Ebb-and-Flow Subirrigation Improves Seedling Growth and Root Morphology of Tomato by Influencing Root-Softening Enzymes and Transcript Profiling of Related Genes
Abstract
:1. Introduction
2. Materials and Methods
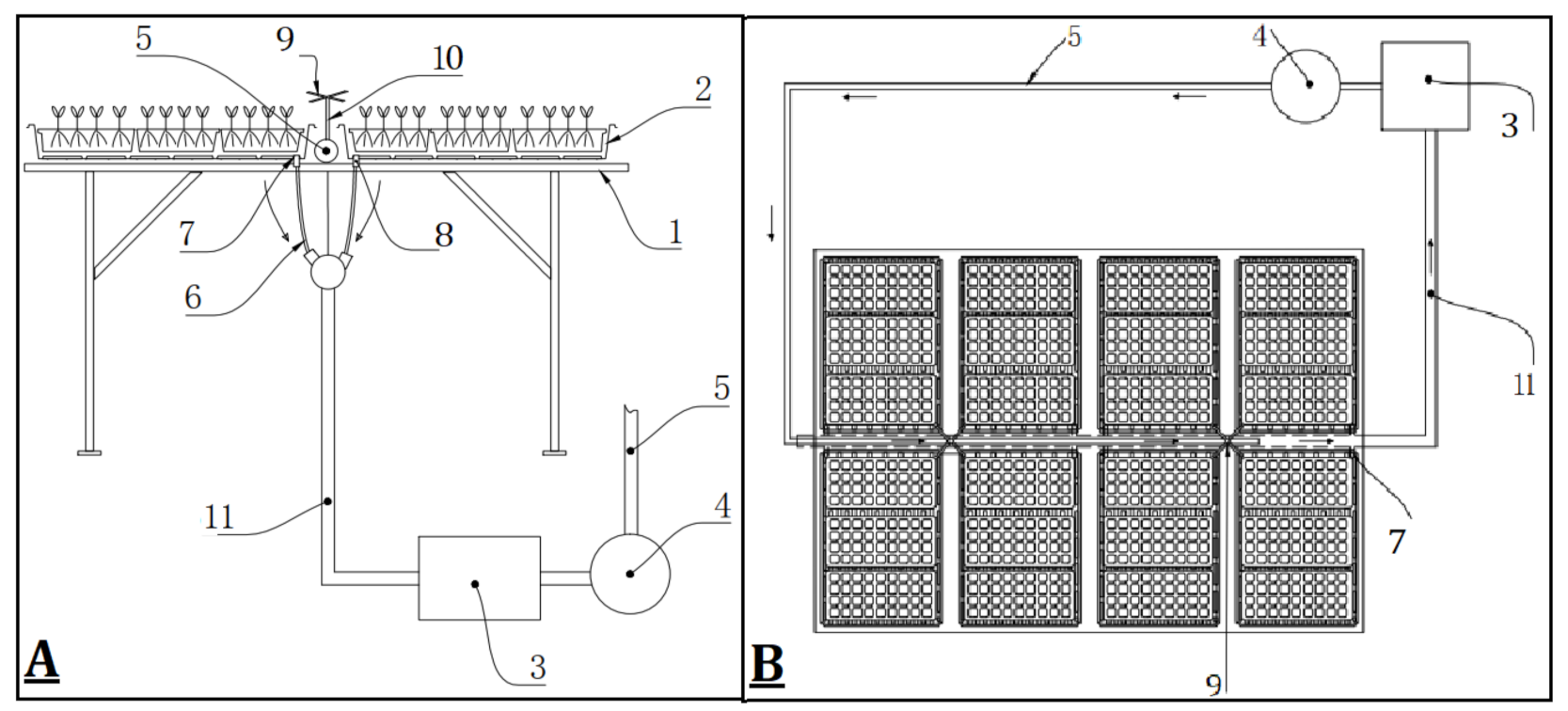
2.1. Plant Source, Experimental Design and Irrigation Treatments
2.2. Morphological Attributes
2.3. Physiological Attributes
2.4. Enzymes Activity Assay
2.5. RNA Extraction, Illumina Sequencing and Gene Expression
2.6. Statistical Analysis
3. Results
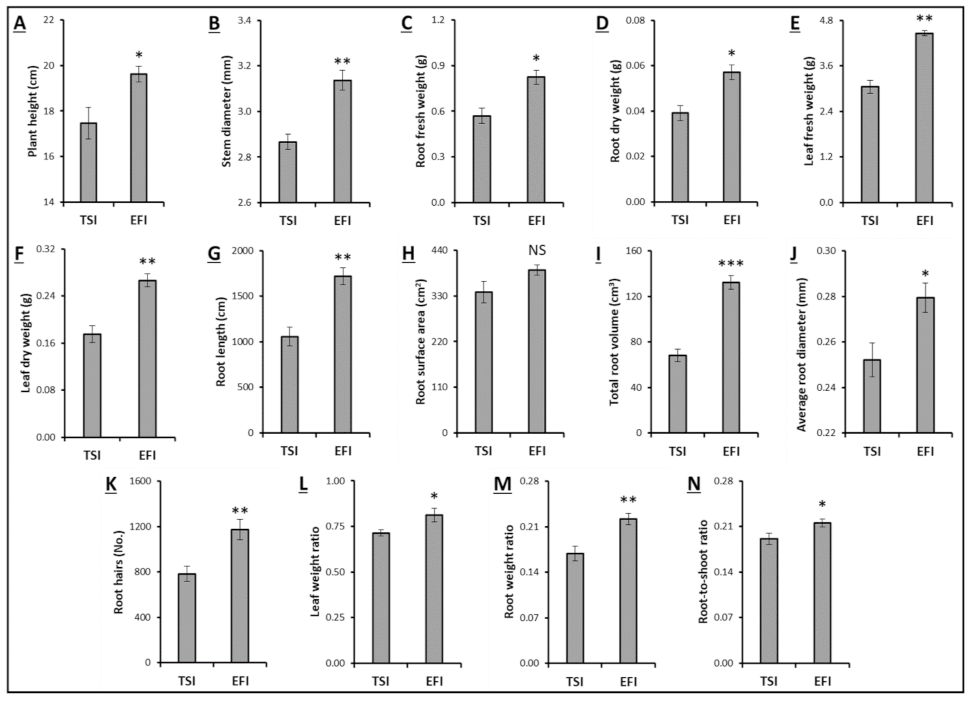
3.1. Morphological Attributes
3.2. Physiological Attributes
3.3. Enzymes Activity Assay
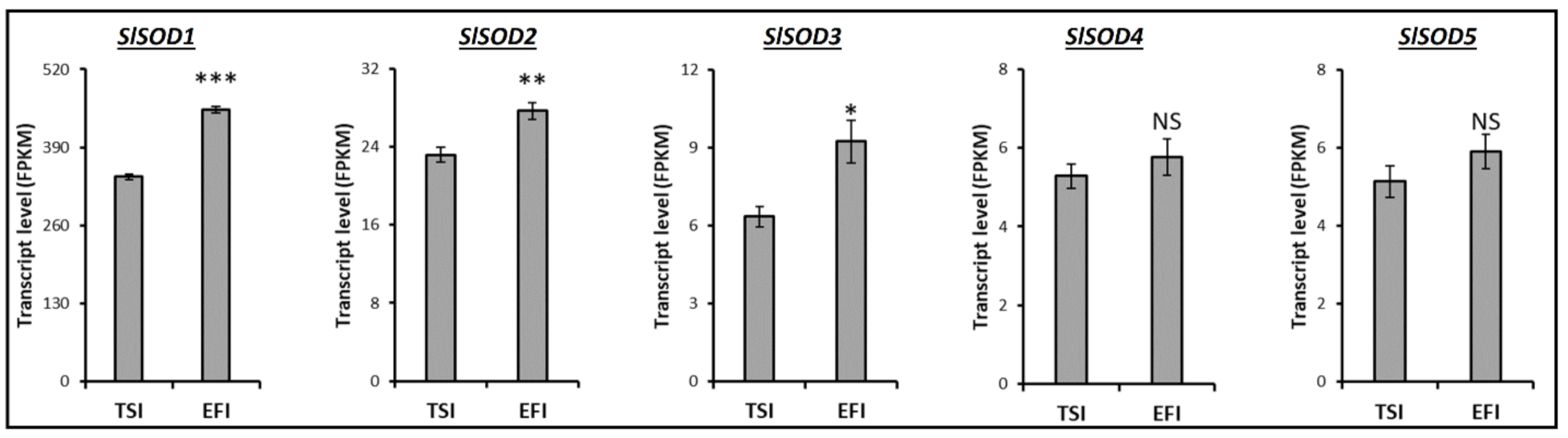
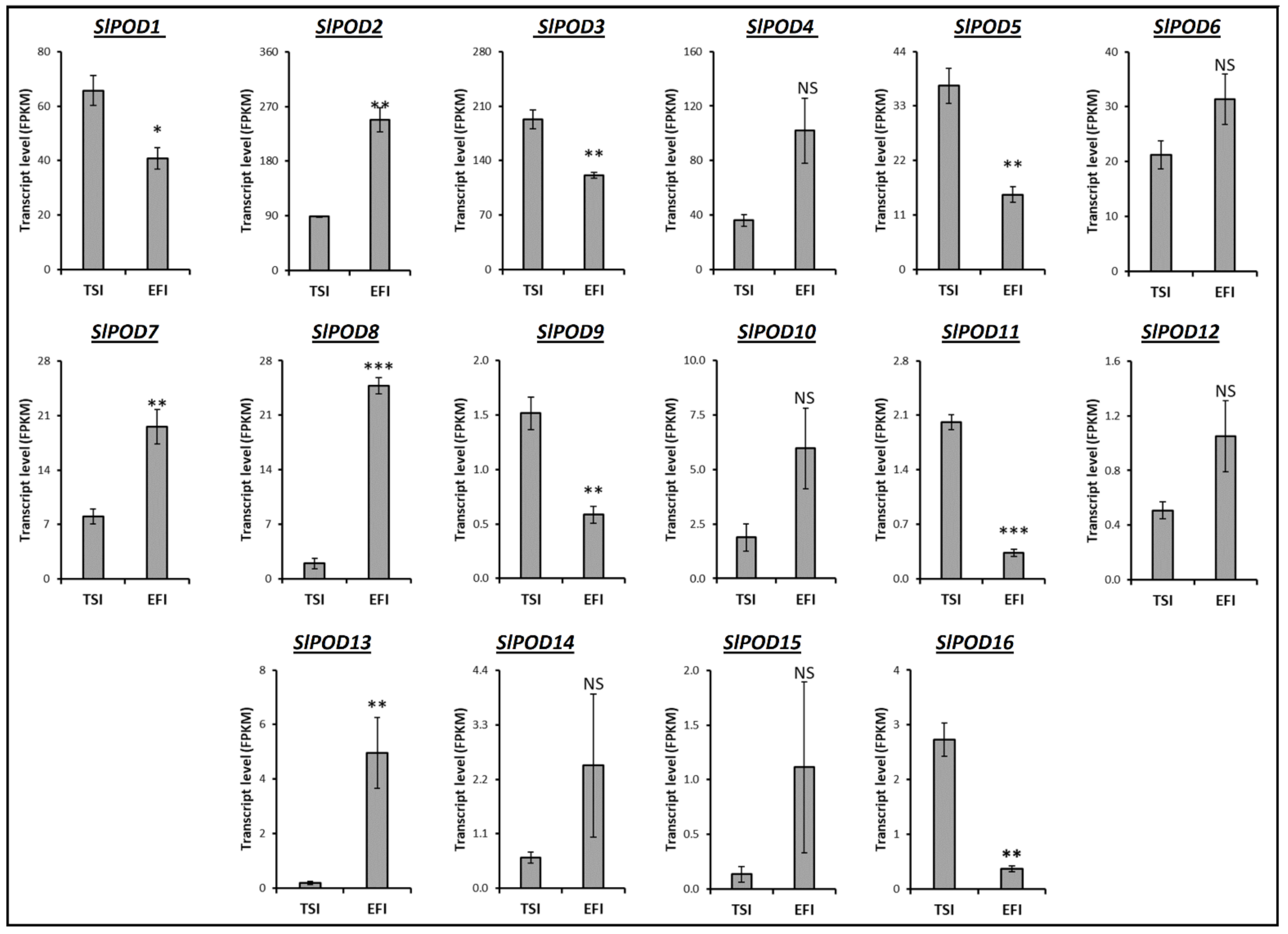
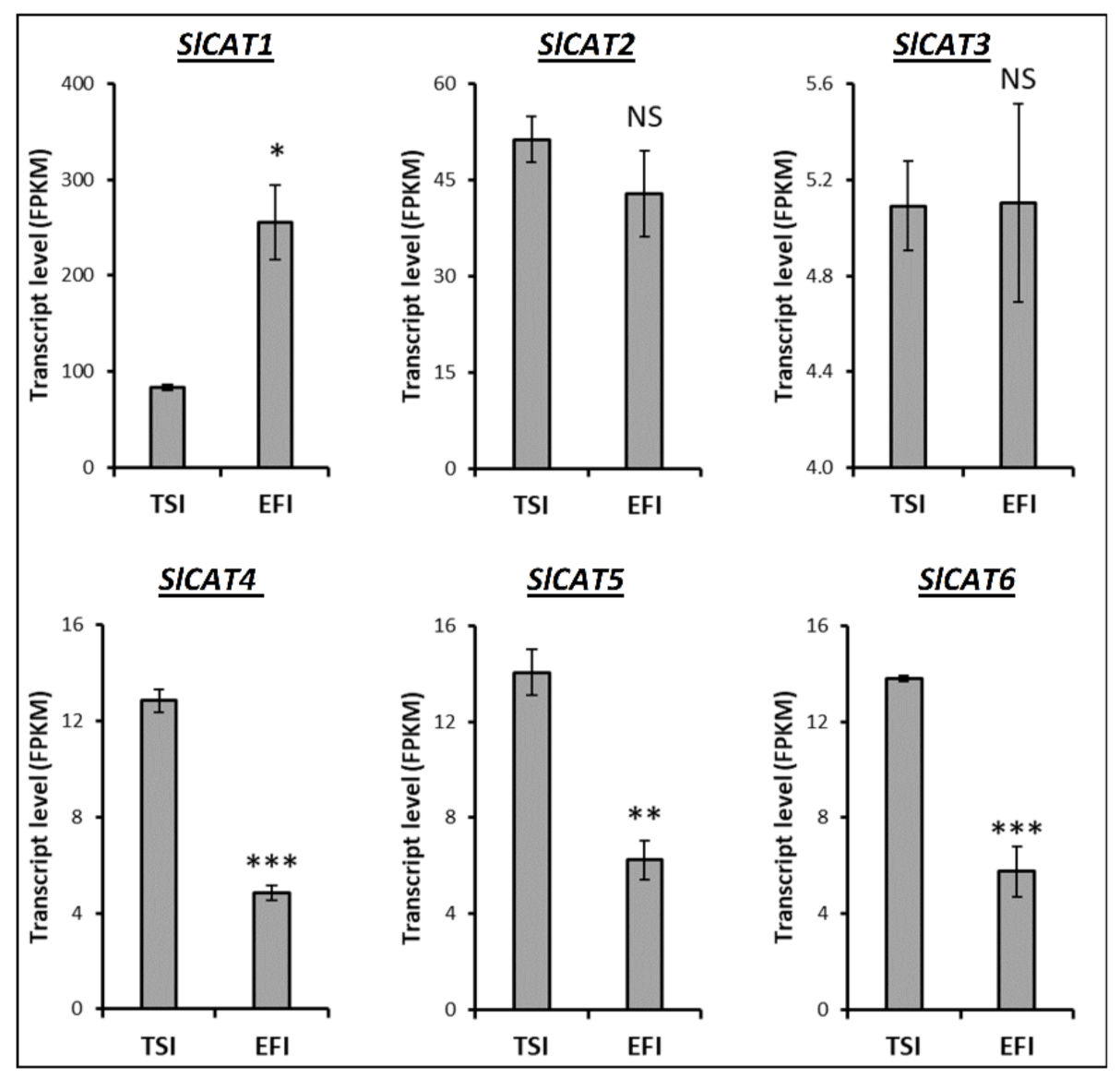
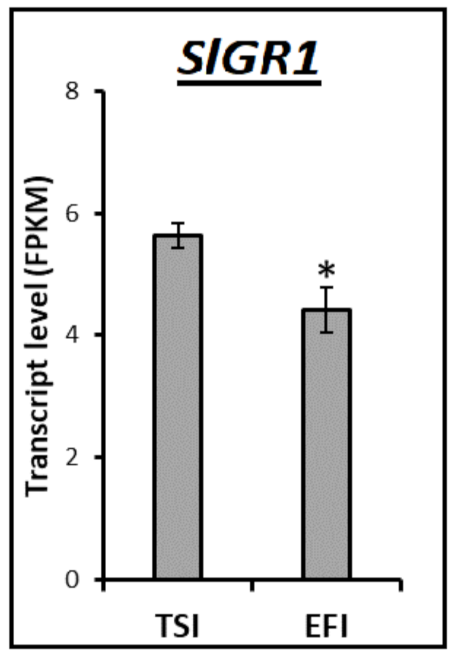
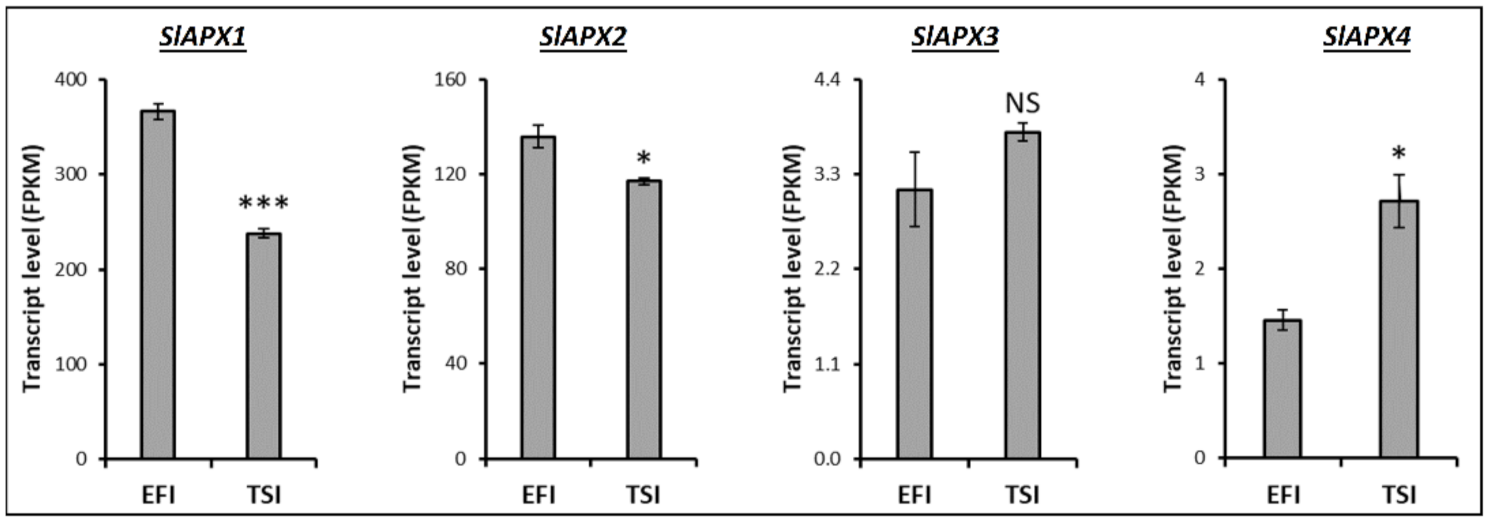
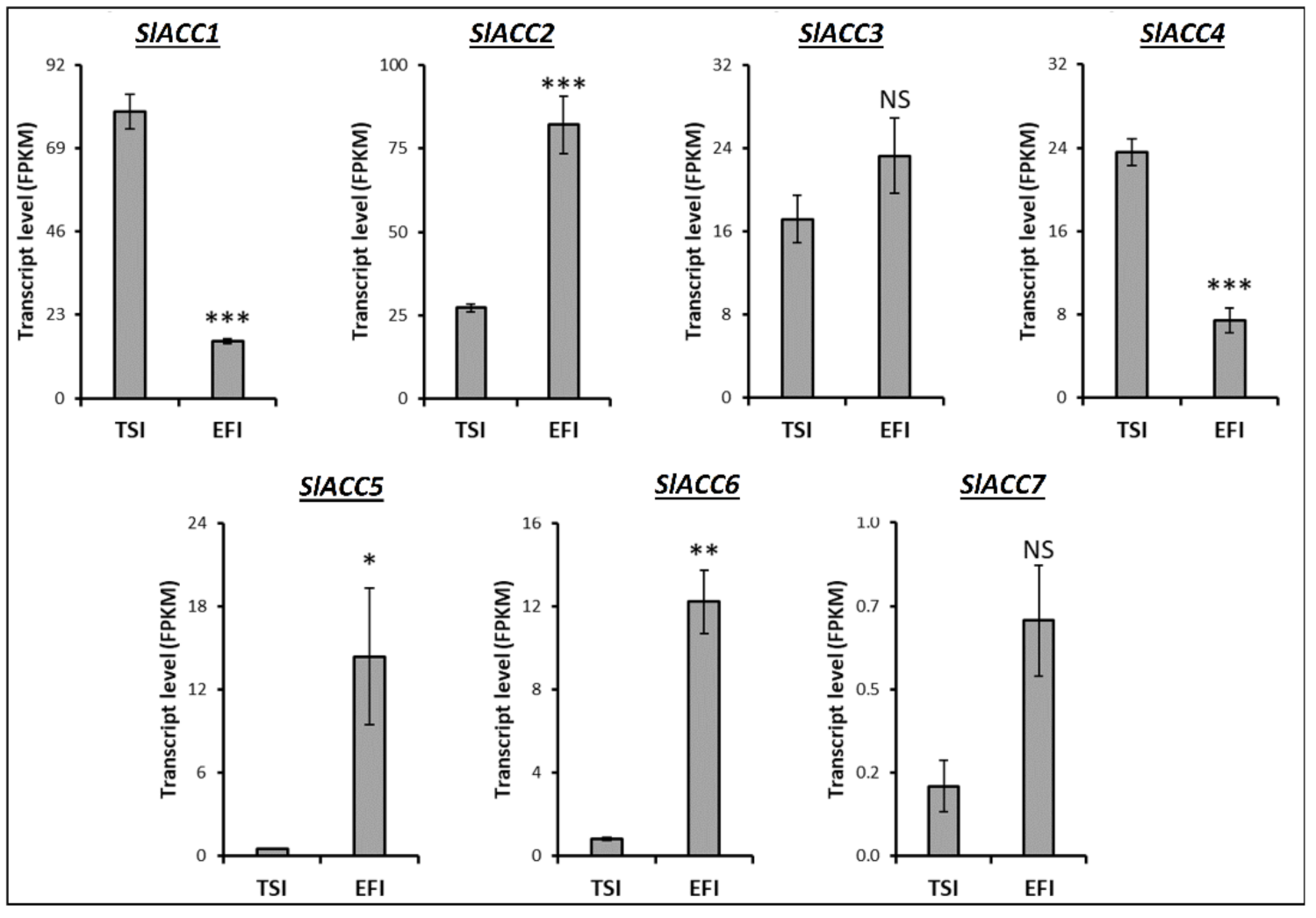
3.4. Transcript Profiling of Genes Related to the Metabolism of Tomato Root Softening Enzymes
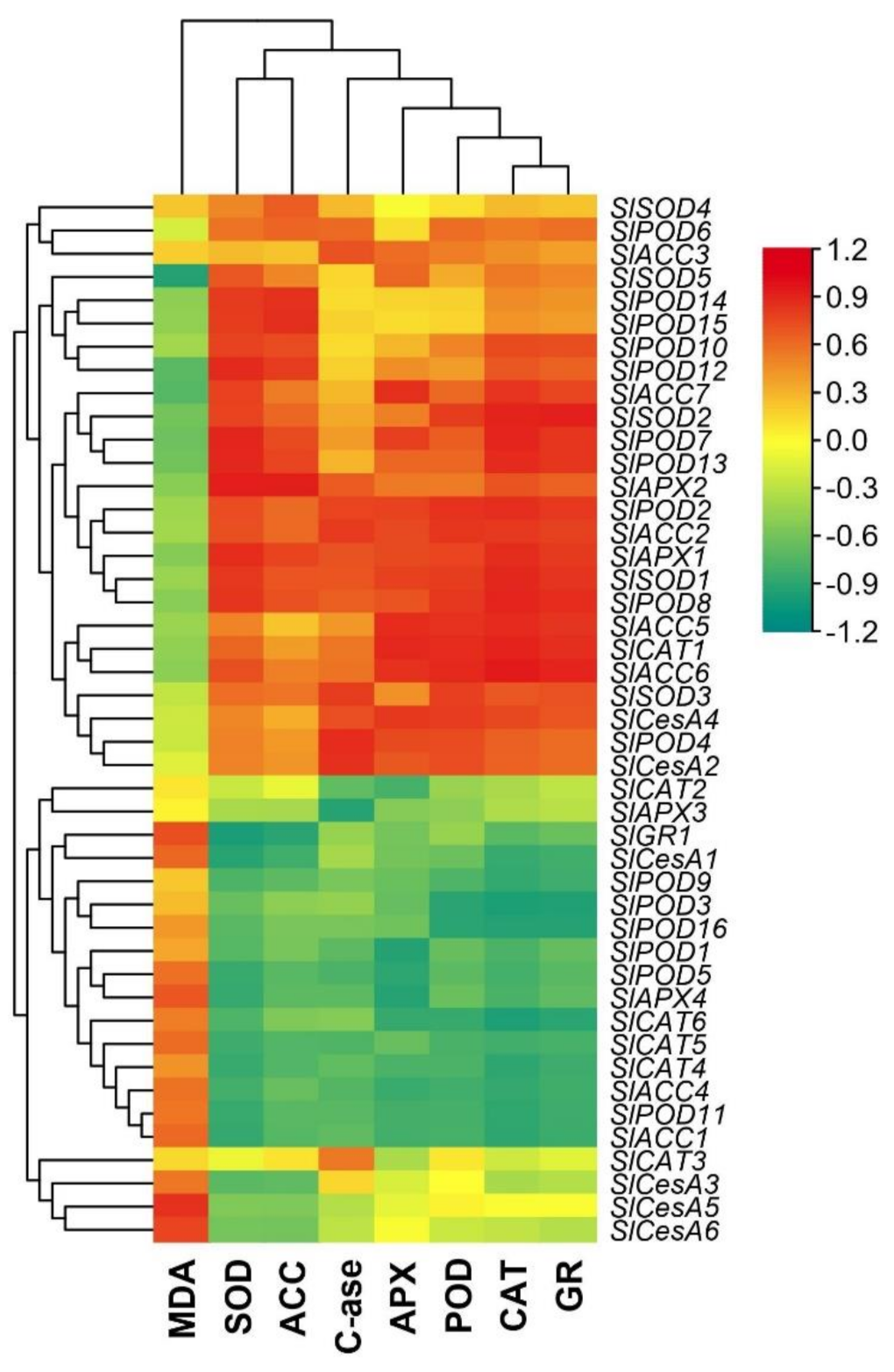
3.5. Pearson (n) Correlation between Root-Softening Enzymes Activities and Transcript Profiling of Their Metabolism-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bai, Y.; Lindhout, P. Domestication and Breeding of Tomatoes: What have We Gained and What Can We Gain in the Future? Ann. Bot. 2007, 100, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.F.; Ali, M.M.; Rizwan, H.M.; Gad, A.G.; Liang, D.; Binqi, L.; Kalaji, H.M.; Wróbel, J.; Xu, Y.; Chen, F. Light quality and quantity affect graft union formation of tomato plants. Sci. Rep. 2021, 11, 9870. [Google Scholar] [CrossRef]
- Al-Muhtaseb, A.H.; Al-Harahsheh, M.; Hararah, M.; Magee, T.R.A. Drying characteristics and quality change of unutilized-protein rich-tomato pomace with and without osmotic pre-treatment. Ind. Crops Prod. 2010, 31, 171–177. [Google Scholar] [CrossRef]
