The Evaluation of the Effects of Zn, and Amino Acid-Containing Foliar Fertilizers on the Physiological and Biochemical Responses of a Hungarian Fodder Corn Hybrid
Abstract
:1. Introduction
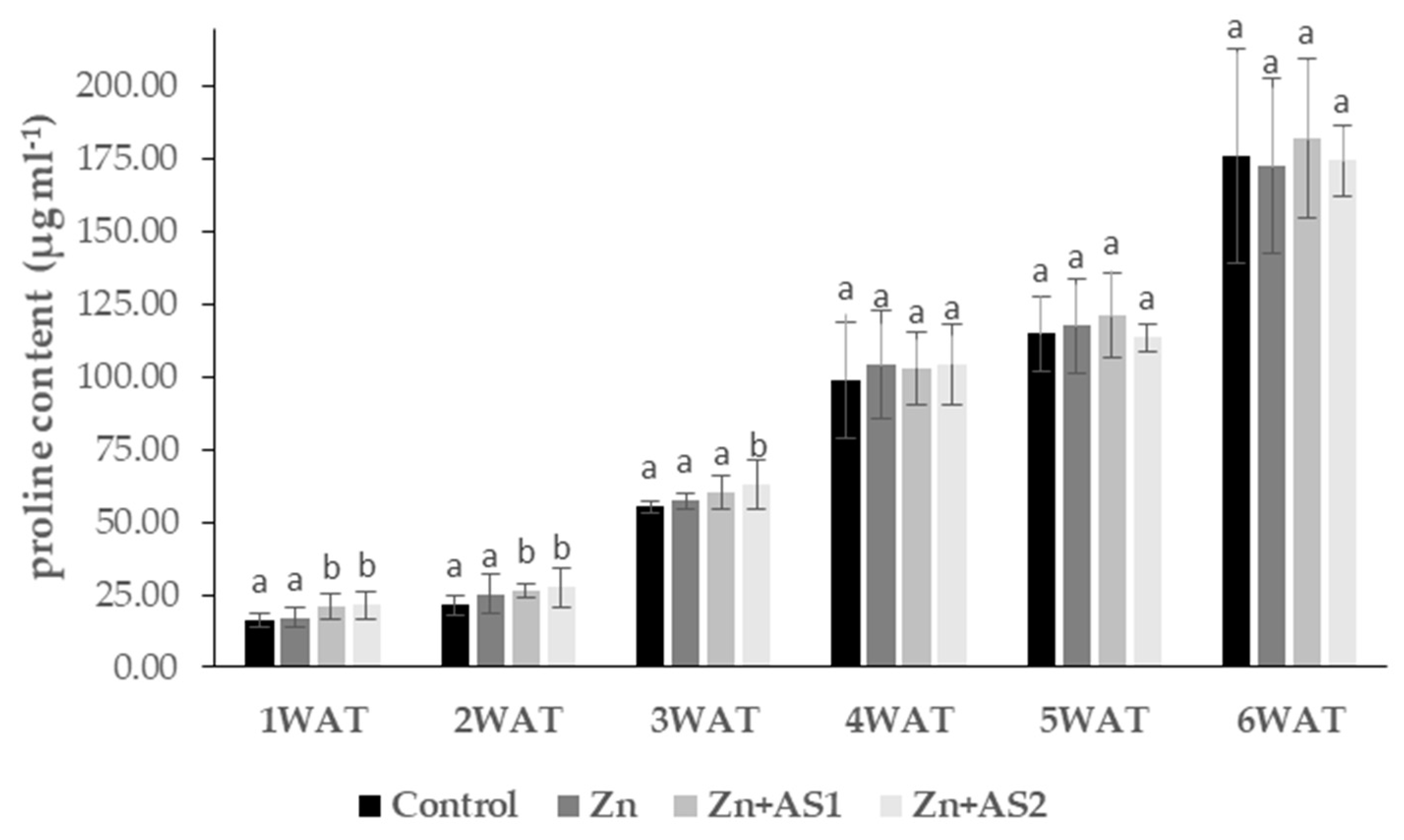
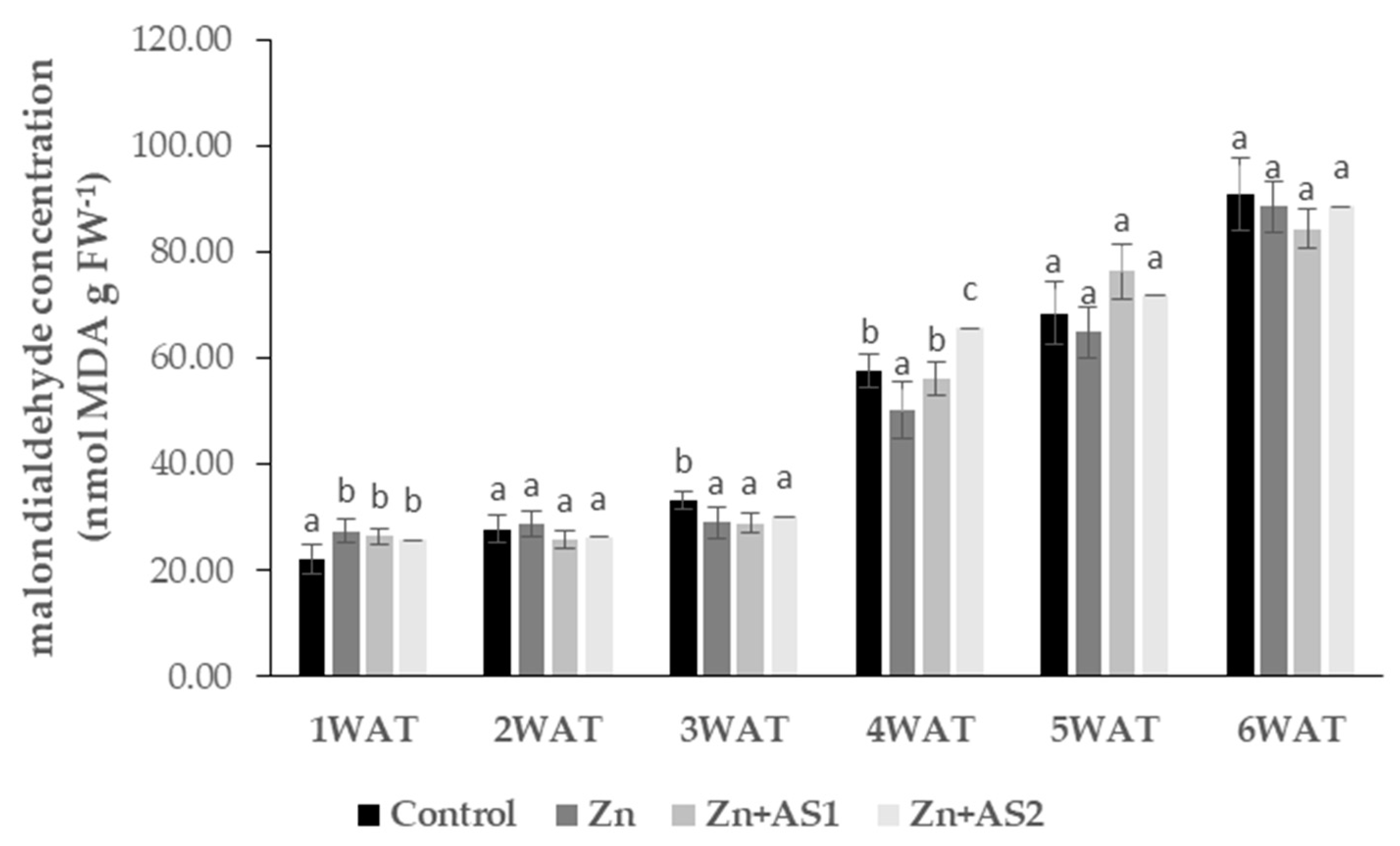
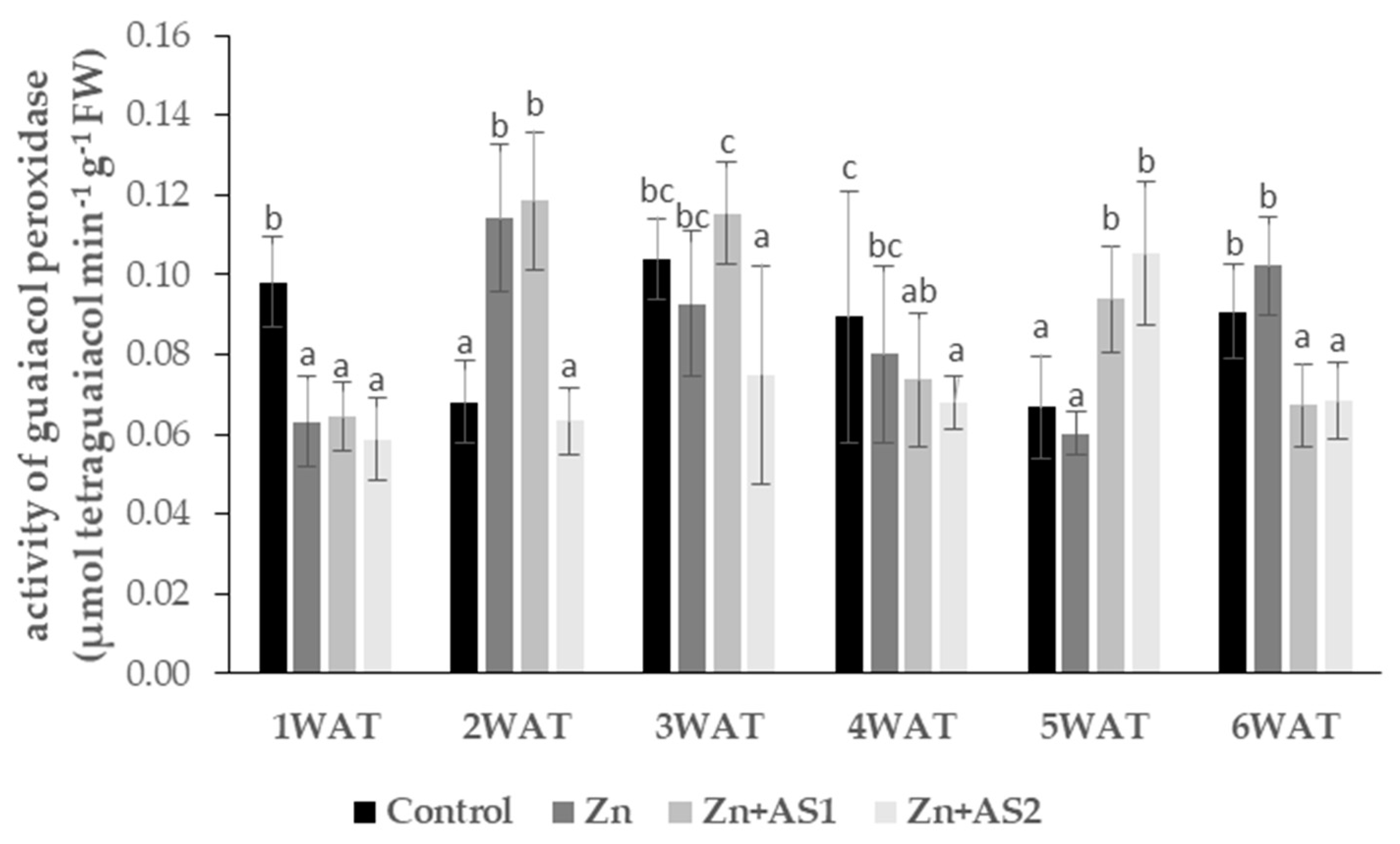
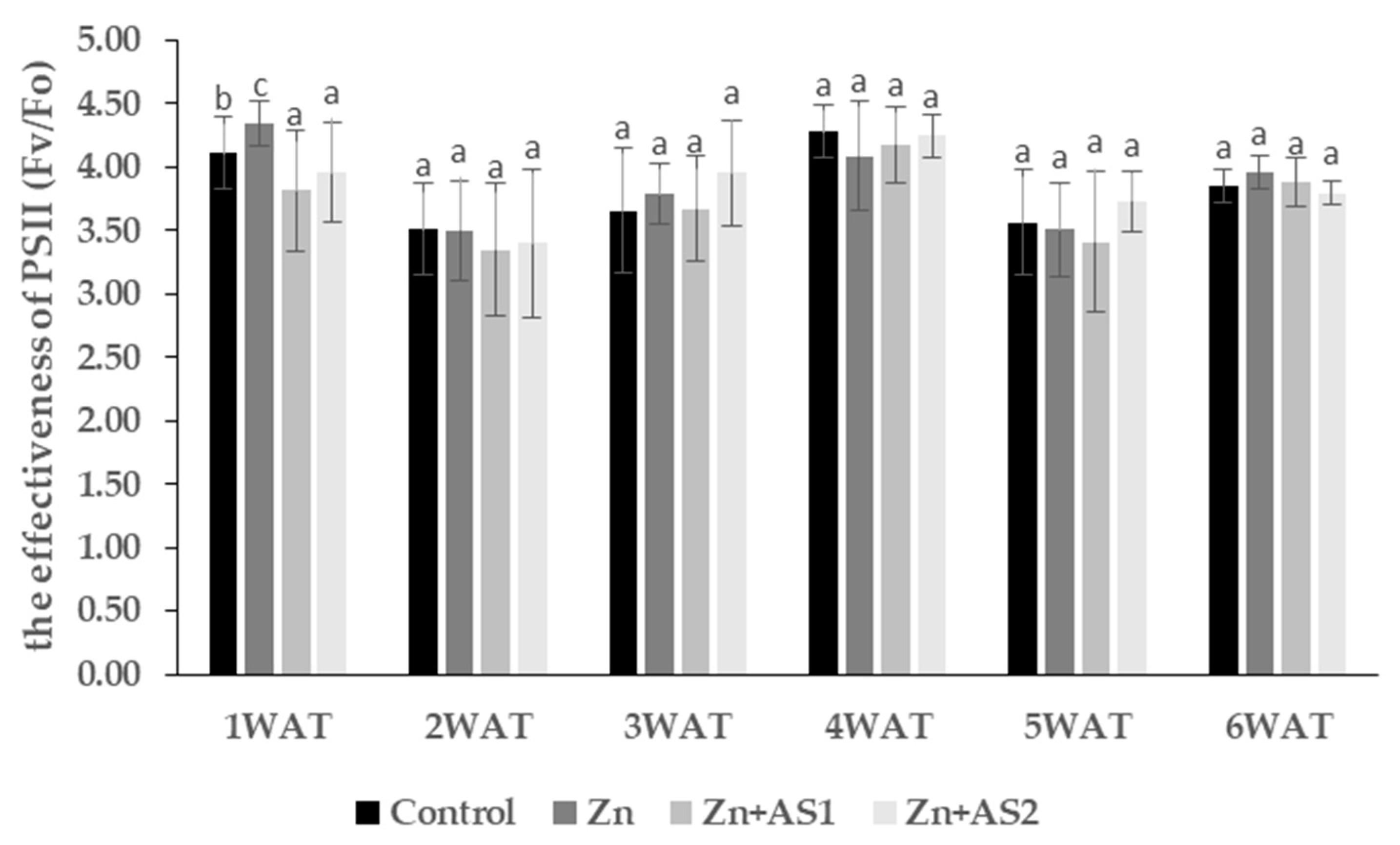
2. Results
3. Discussion
4. Materials and Methods
4.1. Details of the Field Experiment
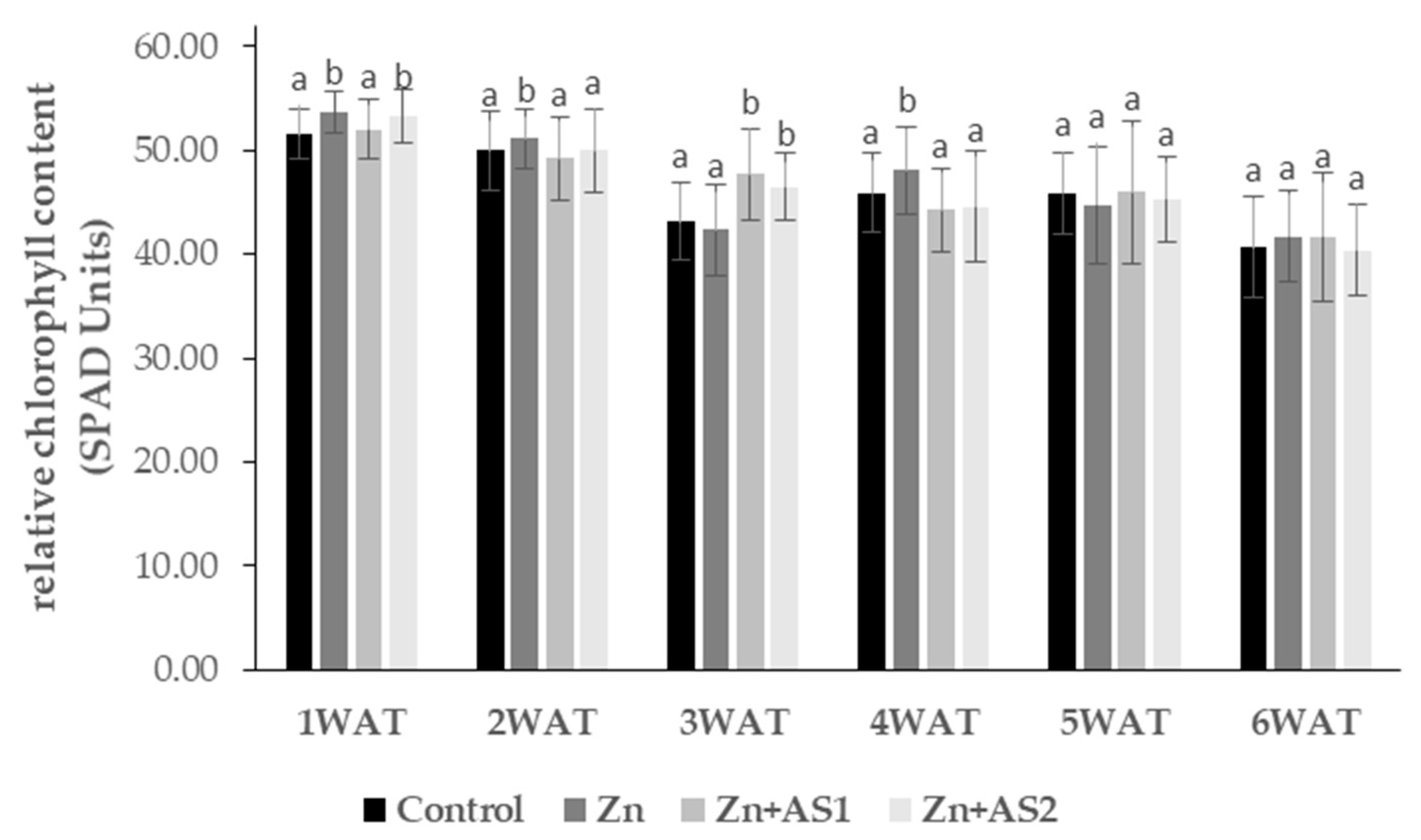
4.2. Relative Chlorophyll Content (SPAD Units)
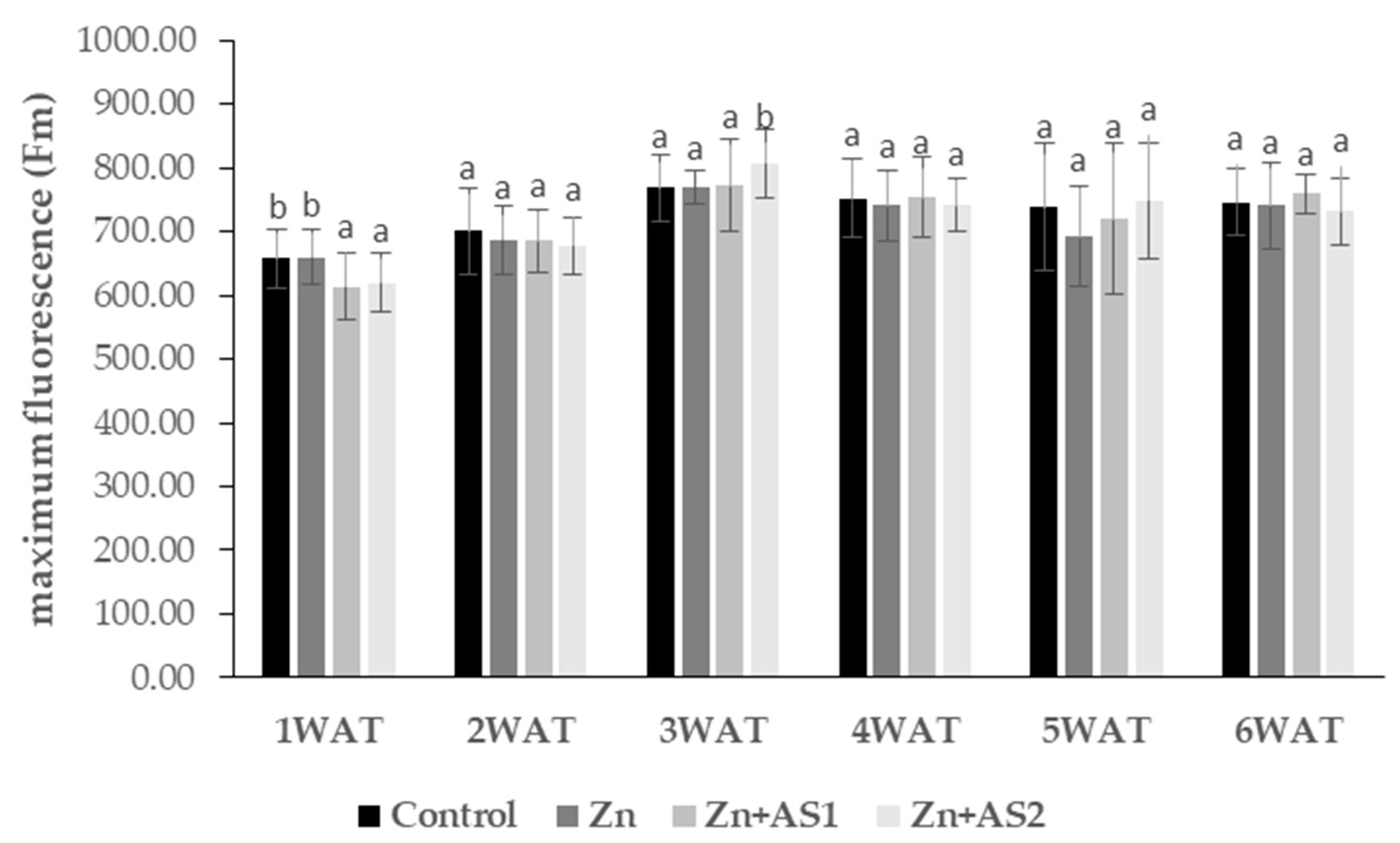
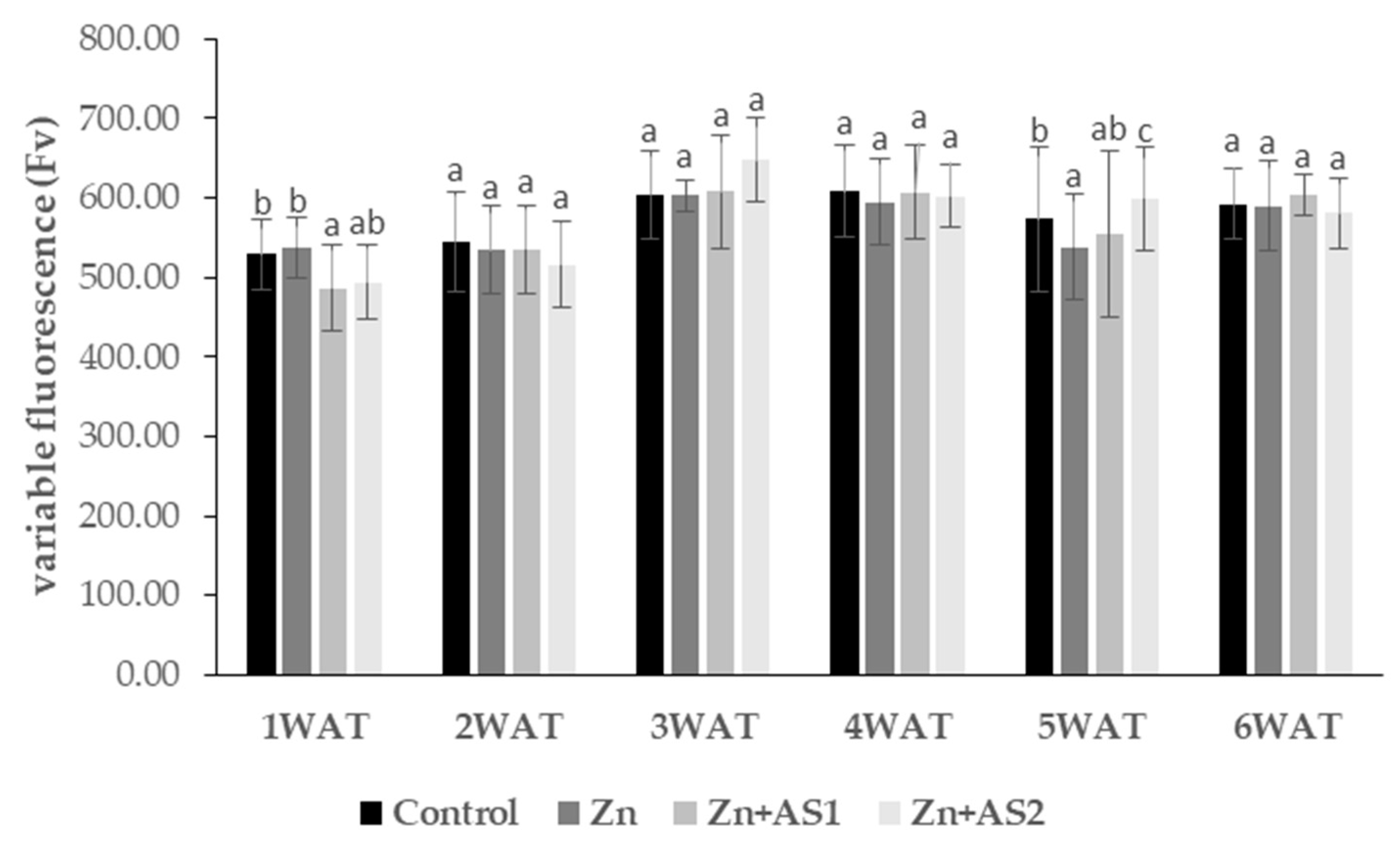
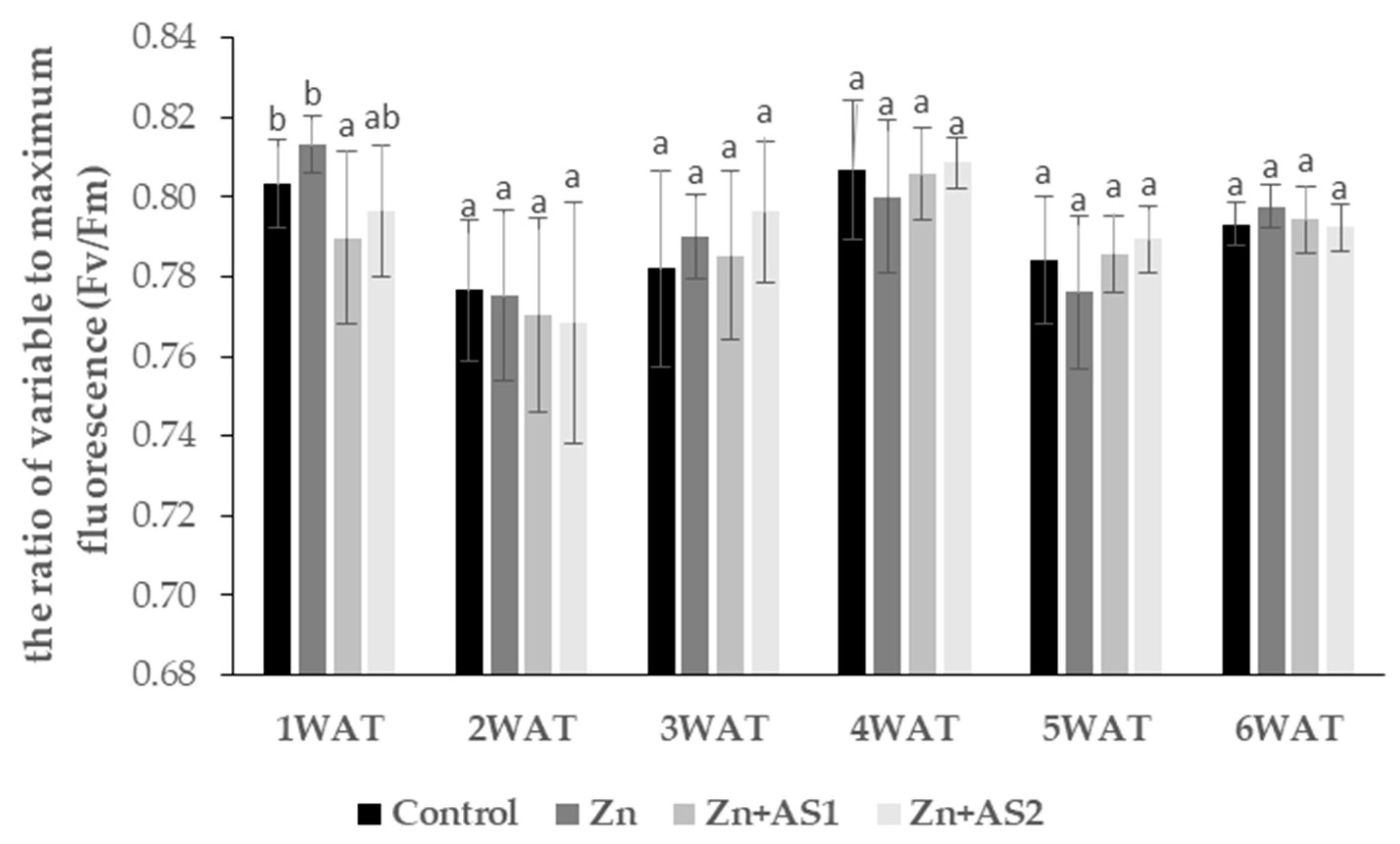
4.3. The Photochemical Efficiency of Photosystem II (PSII)
4.4. Determination of Proline Content of Leaves
4.5. Determination of Malondialdehyde (MDA) Concentration
4.6. Antioxidant Enzymes Assays
4.7. Measurements of Quality Parameters and Yield
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zhu, J.K. Abiotic stress signaling and response in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.; He, C.Q.; Ding, N.Z. Abiotic stresses: General defenses of land plants and chances for engineering multi stress tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Igbal, M.S.; Hashem, A.; Allah, E.F.A.; Ansari, M.I. Plant defence response to biotic stress and its interplay with fluctuating dark/light conditions. Front. Plant Sci. 2021, 12, 631810. [Google Scholar] [CrossRef]
- Szőke, L.; Moloi, M.J.; Kovács, G.E.; Biró, G.; Radócz, L.; Hájos, T.M.; Kovács, B.; Rácz, D.; Danter, M.; Tóth, B. The application of phytohormones as biostimatlants in corn smut infected Hungarian sweet and fodder corn hybrides. Plants 2021, 10, 1822. [Google Scholar] [CrossRef]
- Lynch, J.P.; Clair, S.B. Mineral stress: The missing link in understanding how global climate change will affect plants in real world soils. Field Crop Res. 2004, 90, 101–115. [Google Scholar] [CrossRef]
- Tóth, B.; Moloi, M.J. Interdependence between low pH and heavy metal stress is crucial for crop production efficiency. In Contemporary Studies in Scineces; Efe, R., Cürebal, I., Eds.; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2020; pp. 2–17. ISBN 1-5275-5424-4. [Google Scholar]
