Abstract
Mining severely affects ecosystems and threatens local food security. Remediation practices, however, are a viable way of reducing the negative impacts on post-mining lands. In this study we aim to improve crop yields and drought resistance on a post-tin-mining site located in Bangka Island, Indonesia, with locally available resources. Plots with five different soil amendments: (1) dolomite; (2) compost; (3) charcoal; combinations of (4) charcoal + compost; and (5) charcoal + sawdust; and a control were established. An intercropping system with cassava and centrosema was employed, and yields were determined. Drought resistance was evaluated by carbon isotope discrimination (∆13C) from crop parts of cassava and centrosema’s shoot. Soil physicochemical properties were determined at harvesting time. Soil amendments significantly improved cassava and centrosema yields. In particular, the compost and combined (charcoal + compost) treatments enhanced centrosema yields (1.18 and 1.99 kg·plot−1, respectively) and were related to higher nutrient availability. Similarly, compost, charcoal, and combined treatments showed positive effects on the cassava yield (0.15–0.16 kg·plant−1) and a higher drought resistance in the charcoal treatment (∆13C= 21.48‰). Increased water-holding capacity (WHC) reduced the water deficiency and boosted yields of cassava and centrosema when the soil was treated with dual amendments (charcoal + compost). Charcoal, compost, and their combination turned out to be the most sustainable amendments in degraded post-mining tropical soils.
1. Introduction
Mineral mining contributes to economic growth, infrastructure development and industrial expansion, and creates job opportunities in several countries [1,2]. Mineral commodities such as tin, copper, nickel, gold, and coal are increasingly contributing to the national economy in Indonesia [3]. Tin is used predominantly in the information technology and electronic industry worldwide. More than one third of global tin is mined from the Bangka–Belitung Archipelago; this is also where 90% of Indonesia’s tin comes from [4]. Despite the fact that mining is clearly valuable for economic growth, it has detrimental effects on the surrounding environment such as land-use changes and pollution, causing dramatic losses of biodiversity [2,5,6].
Post-tin-mining areas are characterized by a strong separation of mineral soil particles of different size fractions, with generally a high portion of sand (>90%) with a low content of (highly weathered) clay minerals (< 3%) [7]. The poor organic matter content, coupled with acidic soil conditions as well as low cation exchange capacity (CEC), and nutrient contents, in post-mining areas result in unfavourable soil conditions for agricultural production [5,6,8,9]. These soils typically show high porosity, good aeration, and low water-holding capacity (WHC) [10]; thus, water drains rapidly through the soil. Poor WHC and, hence, soil moisture availability for crops can impair crop growth and development, specifically during drought events [11,12,13].
Although Bangka Island is major producer of tin in Indonesia [5,14,15], the agricultural area is also of great importance. The value of agriculture on the Island is being increasingly recognized, in particular in regard to the pandemic crisis where food supply was limited due to limitations on several sectors such as transportation and trading [16]. The local government recently placed agriculture and tourism as the centrepiece in the development plans since tin mining on land is becoming less profitable [17]. Together with researchers, the local government developed sustainable scientifically proven methods with a high application potential, by using locally available and economically justifiable resources for soil remediation. The research conducted at the experimental field site, is combined with a social science approach to evaluate its applicability [17].
Soil amendments have been proven to be a practical measure to mitigate land degradation and improve drought resistance of crops [18,19]. This is important, since farmers face more and more droughts and these will increase in frequency in this area because of climate change. Organic amendments such as compost and charcoal have been used in post-mining areas to improve soil fertility [20]. These amendments have positively affected crop yields in tropical climates [21,22]. As a soil amendment, charcoal also improved plant water-availability in sandy and sandy loam soils [23,24]. Another possibility for mitigating acidity in mining soils is the application of lime or dolomite which can also improve soil aggregate stability and permeability [25,26]. A further soil amendment that is suggested to improve soil structure is sawdust, which is degraded slowly due to a lack in nutrient availability for plants. Sawdust can improve soil hydro-physical properties and retain water for plants’ supply [27]. While all soil amendments improve soil structure and, hence, possibly water retention in a sandy soil, this could mitigate yield losses by better drought resistance.
In this study, we further selected local crop varieties and followed organic farming practices (no application of pesticides and inorganic fertilizers). These practices allow farmers to be independent of external supplies, as agrochemicals are often unavailable to the extent they are needed. We also aimed for an intercropping system to reduce pest pressure. To our knowledge, this is the first field experiment that investigates crop yield and drought resistance with locally available soil amendments in a tropical post-tin-mining area, using organic farming practices. Cassava (Manihot esculenta Crantz) was selected as main crop because it is an important crop for smallholder farmers in Indonesia [28] and is deployed to support local food diversification in Bangka Island (Minister of Agriculture of Indonesia, [29]). Cassava is also reported to grow well on poor soils with water deficiency [4,30,31,32]. Smallholder farmers in Indonesia primarily grow cassava in intercropping systems with legumes [33,34]. The local legume variety Centrosema pubescens was mentioned as a potential option for cultivation on former tin mining areas, in particular, in combination with compost application because of its ability to supply nitrogen on tailings [7]. Since these legumes can fix atmospheric N and develop quickly even on poor soils, the advanced biomass production compared to non-legume species can increase soil organic matter (SOM) through enhanced biomass quality and quantity [35,36,37]. Furthermore, fast growing intercrops prevent soil erosion and protect SOM during early growth of cassava in the rainy season [38].
Although cassava can grow under limited water supply, prolonged water scarcity due to irregular rainfall and long drought periods can induce drought stress in cassava and affect crop performance [39]. Drought stress has also been shown to impair nitrogen-fixing nodule production and crop yields in centrosema plants [40]. We suggest that it is critical to assess drought resistance in crops when different local soil amendments are applied. Even though drought resistance in different crops is widely investigated using stable isotope measurement and the discrimination of (∆13C) value [41,42,43,44], there is a lack of data from tropical field experiments, specifically on land remediation using local soil amendments. Here, the effect of different local soil amendments applied to post-mining soils, on cassava (Manihot esculenta Crantz) and centrosema (Centrosema pubescens) yields and drought resistance was evaluated. We hypothesized that (i) locally available soil amendments including compost, charcoal, and sawdust would improve crop yields due to better nutrient availability and water supply in soils, and (ii) local charcoal could increase drought resistance of crops by improving soil structure.
2. Materials and Methods
2.1. Experimental Site
The study was conducted in a post-tin-mining area located in Sinar Jaya village, Sungailiat district, Bangka Regency, Kepulauan Bangka Belitung Province, Indonesia (1°47′22.9085 S and 106°5′47.0461 E, Figure S1). The climate is humid tropical with a mean annual precipitation (MAP) of 2288 mm and mean annual temperature (MAT) of 27 °C. Daily precipitation and temperature values at the experimental site from August 2018 to July 2019 are shown in Figure 1.