- Ali, M.M.; Waleed Shafique, M.; Gull, S.; Afzal Naveed, W.; Javed, T.; Yousef, A.F.; Mauro, R.P. Alleviation of Heat Stress in Tomato by Exogenous Application of Sulfur. Horticulturae 2021, 7, 21. [Google Scholar] [CrossRef]
- Yousef, A.F.; Xu, Y.; Chen, F.; Lin, K.; Zhang, X.; Guiamba, H.; Ibrahim, M.M.; Rizwan, H.M.; Ali, M.M. The influence of LEDs light quality on the growth pigments biochemical and chlorophyll fluorescence characteristics of tomato seedlings (Solanum lycopersicum L.). Fresenius Environ. Bull. 2021, 30, 3575–3588. [Google Scholar]
- Yousef, A.F.; Ali, M.M.; Rizwan, H.M.; Tadda, S.A.; Xu, Y.; Kalaji, H.M.; Yang, H.; Ahmed, M.A.A.; Wro, J.; Chen, F. Photosynthetic apparatus performance of tomato seedlings grown under various combinations of LED illumination. PLoS ONE 2021, 16, 1–17. [Google Scholar] [CrossRef]
- Ali, M.M.; Javed, T.; Mauro, R.P.; Shabbir, R.; Afzal, I.; Yousef, A.F. Effect of Seed Priming with Potassium Nitrate on the Performance of Tomato. Agriculture 2020, 10, 498. [Google Scholar] [CrossRef]
- Richards, D.L.; Reed, D.W. New Guinea Impatiens Growth Response and Nutrient Release from Controlled-release Fertilizer in a Recirculating Subirrigation and Top-watering System. HortScience 2004, 39, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Graham, T.; Richard, S.; Dixon, M. Potted Gerbera Production in a Subirrigation System Using Low-concentration Nutrient Solutions. HortScience 2004, 39, 1283–1286. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Graham, T.; Richard, S.; Dixon, M. Can Low Nutrient Strategies be Used for Pot Gerbera Production in Closed-Loop Subirrigation? Acta Hortic. 2005, 365–372. [Google Scholar] [CrossRef]
- Ferrarezi, R.S.; Weaver, G.M.; van Iersel, M.W.; Testezlaf, R. Subirrigation: Historical Overview, Challenges, and Future Prospects. Horttechnology 2015, 25, 262–276. [Google Scholar] [CrossRef] [Green Version]
