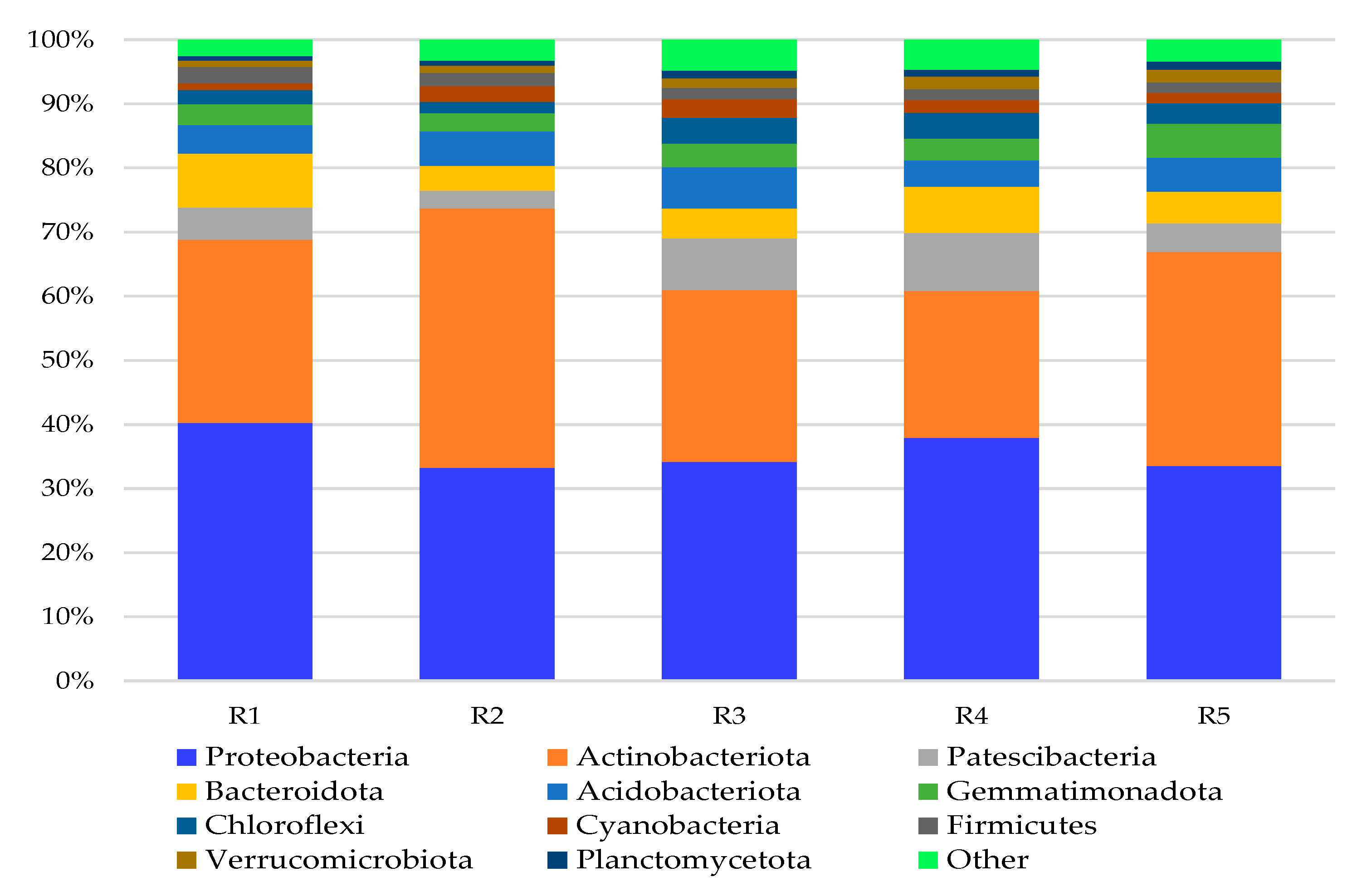
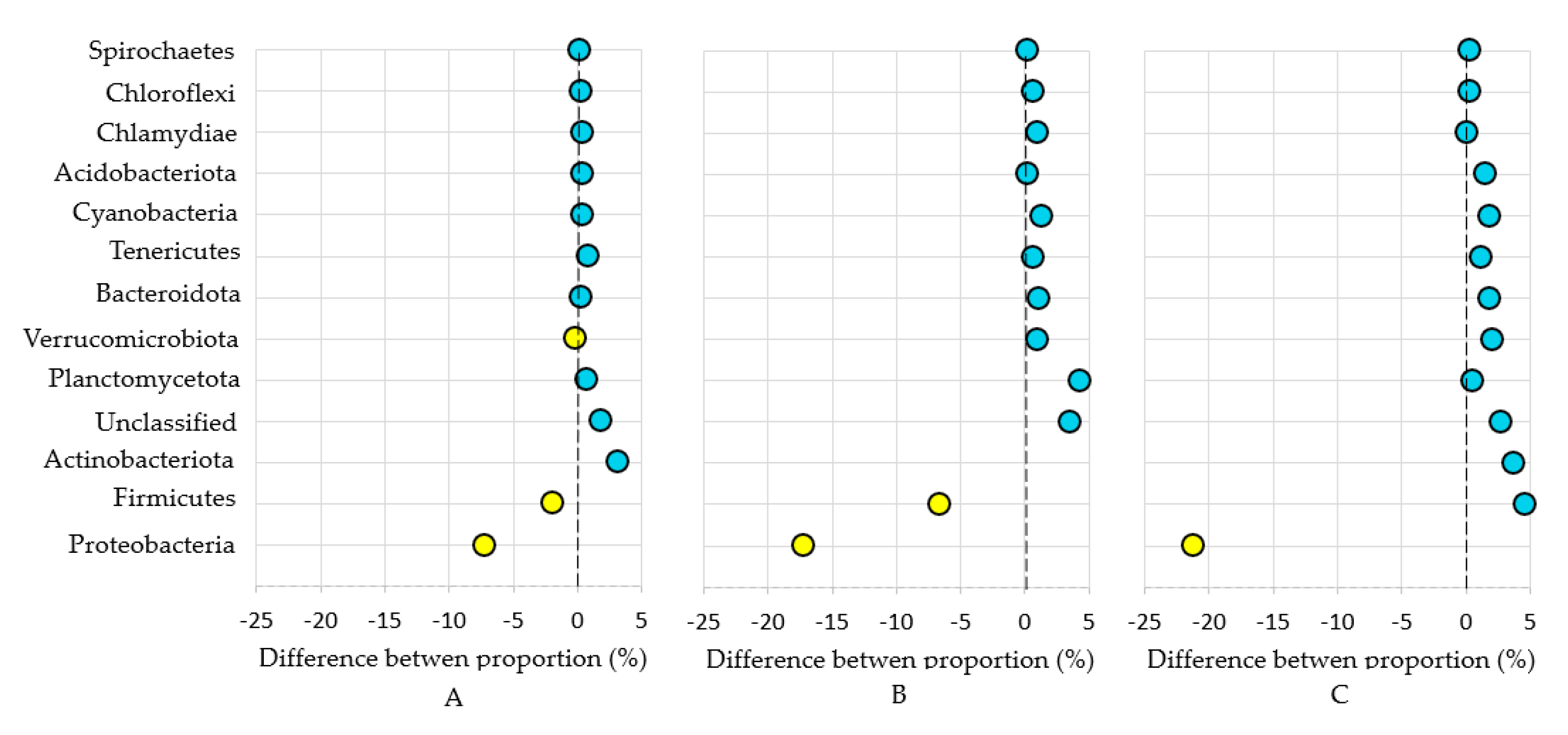



| Properties of the Soil | R1 | R2 |
|---|---|---|
| pH (H2O) | 7.6 | 5.8 |
| Bulk density (g dm−3) | 1600 | 1830 |
| Salinity (g Na Cl dm−3) | 0.23 | 0.23 |
| Humus content (%) | 4.88 | 1.70 |
| Mineral content (mg dm−3): N-NO3 | <3.9 | <3.9 |
| P | 127 | 30 |
| K | 229 | 89 |
| Ca | 1333 | 240 |
| Mg | 188 | 38 |
| Cl | <21.3 | <21.3 |
| Taxonomic Units | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| 2019 year | |||||
| Phylum | 20 | - | 22 | 23 | 23 |
| Class | 44 | - | 48 | 50 | 47 |
| Order | 87 | - | 96 | 93 | 96 |
| Family | 180 | - | 201 | 197 | 205 |
| Genus | 378 | - | 441 | 407 | 470 |
| Species | 456 | - | 591 | 532 | 704 |
| 2020 year | |||||
| Phylum | 30 | 32 | 31 | 32 | 31 |
| Class | 84 | 89 | 89 | 90 | 85 |
| Order | 190 | 201 | 216 | 224 | 207 |
| Family | 280 | 294 | 320 | 338 | 317 |
| Genus | 457 | 499 | 554 | 510 | 513 |
| Species | 832 | 906 | 1007 | 935 | 940 |