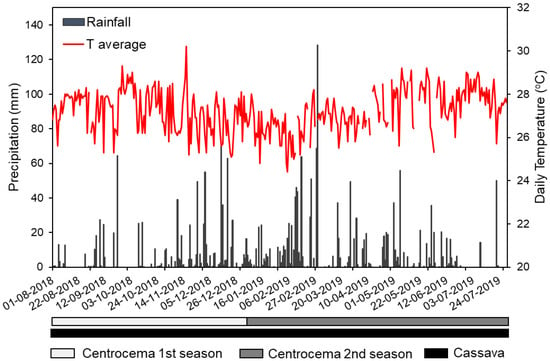
Figure 1.
Daily precipitation (mm) and temperature (°C) during the 2018–2019 growing season. Data were obtained from the Weather Station of Depati Amir, Pangkal Pinang city, Bangka Belitung Islands Province, Indonesia [45].
The common soil type (naturally occurring) in Bangka Regency is a Dystric Cambisol [46], which is dominated by the sand fraction and developed from granite parent material; while in the post-tin-mining area in Bangka, the soil is characterized by enormous anthropogenic impacts due to on-land mining activities and, hence, changes in the landforms and soils classification [3]. Based on IUSS Working Group WRB [46], the soil type at the experimental site is a Technosol (Figures S2 and S3). The soil’s texture in the experimental site is sandy loam with low nutrient contents (Table 1) and low water availability for plants (Table 2).

Table 1.
Physicochemical properties of the soils in the experimental site. Cation exchange capacity, CEC; total organic carbon, TOC; dissolved organic carbon, DOC; total phosphate, TP; available phosphate, AP; total potassium, TK; available potassium, AK; and electrical conductivity, EC. Given are mean ± SE (n = 6).

Table 2.
Soil hydraulic properties. Permanent wilting point, PWP; field capacity, FC; and plant available water, PAW. Given are mean (n = 2).
2.2. Experimental Design
The field trial was set up using a randomized complete block design (RCBD) with five different soil amendments and control plots for comparison, with a size of 2 × 2 m in four replicates. The treatments include (1) dolomite, (2) compost, (3) charcoal, and combinations of (4) charcoal + compost, (5) charcoal + sawdust, and control plot [17]. The soil was amended with 10 t·ha−1 for the single amendments (treatments 2–4) and with a ratio of 1:1 (20 t·ha−1 in total) for combined amendments (treatments 5 and 6). The basic properties of soil amendments are presented in Table 3. The field was established in the post-tin-mining area at the end of July 2018. The plots were used to grow a local variety of cassava (Manihot esculenta Crantz) with four plants per plot. In addition, Centrosema pubescens seeds were planted in a 25 × 25 cm grid as an intercrop to avoid soil erosion, according to local recommendations. These crops were planted on 1 August 2018. The planting season during this study is shown in Figure 1.

Table 3.
Physicochemical properties of the soil amendments used in the experimental site. Cation exchange capacity, CEC; total nitrogen, TN; total organic carbon, TOC; dissolved organic carbon, DOC; total phosphate, TP; available phosphate, AP; total potassium, TK; available potassium, AK; and electrical conductivity, EC. Given are mean (n = 2).
2.3. Soil Physicochemical Properties
Before setting up the experimental field, soil samples were collected in 2018 at a depth of 0–20 cm (topsoil) to assess the conditions prior to amending. Soil samples were also taken at harvest time in the first year growing season (2019) from each treatment. The samples were air dried at room temperature and sieved < 2 mm. The particle size distribution in mineral soil was determined with a combined wet sieving and sedimentation method [47,48]. Accordingly, soil texture was determined [49].
The permanent wilting point (PWP) was measured by dew-point potentiometer (WP4C, METER Group, NE Hopkins Court Pullman, WA, USA). Based on the soil particle size distribution, the field capacity (FC) was determined using an iterative model of diagnostic value tables [50]. The plant available water (PAW) was calculated from the difference of the estimated FC and the PWP. Water-holding capacity (WHC) was determined according to Dane et al. [51].
Soil samples were homogenized using a ball mill to obtain a finely ground powder for elemental analyses. Total organic carbon (TOC) and total nitrogen (TN) were measured by dry combustion [52], using a Thermo Scientific FlashSmart analyzer (Bremen, Germany).
Soil and amendments were extracted in a 1:10 (w/v) ratio of water. The pH and electrical conductivity (EC) were measured by pH and conductometer, respectively (WTW Multi 9620 IDS, Weilheim, Germany), while dissolved organic carbon (DOC) was measured using a UV-VIS spectrophotometer (Agilent 8453 Diode Array UV-VIS, Santa Clara, CA, USA) at a wavelength of 254 nm [53]. Cation exchange capacity (CEC) was determined from the sum of Ca2+, Mg2+, K+, and Na+ by extraction at a 1:20 (w/v) ratio with 0.1 M BaCl2 solution [54]. Element concentrations were measured with atomic absorption spectrometry (AAS) PerkinElmer PinAAcle 900T in flame mode (Waltham, MA, USA). Total phosphate (TP) and potassium (TK) were determined after aqua regia extraction in a 1:50 (w/v) ratio according to ÖNORM L 1085 [55]. Available phosphate (AP) and potassium (AK) were measured in calcium acetate lactate extracts (1:20 w/v ratio, [56]. Total and available PO4− was measured with a UV-VIS spectrophotometer (Agilent 8453 Diode Array UV-VIS, Santa Clara, CA, USA) at a wavelength of 710 nm, potassium was quantified via flame atomic absorption spectrometry (AAS, PerkinElmer PinAAcle 900T, Waltham, MA, USA)
2.4. Crop Yields
At plant maturity, all plants from each treatment were harvested to determine yields. The intercrop (Centrosema pubescens) was harvested in December 2018 and replanted for a second season for harvesting in July 2019 (Figure 1). In contrast, cassava was harvested 12 months after planting (July 2019). Weed was also harvested, and yield measured. Samples from each plot were weighed on-site using a spring balance for centrosema; while individual shoot and tuber from each cassava plant were weighed individually. Dry matter was determined from dried plant parts (65 °C for 48 h).
2.5. Carbon Isotope Discrimination as a Proxy for Drought Resistance
Subsamples from yield determination (see above) were used for isotope analysis. Isotope analyses were conducted on individual plant parts. Three individual plant parts of cassava (leaf, stem, and tuber) and Centrosema’s soot were analysed. The samples were washed to remove soil residues, cut, and oven-dried for 48 h at 65 °C. The plants’ materials were homogenized using a ball mill to obtain a finely grounded powder. From each plant part, three mg were weighed into tin cups and measured for elemental carbon composition and carbon isotope discrimination by using an elemental analyser isotope ratio mass spectrometer (Delta PLUS, Thermo Finnigan, Bremen, Germany), connected via a ConFlo III interface (Thermo Fisher, Bremen, Germany) to a Thermo Delta V (Bremen, Germany). Isotopic internal and external international standards were included in each run. The uncertainty of sample measurement for the whole system was 0.2‰. Carbon isotope composition was expressed as δ13C (‰) [42] computed as:
where R was the 13C/12C ratio in this case Vienna Peedee Belemnite. Δ was calculated according to Farquhar et al. [42] as:
where δa and δp refer to δ atmosphere and plant, respectively. δa (−8.6‰) is the measured value of atmospheric CO2 in the tropical region in 2018–2019, Mauna Loa Observatory, Hawaii (20° N, 156° W) [57].