- Araus, J.L.; Rezzouk, F.Z.; Thushar, S.; Shahid, M.; Elouafi, I.A.; Bort, J.; Serret, M.D. Effect of irrigation salinity and ecotype on the growth, physiological indicators and seed yield and quality of Salicornia europaea. Plant Sci. 2021, 304, 110819. [Google Scholar] [CrossRef] [PubMed]
- Fereres, E.; Soriano, M.A. Deficit irrigation for reducing agricultural water use. J. Exp. Bot. 2006, 58, 147–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morison, J.I.; Baker, N.; Mullineaux, P.; Davies, W. Improving water use in crop production. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 639–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research Progress and Perspective on Drought Stress in Legumes: A Review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkash, V.; Singh, S.; Deb, S.K.; Ritchie, G.L.; Wallace, R.W. Effect of deficit irrigation on physiology, plant growth, and fruit yield of cucumber cultivars. Plant Stress 2021, 1, 100004. [Google Scholar] [CrossRef]
- Leskovar, D.I. Root and Shoot Modification by Irrigation. Horttechnology 1998, 8, 510–514. [Google Scholar] [CrossRef] [Green Version]
- Elmer, W.H.; Gent, M.P.N.; McAvoy, R.J. Partial saturation under ebb and flow irrigation suppresses Pythium root rot of ornamentals. Crop Prot. 2012, 33, 29–33. [Google Scholar] [CrossRef]
- James, E.; van Iersel, M. Ebb and Flow Production of Petunias and Begonias as Affected by Fertilizers with Different Phosphorus Content. HortScience 2001, 36, 282–285. [Google Scholar] [CrossRef]
- Buwalda, F.; Baas, R.; van Weel, P.A. A soilless ebb-and-flow system for all-year-round chrysanthemums. Acta Hortic. 1994, 361, 123–132. [Google Scholar] [CrossRef]
- Naghedifar, S.M.; Ziaei, A.N.; Ansari, H. Numerical analysis of sensor-based flood-floor ebb-and-flow subirrigation system with saline water. Arch. Agron. Soil Sci. 2021, 67, 1285–1299. [Google Scholar] [CrossRef]