| 2021 year | |||||
| Phylum | 32 | 30 | 34 | 33 | 30 |
| Class | 80 | 76 | 79 | 85 | 79 |
| Order | 180 | 170 | 195 | 200 | 189 |
| Family | 272 | 255 | 302 | 301 | 280 |
| Genus | 480 | 458 | 552 | 553 | 505 |
| Species | 838 | 774 | 953 | 942 | 988 |
| R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|
| Number of Unique Taxa | ||||
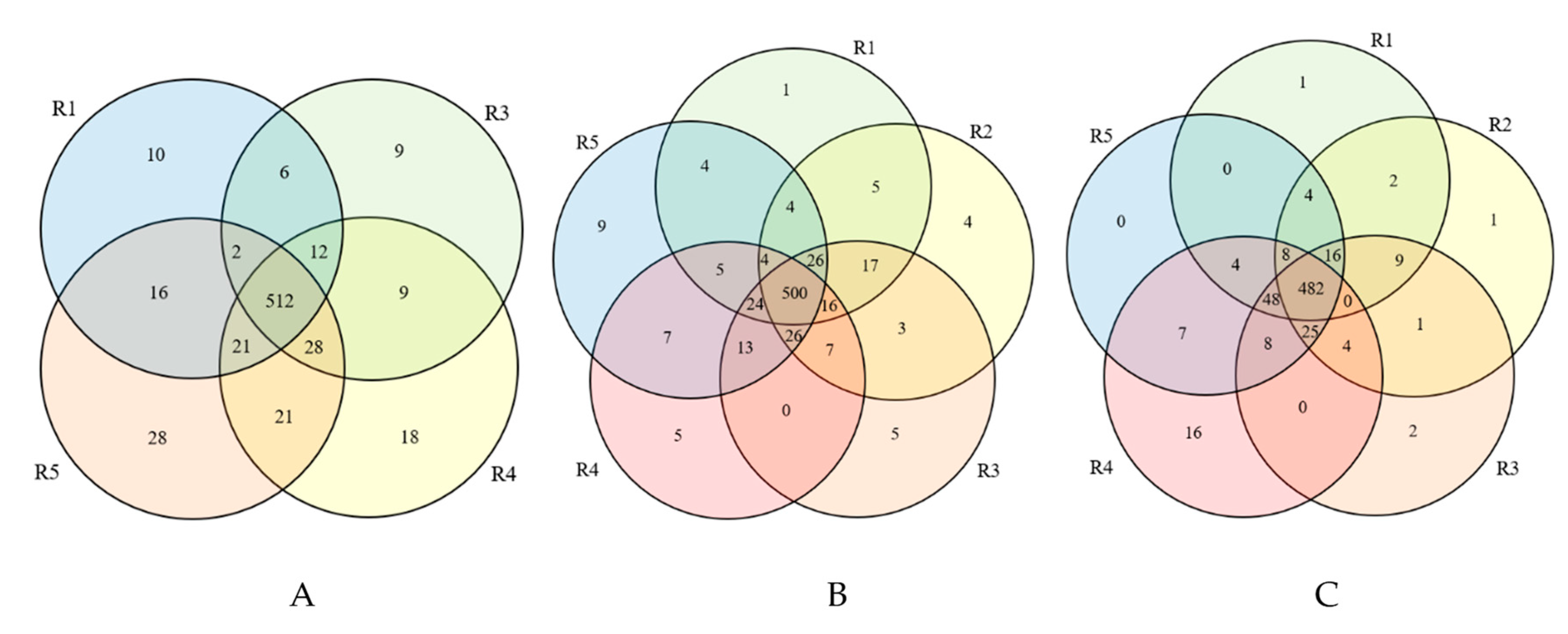
| 10 | 0 | 9 | 18 | 28 |
| Anaerococcus Dorea Eggerthella Enhydrobacter Fructobacillus Mitsuokella Paraprevotella Sarcina Thiocystis Verminephrobacter | _ | Chlamydia Desulfonatronovibrio Desulfotalea Flectobacillus Helcococcus Muricauda Roseateles Sinomonas Spirochaeta | Citricoccus Cryobacterium Dehalobacterium Desulfomicrobium Entomoplasma Fusobacterium Jeotgalicoccus Kibdelosporangium Kytococcus Octadecabacter Odoribacter Peptostreptococcus Roseococcus Saccharomonospora Salinivibrio Sporanaerobacter Thermococcus Xenococcus | Antarctobacter Aureispira Bulleidia Butyricimonas Coprococcus Haliscomenobacter Lachnobacterium