2.6. Statistical Analysis
To assess differences among locally available soil amendments on yield and drought resistance, analysis of variance (ANOVA) was applied. Normality and homogeneity of variance were tested by Shapiro–Wilk method. Parametric ANOVA was used when normality and homogeneity of variance were given. Robust ANOVA (WRS2 package) was used accordingly if data normality and homogeneity of variance were violated.
Parametric one-way analysis of variance (ANOVA) was performed to analyse the effect of treatments on soil physicochemical properties at harvest time (pH, CEC, DOC, TP, AP, TK, AK, EC, and WHC), main crop yield (cassava tuber and shoot), and intercrop yield (centrosema first season). The same test also was applied for carbon isotope discrimination (Δ13C) of centrosema from first and second season.
Robust ANOVA (t1way, WRS2 package) in R [58] was used accordingly for soil properties (TOC and TN), centrosema second season yield, and weed yield due to not normally distributed data. The two-way ANOVA was used to analyse significant differences of treatments and plant parts or season in Δ13C of cassava or centrosema. Statistically significant differences between factor groups were evaluated with Tukey’s HSD for parametric ANOVA and Lincon for robust ANOVA at p < 0.05. Pearson correlation was used to analyse linear correlation between crop yield and soil properties at harvest time at p < 0.05. Statistical analysis and data visualization were conducted using R version 4.1.3 (R core Team, Vienna, Austria) and SigmaPlot 14.5 (Systat, Inc, San Jose, CA, USA).
3. Results
3.1. Effect of Soil Amendments on Crop Yields
Application of soil amendments in the study site affected the yield of cassava and centrosema (Figure 2, Table S1). Compared to the control, organic amendments such as compost, charcoal and its combination significantly improved the yield of cassava (p < 0.05, Figure 2a). Tuber yield increased about twofold in these treatments (0.15–0.16 kg·plant−1). Similarly, cassava’s shoot biomass profited from the application of organic amendments, with highest values for the combined treatment with charcoal + sawdust (0.18 kg·plant−1).
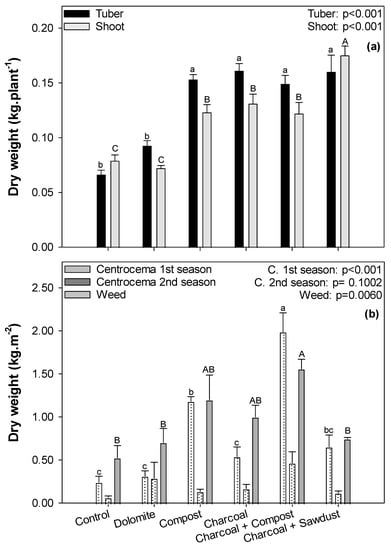
Figure 2.
Yield of cassava tuber and shoot (a) and yield of centrosema 1st season, centrosema 2nd season, as well as weed (b) in different soil amendment treatments. Given are mean ± SE (n = 4). Different uppercase letters and lowercase letters indicate significant differences in cassava’ s tuber, centrosema 1st season, cassava’s shoot and weed, respectively as revealed by one-way ANOVA followed by post hoc test at p < 0.05.
The yields of the intercrop varied between the first and the second season of centrosema (Figure 2b). In the first season, centrosema grew significantly better with soil amendments than in the second season. The yield from the compost treatment significantly improved almost fivefold compared to the control (1.18 and 0.24 kg·plot−1, respectively). The combined treatment of compost + charcoal also increased yield eightfold compared to the control (1.99 and 0.24 kg·plot−1, respectively). Similar to the first season, soil amendments also improved the second harvest of centrosema, although not significantly (Figure 2b, Table S1). Since we realized that centrosema suffered from heavy competition with weed during the planting season, we decided to harvest weed in addition to centrosema at the second harvest and determine yields for both, crop and weed. The weed grew better than centrosema during the second season, where combined treatment (compost + charcoal) significantly increased weed yield (p < 0.05, Figure 2b, Table S1) threefold compared to control (1.56 and 0.53 kg·plot−1, respectively).
3.2. Drought Stress Indication via Carbon Isotope Discrimination
Significant differences were found in carbon isotope discrimination (∆13C) of cassava amongst treatments (p < 0.05, Table 4; Figure S4a). The application of soil amendments slightly increased ∆13C with charcoal showing the highest ∆13C values (Table 4). Cassava plant parts showed significant differences (Table 4), with highest ∆13C values for stem and leaves. The dolomite treatment indicated the highest ∆13C value in the leaves. However, the combined effect of treatment and plant part on ∆13C of cassava was not significant (p > 0.05, Table 4). As given in Figure S4a, carbon isotope discrimination increased in all plant parts by applying soil amendments, except for the tuber in the combined treatment charcoal + compost.

Table 4.
Comparison of mean carbon isotope discrimination (∆13C) in different soil amendment treatments and plant parts of cassava. Given are mean ± SE (n = 4). Different letters indicate significant differences in treatment and plant part as revealed by robust two-way ANOVA followed by linear contrast post hoc test with associated p-values for treatment (T), plant part (P), and their interaction (T × P).
Centrosema showed significantly higher ∆13C values ranging from 22.33‰ to 23.06‰ in the first season compared to the second, ranging from 21.03‰ to 22.41‰ (Figure S4b, Table 5). The combined treatment (charcoal + compost) had the highest ∆13C value for both the first and second harvest of centrosema (Figure S4b) with a minor-non-significant (Table S1)—trend for increased ∆13C values in the centrosema plants in amended soils. When we compared all seasons of centrosema, there is a significant effect of treatment, most notably for “charcoal + compost” (Table 5).

Table 5.
Comparison of mean carbon isotope discrimination (∆13C) in different soil amendment treatments and season of centrosema. Given are mean ± SE (n = 4). Different letters indicate significant differences in treatment and season as revealed by two-way ANOVA followed by linear contrast post hoc test with associated p-values for treatment (T), season (S), and their interaction (T × S).
3.3. Effect of Soil Amendments on Soil Properties
Most of the measured soil properties at harvest time were significantly affected by soil amendments, except for total nitrogen (TN) and total potassium (TK) (see Table 6, Table S2). Compared to the control, inorganic amendment (dolomite) treatment strongly increased pH, cation exchange capacity (CEC), dissolved organic carbon (DOC), and electrical conductivity (EC) (p < 0.05). It was about tenfold for CEC (14.58 mmol·kg−1), twofold for DOC (20.30 mg·L−1), and fivefold for EC (0.06 S·cm−1).