- Poole, R.T.; Conover, C.A. Fertilizer Levels and Medium Affect Foliage Plant Growth in an Ebb and Flow Irrigation System. J. Environ. Hortic. 1992, 10, 81–86. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Rea, E.; Colla, G. The influence of irrigation system and nutrient solution concentration on potted geranium production under various conditions of radiation and temperature. Sci. Hortic. 2008, 118, 328–337. [Google Scholar] [CrossRef]
- Leskovar, D.I.; Boales, A.K. Plant Establishment Systems Affect Yield of Jalapeno Pepper. Acta Hortic. 1995, 412, 275–280. [Google Scholar] [CrossRef]
- Leskovar, D.I.; Cantliffe, D.J. Comparison of Plant Establishment Method, Transplant, or Direct Seeding on Growth and Yield of Bell Pepper. J. Am. Soc. Hortic. Sci. 1993, 118, 17–22. [Google Scholar] [CrossRef]
- Leskovar, D.I.; Cantliffe, D.J.; Stoffella, P.J. Transplant Production Systems Influence Growth and Yield of Fresh-market Tomatoes. J. Am. Soc. Hortic. Sci. 1994, 119, 662–668. [Google Scholar] [CrossRef] [Green Version]
- Mahgoub, N.A.; Ibrahim, A.M.; Ali, O.M. Effect of different irrigation systems on root growth of maize and cowpea plants in sandy soil. Eurasian J. Soil Sci. 2017, 6, 374–379. [Google Scholar] [CrossRef] [Green Version]
- Xuewen, X.; Huihui, W.; Xiaohua, Q.; Qiang, X.; Xuehao, C. Waterlogging-induced increase in fermentation and related gene expression in the root of cucumber (Cucumis sativus L.). Sci. Hortic. 2014, 179, 388–395. [Google Scholar] [CrossRef]
- Zhang, P.; Lyu, D.; Jia, L.; He, J.; Qin, S. Physiological and de novo transcriptome analysis of the fermentation mechanism of Cerasus sachalinensis roots in response to short-term waterlogging. BMC Genom. 2017, 18, 649. [Google Scholar] [CrossRef] [Green Version]