- Azimi, S.; Kaur, T.; Gandhi, T.K. A deep learning approach to measure stress level in plants due to nitrogen deficiency. Measurements 2021, 173, 108650. [Google Scholar] [CrossRef]
- Demirel, U.; Morris, W.L.; Ducreux, L.J.; Yavuz, C.; Asim, A.; Tindas, I.; Campbell, R.; Morris, J.A.; Verrall, S.R.; Hedley, P.E.; et al. Physiological, biochemical, and transcriptional responses to single and combined abiotic stress in stress-tolerant and stress-sensitive potato genotypes. Front. Plant Sci. 2020, 11, 169. [Google Scholar] [CrossRef]
- Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic stress in crop species: Improving tolerance by applying plant metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef]
- Khatibi, A.; Omrani, S.; Omrani, A.; Shojaei, S.H.; Mousavi, S.M.N.; Illés, Á.; Bojtor, C.; Nagy, J. Response of Maize Hybrids in Drought-Stress Using Drought Tolerance Indices. Water 2022, 14, 1012. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2017, 69, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Bojtor, C.; Illés, Á.; Mousavi, S.M.N.; Széles, A.; Tóth, B.; Nagy, J.; Marton, C.L. Evaluation of the Nutrient Composition of Maize in Different NPK Fertilizer Levels Based on Multivariate Method Analysis. Int. J. Agron. 2021, 2021, 5537549. [Google Scholar] [CrossRef]
- Ding, L.; Wang, K.J.; Jiang, G.M.; Biswas, D.K.; Xu, H.; Li, L.F.; Li, Y.H. Effects of nitrogen deficiency on photosynthetic traits of maize hybrids released in different years. Ann. Bot. 2005, 96, 925–930. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.M.; Chen, H.; He, C.L.; Wang, Q. Nitrogen starvation induced oxidative stress in an oil-producing green alga Chlorell sorokiniana C3. PLoS ONE 2013, 8, e69225. [Google Scholar] [CrossRef]
- Tewari, R.K.; Yadav, N.; Gupta, R.; Kumar, P. Oxidative stress under macronutrient deficiency in plants. J. Soil Sci. Plant Nutr. 2021, 21, 832–859. [Google Scholar] [CrossRef]
- Horváth, É.; Gombos, B.; Széles, A. Evaluation phenology, yield and quality of maize genotypes in drought stress and non-stress environments. Agron. Res. 2021, 19, 408–422. [Google Scholar] [CrossRef]