Table 6.
Some physicochemical properties of the soils at harvest time. Cation exchange capacity, CEC; total nitrogen, TN; total organic carbon, TOC; dissolved organic carbon, DOC; total phosphate, TP; available phosphate, AP; total potassium, TK; available potassium, AK; and electrical conductivity, EC. Given are mean ± SE (n = 4). Different letters indicate significant differences in treatment as revealed by one-way ANOVA followed by post hoc test at p < 0.05.
Organic amendments such as compost, charcoal, and its combination were significantly related to available potassium (AK) in soil, and showed almost threefold higher values than the control treatment. Single application of compost amendment showed a significantly higher value (p < 0.05) of total phosphate (TP, 44.60 mg·kg−1) and available phosphate (AP, 20.52 mg·kg−1) compared to the control (13.00 and 3.21 mg·kg−1 for TP and AP). Similarly, the combined treatment of charcoal + compost amendment also significantly increased TP by a factor three (41.16 mg·kg−1) and AP by a factor seven (23.65 mg·kg−1). Soil organic carbon was relatively low among all treatments, but the combined treatments of charcoal with compost or sawdust showed the highest increase (Table 6). The addition of compost in combination with charcoal considerably raised the CEC and DOC concentrations in soils, which is due to the higher intrinsic CEC in compost (Table 3).
Soil water-holding capacity (WHC) was also associated with the soil amendments. In particular, the charcoal treatment showed higher WHC values while it only significantly improved when applied together with compost (Table 6).
4. Discussion
4.1. Crop Yields
The yield of cassava tubers (ranging from 0.45 to 1.08 kg per plant) under intercropping system with centrosema were low compared to the other studies in Indonesia (i.e., Suwarto et al. [59]) regardless of the soil amendments. Previously, Suwarto et al. [59] reported that cassava’s yield was around 4.8 kg of fresh tuber under intercropping cultivation with legumes in acidic soils. The low yields observed in the present study might be related to poor nutrient availability, especially nitrogen (N) content which is very low both in the initial condition and all treatments at harvest time. However, the yield of our intercrop centrosema which ranged from 0.13–4.28 kg of fresh biomass per m2, was higher compared to Suwarto et al. [59] values of about 0.42 kg per m2. The highest yield of intercrop in the first season might be affected by nutrient supply. Quick development of the legume centrosema after seeding suggests a faster nutrient uptake than cassava, which may have resulted in nutrient depletion during the progression of the planting season. The intense competition between centrosema, cassava, and weed for soil nutrients and water during the dry season may inhibit the growth of both centrosema and cassava. Intercrops are advantageous for preventing soil erosion when the main crop has not yet developed, but the effect of legume intercrops on the soil’s water balance is controversial and often discussed [60]. Intercrops improve soil physical structure and water infiltration, on the one hand [61,62]; however, the subsequent crops may suffer from reduced water availability and nutrient depletion [62,63].
Organic amendments significantly boosted cassava yield most notably in the charcoal + sawdust treatment, whereas significant increases in centrosema yield were only found in compost and combined treatment of charcoal + compost. This corroborates a previous study, that reported application of 20 t·ha−1 organic manure or charcoal significantly improved cassava and intercrop peanut yields under tropical climate conditions [34]. The combination of charcoal and compost treatment has also been reported to improve legume yields [64]. The beneficial effect of compost on legume yields is probably due to better micronutrient availability which is crucial for the mutualistic symbiosis for nitrogen fixation [65]. Compost application was further found to result in improved maize yields in a post-tin-mining area [6]. In the present study, crop yields were significantly correlated with the soil physicochemical properties. Total organic carbon (TOC), total phosphate (TP), available phosphate (AP), available potassium (AK), and water-holding capacity (WHC) were found to be highly correlated with cassava and intercrop yields (Figure 3). The high carbon (C) content of charcoal, sawdust, and compost in this study caused a higher organic carbon supply to the amended soils. Organic C from compost is mostly decomposed quickly under wet tropical climate, while organic C from charcoal remains relatively stable due to the presence of specific aromatic compounds [66,67].
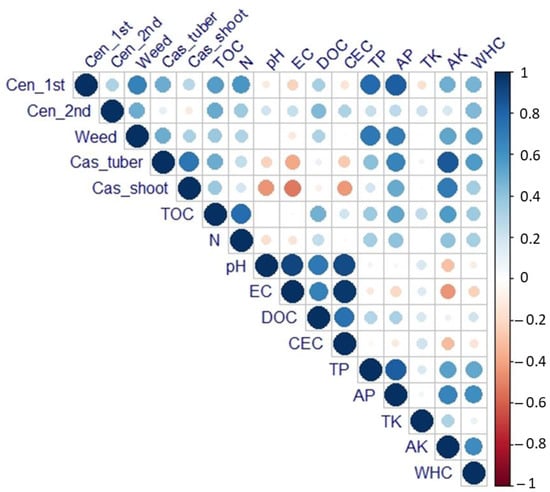
Figure 3.
Heat map showing the relationships among crop yields and some soil physicochemical properties at harvest time. The color gradient represents Pearson correlation coefficients when significant (n = 24, p < 0.05).
As compost had a high PO4− and K contents, this treatment greatly affected these nutrient concentrations in amended soils. Charcoal and its combination (with compost or sawdust) also resulted in higher AP and AK. These results corroborated that in this study organic amendments had a beneficial effect on PO4− and K content, and therefore resulted in higher crop yields compared to dolomite. From an agronomic perspective, the increase in the macronutrient status of the soil after application of organic soil amendments is an important driver for plant growth and yield. The potential of charcoal for improving P and K availability has been reported previously from different soil types [68,69]. Combined treatment (charcoal + compost) has resulted in higher P and K contents than compost alone when applied to a sandy soil [68]. The latter is supported by the fact that compost showed the highest CEC, whereas charcoal had the best liming potential by displaying the highest pH. However, the soils after crop harvest showed a different picture. Improvement of soil pH, as well as CEC and EC, was observed only in the dolomite treatment. A reduction of soil acidity in limed soils can increase the nutrient availability, while dolomite does not supply nutrients beside Ca and Mg, and, hence, the improvement in nutrient availability is also low in the studied soil.
The combined application of charcoal + sawdust improved cassava yields with no effects on the intercrop centrosema. Similarly, no effects on legume yields for a mixture of sawdust, manioc peel and charcoal have been observed under tropical climate [70]. Fresh sawdust is a poor nutrient resource due to its low N and wide C to N ratio; furthermore, a high phenolic content can inhibit or decline microbial decomposition and, hence, nutrient mineralization [27,71]. The poor quality of this substrate may cause delayed mineralization and, hence, a later increase in soil nutrient availability in the sawdust treatment that could positively affect cassava yields.