- Hattori, Y.; Nagai, K.; Furukawa, S.; Song, X.J.; Kawano, R.; Sakakibara, H.; Wu, J.; Matsumoto, T.; Yoshimura, A.; Kitano, H.; et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 2009, 460, 1026–1030. [Google Scholar] [CrossRef]
- Kuroha, T.; Nagai, K.; Gamuyao, R.; Wang, D.R.; Furuta, T.; Nakamori, M.; Kitaoka, T.; Adachi, K.; Minami, A.; Mori, Y.; et al. Ethylene-gibberellin signaling underlies adaptation of rice to periodic flooding. Science 2018, 361, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of Waterlogging Tolerance in Plants: Research Progress and Prospects. Front. Plant Sci. 2021, 11, 2319. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Barrera-Figueroa, B.E.; Juntawong, P.; Peña-Castro, J.M. Submergence and Waterlogging Stress in Plants: A Review Highlighting Research Opportunities and Understudied Aspects. Front. Plant Sci. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Anee, T.I.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Bhuiyan, T.F.; Alam, M.U.; Fujita, M.; Hasanuzzaman, M. Oxidative Damage and Antioxidant Defense in Sesamum indicum after Different Waterlogging Durations. Plants 2019, 8, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habibullah, M.; Sarkar, S.; Islam, M.M.; Ahmed, K.U.; Rahman, M.Z.; Awad, M.F.; ElSayed, A.I.; Mansour, E.; Hossain, M.S. Assessing the Response of Diverse Sesame Genotypes to Waterlogging Durations at Different Plant Growth Stages. Plants 2021, 10, 2294. [Google Scholar] [CrossRef] [PubMed]
- Kelei, W.; Youhe, Z.; Jianlei, S.; Zong’an, H.; Longjing, Z.; Jian, X. Application of dynamic water level management of ebb and flow irrigation for cucumber seedlings. Acta Agric. Zhejiangensis 2017, 29, 408–413. [Google Scholar]
- Ali, M.M.; Anwar, R.; Malik, A.U.; Khan, A.S.; Ahmad, S.; Hussain, Z.; Hasan, M.U.; Nasir, M.; Chen, F. Plant Growth and Fruit Quality Response of Strawberry is Improved After Exogenous Application of 24-Epibrassinolide. J. Plant Growth Regul. 2021, 1–14. [Google Scholar] [CrossRef]