- Rácz, D.; Szőke, L.; Tóth, B.; Kovács, B.; Horváth, É.; Zagyi, P.; Duzs, L.; Széles, A. Examination of the Productivity and Physiological Responses of Maize (Zea mays L.) to Nitrapyrin and Foliar Fertilizer Treatments. Plants 2021, 10, 2426. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Kielland, K. Soil amino acid turnover dominates the nitrogen flux in permafrost-dominated taiga forest soils. Soil Biol. Biochem. 2002, 34, 209–219. [Google Scholar] [CrossRef]
- Sadak, M.S.H.; Abdelhamid, M.T.; Schmidhalter, U. Effect of foliar application of amino acids on plant yield and some physiological parameters in bean plants irrigated with seawater. Acta Biol. Colomb. 2014, 20, 141–152. [Google Scholar] [CrossRef]
- Koukounara, A.; Tsouvaltzis, P.; Siomos, A. Effect of root and foliar application of amino acids ont he growth and yield of greenhouse tomato in different fertilization levels. J. Food Agric. Environ. 2013, 11, 644–648. [Google Scholar]
- Ge, T.; Song, S.; Roberts, P.; Jones, D.L.; Huang, D.; Iwasaki, K. Amino acids as a nitrogen source for tomato seedlings: The use of dual-labeled (13C, 15N) glycine to test for direct uptake by tomato seedlings. Environ. Exp. Bot. 2009, 66, 357–361. [Google Scholar] [CrossRef]
- Gioseffi, E.; de Neergaard, A.; Schjoerring, J.K. Interactions between uptake of amino acids and inorganic nitrogen in wheat plants. Biogeosciences 2012, 9, 1509–1518. [Google Scholar] [CrossRef] [Green Version]
- Brankov, M.; Simic, M.; Dolijanovic, Z.; Rajkovic, M.; Mandic, V.; Dragicevic, V. The response of maize lines to foliar fertilizing. Agriculture 2020, 10, 365. [Google Scholar] [CrossRef]
- Bahari, A.; Pirdashti, H.; Yaghubi, M. The effects of amino acids fertilizers spraying on photosynthetic pigments and antioxidant enzymes of wheat (Triticum aestivum L.) under salinity stress. Int. J. Agron. Plant Prod. 2013, 4, 787–793. [Google Scholar]
- Tsonev, T.; Lidon, F.J.C. Zinc in plants—An overview. Emir. J. Food Agric. 2012, 24, 322–333. [Google Scholar]
- Kumar, R.; Rathore, D.K.; Meena, B.S.; Singh, M.; Kumar, U.; Meena, V.K. Enhancing productivity and quality of fodder maize through soil and foliar zinc nutrition. Indian J. Agric. Res. 2016, 50, 259–263. [Google Scholar] [CrossRef] [Green Version]