4.2. Drought Resistance
Plant material carbon isotope discrimination (Δ13C) was measured for plant-growth-related drought stress. Within plant species, higher Δ13C values, enriched in 13C, indicate greater drought resistance [72]. Between the plant parts of the cassava, the lowest Δ13C was found in the cassava tuber (Table 4), which is consistent with a previous study, where lowest Δ13C were found in the sweet potato and the taro tuber [72,73]. Lower Δ13C in starchy tubers of cassava may have occurred due to the function of the tuber as a sink organ for assimilates and as a result of enzymatic processing to storage compounds [73,74]. Furthermore, the differences of Δ13C among plant parts might be affected by photosynthetic activity such as transport process, lipid composition, and metabolites synthesis [75,76]. The higher Δ13C in cassava leaves compared to tuber in our study might be caused by the fractionation during the respiration and substrate conversion processes that can contribute to higher Δ13C in leaves [77].
The second centrosema growth season had higher drought stress level as demonstrated by lower Δ13C values compared to the first season. Plant species competition for water uptake very likely has caused the lower carbon discrimination. This is supported further by a stronger variation of the precipitation pattern during the second centrosema growth season. The growth phase of the first season of centrosema occurred during the rainy season when no drought stress was detected. Furthermore, plant development of centrosema and cassava after the end of the rainy season, in March 2019, might have been diminished by potential competition for water causing drought stress in both crops. The sandy loam soil at our experimental site had low plant available water (PAW) (Table 2) and, consequently, a high potential for drought stress of crops, in particular, when the precipitation was low in terms of quantity and frequency. As described by O’Geen [78], coarse textured soils such as sands and sandy loam have low PAW because of their high portion of large pores with limited ability to retain water.
The ∆13C of individual crops was positively affected by soil amendments. The charcoal treatment showed the highest ∆13C in the cassava (Figure S4a, Table 4), while combined treatment (charcoal + compost) resulted in higher ∆13C in centrosema in both the first and second seasons (Figure S4b, Table 5). Our findings suggested that charcoal improves drought resistance in crops, particularly cassava. This result was also supported by a slight increase in WHC and crop yields following the application of compost, charcoal, and combined charcoal treatment (with charcoal or sawdust). Water availability is the most important factor determining plant growth and nutrient availability in sandy soils [79]. The present study reflects that more available water for plants under organic amendment treatments may contribute to higher drought resistance. The high organic carbon contents from the amendments can cause better WHC in the soils, as strong correlations between organic matter and WHC (Figure 3) were found, and, hence, increased water supply for plants were observed in other studies [80,81,82].
Strong correlations between TOC and WHC (Figure 3) indicate that the high organic carbon contents of the amendments can improve WHC in the soils, resulting in an increased water supply for plants, as observed in other studies [79,80,81]. The potential of charcoal, and, in particular, charcoal in combination with compost can mitigate drought stress, as has been observed for maize [83]. Similarly, charcoal–compost application in a sandy soil has resulted in more water availability than just compost [68].
Application of locally available organic amendments is important for improving soil fertility and water status in the degraded post-mining sandy soils; and, hence, positively affecting crop yield. However, due to the rapid decomposition of organic carbon in a tropical climate, it is necessary to apply soil amendments, particularly compost, on a seasonal basis during each planting season [84]. Our results show that single application of local charcoal significantly improved drought resistance of cassava, while its combination with compost or sawdust had more beneficial impacts on soils’ WHC.
5. Conclusions
In this study, we demonstrated the viability of land remediation after tin mining, using local resources and an organic farming approach. All amendments enhanced the physicochemical properties of the soil. There were also positive effects on yields and drought resistance, in particular, for the dual soil amendments such as charcoal + compost or charcoal + sawdust, which constitutes the most promising approach to increase crop performances in the post-tin-mining area on Bangka Island. These dual amendments strongly increased soil fertility, which was correlated with the yield data. While larger-scale implementation of these practices needs further agronomic evaluation, the ongoing local joint activities between farmers and researchers include biochar production on the household level (during cooking), using different feedstocks such as rise husks and corncobs, provide promising first steps for implementation of remediating post-mining degraded soils.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13010050/s1, Figure S1: Study site in Bangka Regency, Bangka Belitung Islands Province, Indonesia. The red point indicates the location of the plot experiment; Figure S2: Initial condition of the experimental site; Figure S3: Soil profile of the original soil near the experimental site (Dystric Cambisol) and soil after tin mining activities (Technosol) [1,2]. The mining activity influences the topsoil and subsoil of the area. The result is a soil degradation and genesis of an artificial soil; Figure S4: Carbon isotope discrimination (Δ13C) of plant part in cassava (a) and carbon isotope discrimination (Δ13C) of 1st and 2nd season centrosema (b) in different soil amendment treatments. Given are mean ± SE (n = 4). p-values are shown in the upper right corner for cassava and centrosema as revealed by two-way ANOVA; Table S1: Summary of the parametric and robust one-way ANOVA on crop yields and Δ13C in centrosema; Table S2: Summary of the parametric and robust one-way ANOVA on soil parameters at harvest time.
Author Contributions
Conceptualization, all authors.; methodology, formal analysis, visualization R.M., R.H.-N., A.M. and K.M.K.; resources, N.N., M.M., M.G. and A.M.; writing—original draft, R.M.; writing—review and editing, R.M.; supervision, K.M.K., A.M., R.H.-N. and R.M.K.; funding acquisition, R.M.K., R.M., A.M., M.M., N.N. and K.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ASEA-Uninet, grant numbers 2019/BOKU/2 and 2018/BOKU/4, the Austrian Development Agency, grant number 2706-00/2018, Erasmus+ actions (R.M.K. and R.M. 2018/2019), and by the Indonesia-Austria Scholarship Program in collaboration between the Ministry of Education of Indonesia and OeAD GmbH, grant number 75/D3/PG/2019.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank the farmers who are involved as our partners in this study. We would like to thank BPTP Bangka Belitung for providing compost from their research station and for valuable discussions on the project. We thank PT Timah for Centrosema pubescens seeds; Peter Strauss and the Bundesamt für Wasserwirtschaft (BAW) for estimation of hydraulic soil properties; Katharina Schott for carbon isotope discrimination measurement; Elisabeth Kopecky, Karin Hackl, and Astrid Hobel for TOC, TN, and CEC analyses; and Luca Giuliano Bernardini for statistical advice. Special thanks go to Sabine Huber for pre-submission review of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buta, M.; Blaga, G.; Paulette, L.; Păcurar, I.; Roșca, S.; Borsai, O.; Grecu, F.; Sînziana, P.E.; Negrușier, C. Soil Reclamation of Abandoned Mine Lands by Revegetation in Northwestern Part of Transylvania: A 40-Year Retrospective Study. Sustainability 2019, 11, 3393. [Google Scholar] [CrossRef]
- Worlanyo, A.S.; Jiangfeng, L. Evaluating the environmental and economic impact of mining for post-mined land restoration and land-use: A review. J. Environ. Manag. 2021, 279, 111623. [Google Scholar] [CrossRef] [PubMed]
- Sukarman; Gani, R.A. Asmarhansyah Tin mining process and its effects on soils in Bangka Belitung Islands Province, Indonesia. Sains Tanah 2020, 17, 180–189. [Google Scholar]
- Lestari, T.; Apriyadi, R. Genetic potential of cassava biodiversity in Bangka Island, Indonesia TRI. Cell Biol. Dev. 2017, 1, 41–45. [Google Scholar] [CrossRef][Green Version]
- Inonu, I. Pengelolaan Lahan Tailing Timah di Pulau Bangka : Penelitian yang Telah Dilakukan dan Prospek ke Depan. J. Enviagro 2008, 2. Available online: https://garuda.kemdikbud.go.id/documents/detail/273888 (accessed on 8 November 2022).