- Fernandez, G.E.; Butler, L.M.; Louws, F.J. Strawberry Plant Growth Parameters and Yield among Transplants of Different Types and from Different Geographic Sources, Grown in a Plasticulture System. Horttechnology 2002, 12, 100–103. [Google Scholar] [CrossRef] [Green Version]
- Chiariello, N.R.; Mooney, H.A.; Williams, K. Growth, carbon allocation and cost of plant tissues. In Plant Physiological Ecology; Springer: Dordrecht, The Netherlands, 1989; pp. 327–365. [Google Scholar]
- Ogutu, B.O.; Dash, J.; Dawson, T.P. Developing a diagnostic model for estimating terrestrial vegetation gross primary productivity using the photosynthetic quantum yield and Earth Observation data. Glob. Chang. Biol. 2013, 19, 2878–2892. [Google Scholar] [CrossRef]
- Steponkus, P.L.; Lanphear, F.O. Refinement of the Triphenyl Tetrazolium Chloride Method of Determining Cold Injury. Plant Physiol. 1967, 42, 1423–1426. [Google Scholar] [CrossRef] [Green Version]
- Chao, Y.Y.; Chen, C.Y.; Huang, W.D.; Kao, C.H. Salicylic acid-mediated hydrogen peroxide accumulation and protection against Cd toxicity in rice leaves. Plant Soil 2010, 329, 327–337. [Google Scholar] [CrossRef]
- El-Shabrawi, H.; Kumar, B.; Kaul, T.; Reddy, M.K.; Singla-Pareek, S.L.; Sopory, S.K. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 2010, 245, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Zhou, Y.; Ming, D.; Cui, J.; Chen, X.; Wen, Z.; Zhang, J.; Liu, H. Exogenous GSH protects tomatoes against salt stress by modulating photosystem II efficiency, absorbed light allocation and H2O2-scavenging system in chloroplasts. J. Integr. Agric. 