- Cherif, J.; Mediouni, C.; Ammar, W.B.; Jemal, F. Interactions of zinc and cadmium toxicity in their effects on growth and in antioxidative systems in tomato plants (Solanum lycopersicum). J. Environ. Sci. 2011, 23, 837–844. [Google Scholar] [CrossRef]
- Prasad, R.; Shivay, Y.S.; Kumar, D. Agronomic biofortification of cereal grains with iron and zinc. Adv. Agron. 2014, 125, 55–91. [Google Scholar]
- El-Mageed, A.; Taia, A.; Rady, M.O.; Semida, W.M.; Shaaban, A.; Mekdad, A.A. Exogenous micronutrients modulate morpho-physiological attributes, yield, and sugar quality in two salt-stressed sugar beet cultivars. J. Soil Sci. Plant Nutr. 2021, 21, 1421–1436. [Google Scholar] [CrossRef]
- Alloway, B.J. Zinc in Soils and Crop Nutrition; International Zinc Association: Brussels, Belgium; International Fertilizer Industry Association: Paris, France, 2008. [Google Scholar]
- Haslett, B.S.; Reid, R.J.; Rengel, Z. Zinc mobility in wheat: Uptake and distribution of zinc applied to leaves or roots. Ann. Bot. 2001, 87, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Jakab, P.; Zoltan, G.; Komarek, L. The effects of foliar fertilization on the yield and generative factors of maize. Rev. Agric. Rural Dev. 2016, 5, 158–161. [Google Scholar] [CrossRef]
- Ivanov, K.; Tonev, T.; Nguyen, N.; Peltekov, A.; Mitkov, A. Impact of foliar fertilization with nanosized zinc hydroxy nitrate on maize yield and quality. Emir. J. Food Agric. 2019, 31, 597–604. [Google Scholar] [CrossRef] [Green Version]
- Awad, A.A.M.; Rady, M.M.; Semida, W.M.; Belal, E.E.; Omran, W.M.; Al-Yasi, H.M.; Ali, E.F. Foliar nourisgment with different zinc-contatining forms effectively sustains carrot performance in zinc-deficient soil. Agronomy 2021, 11, 1853. [Google Scholar] [CrossRef]
- Fu, X.Z.; Xing, F.; Cao, L.; Chun, C.P.; Ling, L.L.; Jiang, C.L.; Peng, L.Z. Effects of foliar application of various zinc fertilizers with organosilicone on correcting citrus inc deficiency. HortScience 2016, 51, 422–426. [Google Scholar] [CrossRef] [Green Version]
- Afsahi, K.; Nazari, M.; Omidi, H.; Shekari, F.; Bostoni, A.A. The effects of different methods of zinc application on canola seed yield and oil content. J. Plant Nutr. 2020, 43, 8. [Google Scholar] [CrossRef]