- Asmarhansyah, A. Improving Soil Properties and Yield of Corn (Zea mays L.) by Application of Organic Amendment on Abandoned Tin-Mining Land in Bangka Island. J. Trop. Soil 2016, 21, 141–151. [Google Scholar] [CrossRef]
- Budianta, D.; Gofar, N.; Andika, G.A. Improvement of Sand Tailing Fertility Derived from Post Tin Mining Using Leguminous Crop Applied by Compost and Mineral Soil. J. Tanah Trop. 2013, 18, 217–223. [Google Scholar]
- Ashraf, M.A.; Maah, M.J.; Yusoff, I. Analysis of physio-chemical parameters and distribution of heavy metals in soil and water of ex-mining area of Bestari Jaya, Peninsular Malaysia. Asian J. Chem. 2011, 23, 3493–3499. [Google Scholar]
- Nath, T.N. Soil texture and total organic matter content and its influences on soil water holding capacity of some selected tea growing soils in Sivasagar district of Assam, India. Int. J. Chem. Sci. 2014, 12, 1419–1429. [Google Scholar]
- Mulcahy, D.N.; Mulcahy, D.L.; Dietz, D. Biochar soil amendment increases tomato seedling resistance to drought in sandy soils. J. Arid Environ. 2013, 88, 222–225. [Google Scholar] [CrossRef]
- Avramova, V.; Meziane, A.; Bauer, E.; Blankenagel, S.; Eggels, S.; Gresset, S.; Grill, E.; Niculaes, C.; Ouzunova, M.; Poppenberger, B.; et al. Carbon isotope composition, water use efficiency, and drought sensitivity are controlled by a common genomic segment in maize. Theor. Appl. Genet. 2019, 132, 53–63. [Google Scholar] [CrossRef]
- Christmann, A.; Weiler, E.W.; Steudle, E.; Grill, E. A hydraulic signal in root-to-shoot signalling of water shortage. Plant J. 2007, 52, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, F.; Simonneau, T.; Muller, B. The Physiological Basis of Drought Tolerance in Crop Plants: A Scenario-Dependent Probabilistic Approach. Annu. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef] [PubMed]
- Supriadi, A.; Oktaviani, K.; Kencono, A.W.; Prasetyo, B.E.; Kurniasih, T.N.; Kurniadi, C.B.; Kurniawan, F.; Alwendra, Y.; Rabbani, Q.; Aprillia, R.; et al. Analisis Pembentukan Harga di Bursa Timah Indonesia dan Dunia; Kementerian Energi Dan Sumber Daya Mineral: Jakarta, Indonesia, 2016. [Google Scholar]
- Zglinicki, K.; Małek, R.; Szamałek, K.; Wołkowicz, S. Mining Waste as a Potential Additional Source of HREE and U for the European Green Deal: A Case Study of Bangka Island (Indonesia). Minerals 2022, 12, 44. [Google Scholar] [CrossRef]
- Rozaki, Z. COVID-19, Agriculture, and Food Security in Indonesia. Rev. Agric. Sci. 2020, 8, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Kral, R.M.; Maftukhah, R.; Mentler, A.; Murtiningrum, M.; Ngadisih, N.; Keiblinger, K.M. Citizen science in the field: Co-experimentation at pilot scale for sustainable use of natural resources. Sustainability 2020, 12, 7700. [Google Scholar] [CrossRef]
- Alvarenga, P.; Carneiro, J.P.; Fangueiro, D.; Cordovil, C.M.d.S.; Bernal, M.P. Chapter 5—Managing organic amendments in agroecosystems to enhance soil carbon storage and mitigate climate change. In Climate Change and Soil Interaction; Prasad, M.N.V., Pietrzykowski, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 89–141. [Google Scholar]
- Navarro-Pedreño, J.; Almendro-Candel, M.B.; Zorpas, A.A. The Increase of Soil Organic Matter Reduces Global Warming, Myth or Reality? Sci 2021, 3, 18. [Google Scholar] [CrossRef]
- Larney, F.J.; Angers, D.A. The role of organic amendments in soil reclamation: A review. Can. J. Soil Sci. 2012, 92, 19–38. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Jeffery, S.; Abalos, D.; Prodana, M.; Bastos, A.C.; Van Groenigen, J.W.; Hungate, B.A.; Verheijen, F. Biochar boosts tropical but not temperate crop yields. Environ. Res. Lett. 2017. [CrossRef]
- Sandhu, S.S.; Kumar, S. Impact of Three Types of Biochar on the Hydrological Properties of Eroded and Depositional Landscape Positions. Soil Sci. Soc. Am. J. 2017, 81, 878–888. [Google Scholar] [CrossRef]
- Jeffery, S.; Meinders, M.B.J.; Stoof, C.R.; Bezemer, T.M.; van de Voorde, T.F.J.; Mommer, L.; van Groenigen, J.W. Biochar application does not improve the soil hydrological function of a sandy soil. Geoderma 2015, 251–252, 47–54. [Google Scholar] [CrossRef]
- Becher, H.H. Influence of long-term liming on aggregate stability of a loess-derived soil. Int. Agrophysics 2001, 15, 67–72. [Google Scholar]
- Keiblinger, K.M.; Bauer, L.M.; Deltedesco, E.; Holawe, F.; Unterfrauner, H.; Zehetner, F.; Peticzka, R. Quicklime application instantly increases soil aggregate stability. Int. Agrophysics 2016, 30, 123–128. [Google Scholar] [CrossRef]
- Abd El Halim, A.A.; El Baroudy, A.A. Influence addition of fine sawdust on the physical properties of expansive soil in the middle Nile Delta, Egypt. J. Soil Sci. Plant Nutr. 2014, 14, 483–490. [Google Scholar] [CrossRef][Green Version]
- FAO. Save and Grow: Cassava A Guide to Sustainable Production Intensification; FAO: Rome, Italy, 2013. [Google Scholar]
- Media Indonesia. Mentan SYL Genjot Industri Tepung Tapioka dan Sagu di Babel 2020. Available online: https://mediaindonesia.com/ekonomi/334997/mentan-syl-genjot-industri-tepung-tapioka-dan-sagu-di-babel (accessed on 8 November 2022).