2018, 17, 2257–2272. [Google Scholar] [CrossRef] [Green Version]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Cakmak, I.; Strbac, D.; Marschner, H. Activities of Hydrogen Peroxide-Scavenging Enzymes in Germinating Wheat Seeds. J. Exp. Bot. 1993, 44, 127–132. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Z.; Cai, J.; Liu, Q.; Yang, J.; Gong, Y.; Wu, M.; Shen, Q.; Xu, S. Effect of cadmium on oxidative stress and immune function of common carp (Cyprinus carpio L.) by transcriptome analysis. Aquat. Toxicol. 2017, 192, 171–177. [Google Scholar] [CrossRef]
- Ma, Q.; Yang, J. Transcriptome profiling and identification of functional genes involved in H2S response in grapevine tissue cultured plantlets. Genes Genom. 2018, 40, 1287–1300. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- García-Santiago, J.C.; Valdez-Aguilar, L.A.; Cartmill, A.D.; Cartmill, D.L.; Juárez-López, P.; Díaz-Pérez, J.C. Subirrigation of Container-Grown Tomato I: Decreased Concentration of the Nutrient Solution Sustains Growth and Yield. Water 2019, 11, 2064. [Google Scholar] [CrossRef] [Green Version]
- Pinto, J.R.; Chandler, R.A.; Dumroese, R.K. Growth, Nitrogen Use Efficiency, and Leachate Comparison of Subirrigated and Overhead Irrigated Pale Purple Coneflower Seedlings. HortScience 2008, 43, 897–901. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.J.; Li, Q.; Wang, L.L.; Shang, Q.M. Dynamic changes in bacterial communities in the recirculating nutrient solution of cucumber plug seedlings cultivated in an ebb-and-flow subirrigation system. PLoS ONE 2020, 15, e0232446. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tian, Y.; Shang, Q.; Cao, L.; Dong, C. Effects of Irrigation Height on Water and Nitrogen Use Efficiency of Tomato Plug Seedlings under Ebb-and-Flow Irrigation. Acta Agric. Boreali-Sinica 2019, 34, 126–132. [Google Scholar] [CrossRef]
- Leakey, R.R.B. Agroforestry—Participatory Domestication of Trees. In Multifunctional Agriculture; Elsevier: Amsterdam, The Netherlands, 2017; pp. 297–314. [Google Scholar]