- Samreen, T.; Humaira; Shah, H.U.; Ullah, S.; Javid, M. Zinc effect of growth rate, chlorophyll, protein and mineral contents of hydroponically grown mungbeans plants (Vigna radiata). Arab. J. Chem. 2017, 10, S1802–S1807. [Google Scholar] [CrossRef] [Green Version]
- Kandoliya, R.; Sakarvadiya, H.L.; Kunjadia, B.B. Effect of zinc and iron application on leaf chlorophyll, carotenoid, grain yield and quantity of wheat in calcareous soil of Saurashtra region. Int. J. Chem. Stud. 2018, 6, 2092–2096. [Google Scholar]
- Mohammadi, M.; Hoseini, N.M.; Chaichi, M.R.; Alipour, H.; Dashtaki, M.; Safikhani, S. Influence of nano-iron oxide and zinc sulfate on physiological characteristics of peppermint. Commun. Soil Sci. Plant Anal. 2018, 49, 2315–2326. [Google Scholar] [CrossRef]
- Liu, H.; Gan, W.; Rengel, Z.; Zhao, P. Effects of zinc fertilizer rate and application method on photosynthetic characteristics and grain yield of summer maize. J. Soil Sci. Plant Nutr. 2016, 16, 550–562. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.B.; Mulllins, G.L. Foliar burn and wheat grain yield responses following topdress-applied nitrogen and sulfur fertilizers. J. Plant Nutr. 2004, 27, 921–930. [Google Scholar] [CrossRef]
- Tardos, M.J.; Omari, H.J.; Turk, M.A. The morphological, physiological and biochemical responses of sweet corn to foliar application of amino acids biostimulants sprayed at three growth stages. Aust. J. Crop Sci. 2019, 13, 412–417. [Google Scholar] [CrossRef]
- Żebrowska, J.; Michałek, W. Chlorophyll fluorescence in two strawberry (Fragaria × ananassa Duch.) cultivars. J. Cent. Eur. Agric. 2014, 15, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Dai, Z.; Li, S.; Xin, H. A novel system for evaluating drought-cold tolerance of grapevines using chlorophyll fluorescence. BMC Plant Biol. 2015, 15, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanowska-Duda, Z.; Grzesik, M.; Janas, R. Maximal efficiency of PSII as a marker of sorghum development fertilized with waste from a biomass biodigestion to methane. Front. Plant Sci. 2019, 9, 1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [Green Version]
- El-Zohri, M.; Al-Wadaani, N.A.; Bafeel, S.O. Foliar sprayed green zinc oxide nanoparticles mitigate drought-induced oxidatives stress in tomato. Plants 2021, 10, 2400. [Google Scholar] [CrossRef]