- Burns, A.E.; Gleadow, R.M.; Zacarias, A.M.; Cuambe, C.E.; Miller, R.E.; Cavagnaro, T.R. Variations in the chemical composition of cassava (Manihot esculenta Crantz) leaves and roots as affected by genotypic and environmental variation. J. Agric. Food Chem. 2012, 60, 4946–4956. [Google Scholar] [CrossRef] [PubMed]
- Van Der Eng, P. Cassava in Indonesia: A historical re-appraisal of an enigmatic food crop. Southeast Asian Stud. 1998, 36, 3–31. [Google Scholar]
- El-Sharkawy, M.A. Cassava biology and physiology. Plant Mol. Biol. 2004, 56, 481–501. [Google Scholar] [CrossRef]
- Hairiah, K.; Van Noordwijk, M.; Cadisch, G. Crop yield, C and N balance of three types of cropping systems on an Ultisol in Northern Lampung. NJAS-Wagening. J. Life Sci. 2000, 48, 3–17. [Google Scholar] [CrossRef][Green Version]
- Islami, T.; Guritno, B.; Basuki, N.; Suryanto, A. Biochar for sustaining productivity of cassava based cropping systems in the degraded lands of East Java, Indonesia. J. Trop. Agric. 2011, 49, 40–46. [Google Scholar]
- Olson, K.R.; Ebelhar, S.A.; Lang, J.M. Cover crop effects on crop yields and soil organic carbon content. Soil Sci. 2010, 175, 89–98. [Google Scholar] [CrossRef]
- Steenwerth, K.; Belina, K.M. Cover crops enhance soil organic matter, carbon dynamics and microbiological function in a vineyard agroecosystem. Appl. Soil Ecol. 2008, 40, 359–369. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Alva, A. Growing Cover Crops to Improve Biomass Accumulation and Carbon Sequestration: A Phytotron Study. J. Environ. Prot. 2010, 1, 73–84. [Google Scholar] [CrossRef]
- Sanusi, R.O.; Lordbanjou, D.-T.; Ibrahim, A.O.; Abubakar, M.B.; Oke, O.O. Cassava Production Enterprise in the Tropics. In Tropical Plant Species and Technological Interventions for Improvement; Khan, D.M.S., Ed.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- El-Sharkawy, M.A.; De Tafur, S.M. Comparative photosynthesis, growth, productivity, and nutrient use efficiency among tall- and short-stemmed rain-fed cassava cultivars. Photosynthetica 2010, 48, 173–188. [Google Scholar] [CrossRef]
- Fening, J.O.; Quansah, C.; Sarfo-Kantanka, A. Response of Three Forage Legumes To Soil. J. Sci. Technol. 2009, 29, 24–30. [Google Scholar]
- El-Sharkawy, M.A.; De Tafur, S.M. Genotypic and within canopy variation in leaf carbon isotope discrimination and its relation to short-term leaf gas exchange characteristics in cassava grown under rain-fed conditions in the tropics. Photosynthetica 2007, 45, 515–526. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Shaheen, R.; Hood-nowotny, R.C. Effect of drought and salinity on carbon isotope discrimination in wheat cultivars. Plant Sci. 2005, 168, 901–909. [Google Scholar] [CrossRef]
- Vantyghem, M.; Merckx, R.; Stevens, B.; Hood-Nowotny, R.; Swennen, R.; Dercon, G. The potential of stable carbon isotope ratios and leaf temperature as proxies for drought stress in banana under field conditions. Agric. Water Manag. 2022, 260, 107247. [Google Scholar] [CrossRef]
- BMKG Indonesia Data Online BMKG. Available online: https://dataonline.bmkg.go.id/home (accessed on 14 January 2020).
- IUSS Working Group WRB. World Reference Base for Soil Resources Update2015, International Soil Classification System for Naming Soils and Creating Legends for Soilmaps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- ÖNORM L 1061-1; Physical analysis of soils—Determination of particle size distribution in mineral soils used for agriculture and forestry—Part 1: Coarse soil. Austrian Standards International: Vienna, Austria, 2019; pp. 2–4.
- ÖNORM L 1061-2; Physical analysis of soils—Determination of particle size distribution in mineral soils used for agriculture and forestry—Part 2: Fine soil. Austrian Standards International: Vienna, Austria, 2019.
- ÖNORM L 1050; Soil as plant site—Terms, definitions and test methods. Austrian Standards International: Vienna, Austria, 2016.
- Eckelmann, W. Bodenkundliche Kartieranleitung. KA5; Sponagel, H., Grottenthaler, W., Hartmann, K.-J., Hartwich, R., Janetzko, P., Joisten, H., Kühn, D., Sabel, K.-J., Traidl, R., Eds.; Schweizerbart Science: Stuttgart, Germany, 2005. [Google Scholar]
- Dane, J.H.; Topp, C.; Campbell, G.S.; Horton, R.; Jury, W.A.; Nielsen, D.R.; van Es, H.M.; Wierenga, P. Methods of Soil Analysis. Part 4, Physical Methods; Soil Science Society of America, Inc.: Madison, WI, USA, 2002. [Google Scholar]
- ÖNORM L 1080; Chemical Analyses of Soils—Determination of Organic Carbon by Dry Combustion with and without Consideration of Carbonates. Austrian Standards International: Vienna, Austria, 2013.
- Brandstetter, A.; Sletten, R.S.; Mentler, A.; Wenzel, W.W. Estimating dissolved organic carbon in natural waters by UV absorbance (254 nm). J. Plant Nutr. Soil Sci. 1996, 159, 605–607. [Google Scholar] [CrossRef]
- ÖNORM L 1086-1; Chemical analyses of soils—Determination of exchangeable cations and of effective cations exchange capacity (CECeff) by extraction with bariumchloride solution. Austrian Standards International: Vienna, Austria, 2001.
- ÖNORM L 1085; Chemical analyses of soils—Acid digest for the determination of nutritive and toxic elements. Austrian Standards International: Vienna, Austria, 1999.
- ÖNORM L 1087; Chemical analysis of soils—Determination of “plant-available” phosphorus and potassium by the calcium-acetate-lactate (CAL)-method. Austrian Standards International: Vienna, Austria, 2001.
- Scripps Mauna Loa and South Pole Difference Graphic|Scripps CO2 Program. Available online: https://scrippsco2.ucsd.edu/ (accessed on 19 May 2021).