Limnothrix Luteococcus Nevskia Parabacteroides Porphyromonas Propionigenium Pseudanabaena Psychroflexus Rhodothalassium Roseburia Roseiflexus Roseivivax Salinimicrobium Snowella Streptomonospora Sulfuricurvum Sulfuritalea Teredinibacter Terriglobus Thermacetogenium Zobellia |
| R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|
| Number of Unique Taxa | ||||
| 1 | 4 | 5 | 4 | 3 |
| Amycolatopsis | Haliscomenobacter Novibacillus f_Enterobacteriaceae; Other Hafnia- Obesumbacterium | f_Nostocaceae; Other Archangium Aetherobacter f_Polyangiaceae;g_ uncultured Leptospira | Vicingus Blastopirellula Chitinimona f_Parachlamydiaceae; g_uncultured | o_Babeliales; Other; Lactococcus OM60(NOR5)_clade |
| R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|
| Number of Unique Taxa | ||||
| 1 | 1 | 2 | 16 | 0 |
| Amycolatopsis | Rahnella1 | f_Myxococcaceae;Other Candidatus_Falkowbacteria | Paludibacter WCHB1-32 Vitellibacter Bacteriovorax Pseudarcobacter Candidatus_Megaira Tolumonas Idiomarina Shewanella Noviherbaspirillum Candidatus_Accumulibacter f_Methylococcaceae;g_uncultured Methylophaga Halomonas Alkanindiges f_Criblamydiaceae;g_uncultured | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieczorek, R.; Zydlik, Z.; Wolna-Maruwka, A.; Niewiadomska, A.; Kayzer, D. The Effect of Biofumigation on the Microbiome Composition in Replanted Soil in a Fruit Tree Nursery. Agronomy 2023, 13, 2507. https://doi.org/10.3390/agronomy13102507
Wieczorek R, Zydlik Z, Wolna-Maruwka A, Niewiadomska A, Kayzer D. The Effect of Biofumigation on the Microbiome Composition in Replanted Soil in a Fruit Tree Nursery. Agronomy. 2023; 13(10):2507. https://doi.org/10.3390/agronomy13102507
Chicago/Turabian StyleWieczorek, Robert, Zofia Zydlik, Agnieszka Wolna-Maruwka, Alicja Niewiadomska, and Dariusz Kayzer. 2023. "The Effect of Biofumigation on the Microbiome Composition in Replanted Soil in a Fruit Tree Nursery" Agronomy 13, no. 10: 2507. https://doi.org/10.3390/agronomy13102507