- Tavanti, T.R.; de Melo, A.A.R.; Moreira, L.D.K.; Sanchez, D.E.J.; Silva, R.S.; Silva, R.M.; Reis, A.R. Micronutrient fertilization enhances ROS scavenging system for alleviation of abiotic stresses in plants. Plant Physiol. Biochem. 2021, 160, 386–396. [Google Scholar] [CrossRef]
- Ma, J.Z.; Zhang, M.; Liu, Z.G.; Wang, M.; Sun, Y.; Zheng, W.K.; Lu, H. Copper-based-zinc-boron foliar fertilizer improved yield, quality, physiological characteristics, and mircoelement concentration of celery (Apium graveolens L.). Environ. Pollut. Bioavailab. 2019, 21, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Luo, Q.; Huang, K.; Yang, G.; He, G. Prospecting for microelement function and biosafety assessment of transgenic cereal plants. Front. Plant Sci. 2018, 9, 326. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acids biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Weiland, M.; Mancuso, S.; Baluska, F. Signalling via glutamate and GLRs in Arabidopsis thaliana. Funct. Plant Biol. 2015, 43, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Santi, C.; Zamboni, A.; Varanini, Z.; Pandolfini, T. Growth stimulatory effects and genome-wide transcriptional changes produced by protein hydrolysates in maize seedlings. Front. Plant Sci. 2017, 8, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojtaszek, P. The oxidative burst: A plant’s early response against infection. Biochem. J. 1997, 322, 681–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqur, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2018, 221, 1197–1214. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.J.; Zhu, D.H. Functional roles of the plant superoxide dismutase. Yi Chuan/Hereditas 2003, 25, 225–231. [Google Scholar] [PubMed]
- Bharti, K.; Pandey, N.; Shankhdhar, D.; Srivastava, P.C.; Shankhdhar, S.C. Effect of different zinc levels on activity of superoxide dismutases and acid phosphatases and organic acid exudation on wheat genotypes. Physiol. Mol. Biol. Plants 2014, 20, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Shukla, A.K.; Behera, S.K.; Tiwari, P.K. Zinc application enhances superoxide dismutase and carbonic anhydrase activities in zinc-efficient and zinc-inefficient wheat genotypes. J. Soil Sci. Plant Nutr. 2019, 19, 477–487. [Google Scholar] [CrossRef]