- Mair, P.; Wilcox, R. Robust statistical methods in R using the WRS2 package. Behav. Res. Methods 2020, 52, 464–488. [Google Scholar] [CrossRef]
- Suwarto; Parlindungan, E.S.; Asih, R. Potency legume cover crops as a source of organic material in situ and its effect on the growth and tuber yield of cassava (Manihot esculenta). Plant Arch. 2020, 20, 1484–1490. [Google Scholar]
- Krstić, Đ.; Vujić, S.; Jaćimović, G.; D’Ottavio, P.; Radanović, Z.; Erić, P.; Ćupina, B. The Effect of Cover Crops on Soil Water Balance in Rain-Fed Conditions. Atmosphere 2018, 9, 492. [Google Scholar] [CrossRef]
- Blanco-canqui, H.; Shaver, T.M.; Lindquist, J.L.; Shapiro, C.A.; Elmore, R.W.; Francis, C.A.; Hergert, G.W. Cover Crops and Ecosystem Services : Insights from Studies in Temperate Soils. J. Agron. 2015, 107, 2449–2474. [Google Scholar] [CrossRef]
- Berriel, V.; Perdomo, C.; Monza, J. Carbon Isotope Discrimination and Water-Use Efficiency in Crotalaria Cover Crops under Moderate Water Deficit. J. Soil Sci. Plant Nutr. 2020, 20, 537–545. [Google Scholar] [CrossRef]
- Unger, P.W.; Vigil, M.F. Cover crop effects on soil water relationships. J. Water Soil Conserv. 1998, 53, 200–207. [Google Scholar]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Muirhead, B.; Wright, G.; Bird, M.I. Biochar and biochar-compost as soil amendments: Effects on peanut yield, soil properties and greenhouse gas emissions in tropical North Queensland, Australia. Agric. Ecosyst. Environ. 2015, 213, 72–85. [Google Scholar] [CrossRef]
- Bezabeh, M.W.; Hailemariam, M.H.; Sogn, T.A.; Eich-Greatorex, S. Yield, nutrient uptake, and economic return of faba bean (Vicia faba L.) in calcareous soil as affected by compost types. J. Agric. Food Res. 2021, 6, 100237. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black Carbon Increases Cation Exchange Capacity in Soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Liu, J.; Schulz, H.; Brandl, S.; Miehtke, H.; Huwe, B.; Glaser, B. Short-term effect of biochar and compost on soil fertility and water status of a Dystric Cambisol in NE Germany under field conditions. J. Plant Nutr. Soil Sci. 2012, 175, 698–707. [Google Scholar] [CrossRef]
- Tammeorg, P.; Simojoki, A.; Mäkelä, P.; Stoddard, F.L.; Alakukku, L.; Helenius, J. Biochar application to a fertile sandy clay loam in boreal conditions: Effects on soil properties and yield formation of wheat, turnip rape and faba bean. Plant Soil 2014, 374, 89–107. [Google Scholar] [CrossRef]
- Topoliantz, S.; Ponge, J.F.; Ballof, S. Manioc peel and charcoal: A potential organic amendment for sustainable soil fertility in the tropics. Biol. Fertil. Soils 2005, 41, 15–21. [Google Scholar] [CrossRef]
- Tian, G.; Kang, B.T.; Brussaard, L. Effect of mulch quality on earthworm activity and nutrient supply in the humid tropics. Soil Biol. Biochem. 1997, 29, 369–373. [Google Scholar] [CrossRef]
- Gouveia, C.S.S.; Ganança, J.F.T.; Slaski, J.; Lebot, V.; Pinheiro, M.Â.A.; Carvalho, D. Stable isotope natural abundances (δ 13 C and δ 15 N) and carbon-water relations as drought stress mechanism response of taro (Colocasia esculenta L.). J. Plant Physiol. 2019, 232, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, C.S.S.; Ganança, J.F.T.; Slaski, J.; Lebot, V.; Pinheiro, M.Â.A.; Carvalho, D. Variation of carbon and isotope natural abundances (δ 15 N and δ 13 C) of whole-plant sweet potato (Ipomoea batatas L.) subjected to prolonged water stress. J. Plant Physiol. 2019, 243, 153052. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, L.; Zhang, C.; Ning, Y.; Zhang, Y. Effect of water stress on dry mass accumulation and allocation in sweet potato based on stable isotope analysis. Can. J. Plant Sci. 2015, 95, 263–271. [Google Scholar] [CrossRef]
- Zheng, S.; Cui, N.; Gong, D.; Wang, Y.; Hu, X.; Feng, Y. Relationship between stable carbon isotope discrimination and water use efficiency under deficit drip irrigation of kiwifruit in the humid areas of South China. Agric. Water Manag. 2020, 240, 106300. [Google Scholar] [CrossRef]
- De Souza, C.; Maroco, J.; dos Santos, T.; Rodrigues, M.; Lopes, C.; Pereira, J.; Chaves, M. Impact of deficit irrigation on water use efficiency and carbon isotope composition (d 13 C) of field-grown grapevines under Mediterranean climate. J. Environ. Sci. (China) 2005, 56, 2163–2172. [Google Scholar]
- Tcherkez, G.; Nogués, S.; Bleton, J.; Cornic, G.; Badeck, F.; Ghashghaie, J. Metabolic origin of carbon isotope composition of leaf dark-respired CO2 in French bean. Plant Physiol. 2003, 131, 237–244. [Google Scholar] [CrossRef]
- O’Geen, A.T. Soil Water Dynamics. Nat. Educ. Knowl. 2013, 4, 9. [Google Scholar]
- Bednik, M.; Medyńska-Juraszek, A.; Dudek, M.; Kloc, S.; Kręt, A.; Łabaz, B.; Waroszewski, J. Wheat Straw Biochar and NPK Fertilization Efficiency in Sandy Soil Reclamation. Agronomy 2020, 10, 496. [Google Scholar] [CrossRef]
- Hudson, B.D. Soil organic matter and available water capacity. J. Soil Water Conserv. 1994, 49, 189–194. [Google Scholar]
- Cellier, A.; Gauquelin, T.; Baldy, V.; Ballini, C. Effect of organic amendment on soil fertility and plant nutrients in a post-fire Mediterranean ecosystem. Plant Soil 2014, 376, 211–228. [Google Scholar] [CrossRef]
- Ozores-hampton, M.; Stansly, P.A.; Salame, T.P.; Stansly, P.A.; Salame, T.P.; Ozores-hampton, M.; Stansly, P.A. Soil Chemical, Physical, and Biological Properties of a Sandy Soil Subjected to Long-Term Organic Amendments. J. Sustain. Agric. 2011, 35, 243–259. [Google Scholar] [CrossRef]
- Ullah, N.; Ditta, A.; Imtiaz, M.; Li, X.; Jan, A.U.; Mehmood, S.; Rizwan, M.S.; Rizwan, M. Appraisal for organic amendments and plant growth-promoting rhizobacteria to enhance crop productivity under drought stress: A review. J. Agron. Crop Sci. 2021, 207, 783–802. [Google Scholar] [CrossRef]
- Frimpong, K.A.; Phares, C.A.; Boateng, I.; Abban-Baidoo, E.; Apuri, L. One-time application of biochar influenced crop yield across three cropping cycles on tropical sandy loam soil in Ghana. Heliyon 2021, 7, e06267. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).