- Rengel, Z.; Graham, R.D. Wheat genotypes differ in Zn efficiency when grown in chelate-buffered nutrient solution. I. Vegetative growth. Plant Soil 1995, 176, 307–316. [Google Scholar] [CrossRef]
- Cakmak, I.; Ekiz, H.; Yilmaz, A.; Torum, B.; Köleli, N.; Gültekin, I.; Alkan, A.; Eker, S. Differencet responses of rye, triticale, bread and durum wheats to zinc deficiency in calcaresous soils. Plant Soil 1997, 188, 1–10. [Google Scholar] [CrossRef]
- Mathpal, B.; Srivastava, P.C.; Shankhdhar, D.; Shankhdar, S.C. Zinc enrichmnet in wheat genotypes under various methods of zinc application. Plant Soil Environ. 2015, 61, 171–175. [Google Scholar] [CrossRef] [Green Version]
- Potarzycki, J.; Grzebisz, W. Effect of zinc foliar application on grain yield of maize and its yielding components. Plant Soil Environ. 2009, 55, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Steward, Z.P.; Paparozzi, E.T.; Wortmann, C.S.; Jha, P.K.; Shapiro, C.A. Effect of flioar micronutrients (B, Mn, Fe, Zn) on maize grain yield, micronutrients recovery, uptake, and partitioning. Plants 2021, 10, 528. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, W.; Fagan, E.B.; Soares, L.H.; Soares, J.N.; Reichardt, K.; Neto, D.D. Seed and foliar application of amino acids improves variable of nitrogen metabolism and productivity in soybean crop. Front. Plant Sci. 2018, 9, 396. [Google Scholar] [CrossRef] [Green Version]
- Nemati, H.; Ajam, N.H.; Faragi, A.; Soltani, A. Responses of soybean to micronutrients, amino acid and NPK foliar application under normal and drought stress condition. Environ. Anal. Ecol. Stud. 2019, 6, 623–630. [Google Scholar] [CrossRef]
- MSZ-08-0206-2:1978; Evaluation of Some Chemical Properties of the Soil. Laboratory Tests. Hungarian Standards Institution: Budapest, Hungary, 1978.
- MSZ-08-0205:1978; Determination of Physical and Hydrophysical Properties of Soils. Hungarian Standards Institution: Budapest, Hungary, 1978.
- MSZ 20135:1999; Determination of the Soluble Nutrient Element Content of the Soil. Hungarian Standards Institution: Budapest, Hungary, 1999.
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll Fluorescence as a Nonintrusive Indicator for Rapid Assessment of In Vivo Photosynthesis. In Ecophysiology of Photosynthesis; Springer Study Edition; Schulze, E.D., Caldwell, M.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; Volume 100. [Google Scholar] [CrossRef]
- Carillo, P.; Gibon, Y. Protocol: Extraction and Determination of Proline. 2011. Available online: https://www.researchgate.net/publication/211353600_PROTOCOL_Extraction_and_determination_of_proline (accessed on 15 November 2021).
- Hummel, I.; Pantin, F.; Sulpice, R.; Piques, M.; Rolland, G.; Dauzat, M.; Christophe, A.; Pervent, M.; Bouteillé, M.; Stitt, M.; et al. Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: An integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant Physiol. 2010, 154, 357–372. [Google Scholar] [CrossRef] [Green Version]
- Tóth, B.; Juhász, C.; Labuschagne, M.; Moloi, M.J. The influence of soil acidity on the physiological responses of two bread wheat cultivars. Plants 2020, 9, 1472. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Pukacka, S.; Ratajczak, E. Production and scavenging of reactive oxygen species in Fagus sylvatica seeds during storage at varied temperature and humidity. J. Plant Physiol. 2005, 162, 873–885. [Google Scholar] [CrossRef]
- Mishra, N.P.; Mishra, R.K.; Singhal, G.S. Changes in the activities of anti-oxidant enzymes during exposure of intact wheat leaves to strong visible light at different temperatures in the presence of protein synthesis inhibitors. Plant Physiol. 1993, 102, 903–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeislin, N.; Ben-Zaken, R. Peroxidases, phenylalanine ammonia-lyase and lignification in peduncles of rose flowers. Plant Physiol. Biochem. 1991, 29, 147–151. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [Green Version]
- Mahapoonyanont, N.; Mahapoonyanont, T.; Pengkaew, N.; Kamhangkit, R. Power of the test of One-Way Anova after transforming with large sample size data. Procedia Soc. Behav. Sci. 2010, 9, 933–937. [Google Scholar] [CrossRef] [Green Version]
- Nanda, A.; Mohapatra, B.B.; Mahapatra, A.P.K.; Abiresh Prasad Kumar Mahapatra, A.P.K.; Mahapatra, A.P.K. Multiple comparison test by Tukey’s honestly significant difference (HSD): Do the confident level control type I error. IJAMS 2021, 6, 59–65. [Google Scholar] [CrossRef]




| Parameters | Treatments | |||
|---|---|---|---|---|
| Control | Zn | Zn+AS1 | Zn+AS2 | |
| Moisture % | 12.6 ± 1.30 a | 13.3 ± 1.34 a | 13.2 ± 1.14 a | 13.2 ± 0.59 a |
| Protein % | 5.47 ± 0.49 a | 5.36 ± 0.36 a | 5.42 ± 0.50 a | 5.30 ± 0.33 a |
| Starch % | 64.7 ± 0.51 a | 64.7 ± 0.64 a | 64.6 ± 0.48 a | 64.4 ± 0.57 a |
| Oil % | 3.22 ± 0.42 a | 3.11 ± 0.33 a | 3.25 ± 0.34 a | 3.40 ± 0.36 a |
| Grain/cob (db) | 557 ± 43.2 a | 596 ± 30.8 b,c | 583 ± 32.2 b | 608 ± 43.6 c |
| Grain/cob (g) | 245 ± 30.9 a | 269 ± 21.7 b | 261 ± 19.4 b | 269 ± 19.8 b |
| Thousand-seed weight (g) | 37.0 ± 5.92 a | 30.0 ± 4.79 a | 43.0 ± 6.81 a | 28.5 ± 4.56 a |
| Grain weight/cob (g) 14% | 24 ± 28.3 a | 257 ± 15.9 b | 248 ± 17.6 b | 256 ± 16.09 b |
| Thousand-seed weight at 14% moisture (g) | 416 ± 34.8 a | 430 ± 23.0 a | 425 ± 40.2 a | 422 ± 28.3 a |
| Ear weight (g) | 275 ± 33.6 a | 309 ± 22.9 b | 296 ± 23.9 b | 306 ± 22.5 b |
| Cob weight (g) | 30.6 ± 5.48 a | 36.2 ± 6.15 b | 34.6 ± 4.98 b | 35.3 ± 2.62 b |
| Grain:cob | 12.6 ± 1.30 a | 13.3 ± 1.34 a | 13.2 ± 1.14 a | 13.2 ± 0.59 a |
| Yield t/ha at 14% moisture | 17.3 ± 2.09 a | 18.9 ± 1.25 b | 18.4 ± 1.30 b | 19.0 ± 1.19 b |
| Composition | AS1 | AS2 |
|---|---|---|
| Total amino acids (m/m%) | 47.00 | 21.67 |
| N (m/m%) | 3.20 | 8.0 |
| P (m/m%) | 3.90 | 0.5 |
| K (m/m%) | 3.20 | 0.2 |
| Alanine % | 5.26 | 2.64 |
| Arginine % | 1.52 | <0.10 |
| Aspartic acid % | 3.80 | 1.18 |
| Cysteine % | <0.04 | <0.10 |
| Glycine % | 2.98 | 0.28 |
| Glutamine % | 12.95 | 16.15 |
| Histidine % | 0.96 | 0.13 |
| Leucine + isoleucine % | 2.27 | 0.23 |
| Lysine % | 2.14 | <0.10 |
| Methionine % | 0.90 | <0.10 |
| Phenylalanine % | 1.42 | <0.10 |
| Proline % | 4.23 | 1.04 |
| Serine % | 2.19 | - |
| Threonine % | 2.24 | - |
| Tryptophan % | 0.34 | - |
| Tyrosine % | 1.71 | - |
| Valine % | 2.29 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóth, B.; Moloi, M.J.; Mousavi, S.M.N.; Illés, Á.; Bojtor, C.; Szőke, L.; Nagy, J. The Evaluation of the Effects of Zn, and Amino Acid-Containing Foliar Fertilizers on the Physiological and Biochemical Responses of a Hungarian Fodder Corn Hybrid. Agronomy 2022, 12, 1523. https://doi.org/10.3390/agronomy12071523
Tóth B, Moloi MJ, Mousavi SMN, Illés Á, Bojtor C, Szőke L, Nagy J. The Evaluation of the Effects of Zn, and Amino Acid-Containing Foliar Fertilizers on the Physiological and Biochemical Responses of a Hungarian Fodder Corn Hybrid. Agronomy. 2022; 12(7):1523. https://doi.org/10.3390/agronomy12071523
Chicago/Turabian StyleTóth, Brigitta, Makoena Joyce Moloi, Seyed Mohammad Nasir Mousavi, Árpád Illés, Csaba Bojtor, Lóránt Szőke, and János Nagy. 2022. "The Evaluation of the Effects of Zn, and Amino Acid-Containing Foliar Fertilizers on the Physiological and Biochemical Responses of a Hungarian Fodder Corn Hybrid" Agronomy 12, no. 7: 1523. https://doi.org/10.3390/agronomy12071523