- Kozlowski, T.T.; Pallardy, S.G. Seed Germination and Seedling Growth. In Growth Control in Woody Plants; Elsevier: Amsterdam, The Netherlands, 1997; pp. 14–72. [Google Scholar]
- Ali, M.M.; Yousef, A.F.; Li, B.; Chen, F. Effect of Environmental Factors on Growth and Development of Fruits. Trop. Plant Biol. 2021, 14, 226–238. [Google Scholar] [CrossRef]
- Javed, T.; Ali, M.M.; Shabbir, R.; Anwar, R.; Afzal, I.; Mauro, R.P. Alleviation of Copper-Induced Stress in Pea (Pisum sativum L.) through Foliar Application of Gibberellic Acid. Biology 2021, 10, 120. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 1–31. [Google Scholar] [CrossRef]
- Li, X. FGFs in Injury Repair and Regeneration. In Fibroblast Growth Factors; Elsevier: Amsterdam, The Netherlands, 2018; pp. 17–144. [Google Scholar]
- Roede, J.R.; Fritz, K.S. Hepatotoxicity of Reactive Aldehydes. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Ghanbari, F.; Sayyari, M. Controlled drought stress affects the chilling-hardening capacity of tomato seedlings as indicated by changes in phenol metabolisms, antioxidant enzymes activity, osmolytes concentration and abscisic acid accumulation. Sci. Hortic. 2018, 229, 167–174. [Google Scholar] [CrossRef]
- Ali, S.; Akbar Anjum, M.; Sattar Khan, A.; Nawaz, A.; Ejaz, S.; Khaliq, G.; Iqbal, S.; Ullah, S.; Naveed Ur Rehman, R.; Moaaz Ali, M.; et al. Carboxymethyl cellulose coating delays ripening of harvested mango fruits by regulating softening enzymes activities. Food Chem. 2021, 380, 131804. [Google Scholar] [CrossRef]
- Tang, J.; Wang, S.Q.; Hu, K.D.; Huang, Z.Q.; Li, Y.H.; Han, Z.; Chen, X.Y.; Hu, L.Y.; Yao, G.F.; Zhang, H. Antioxidative capacity is highly associated with the storage property of tuberous roots in different sweetpotato cultivars. Sci. Rep. 2019, 9, 11141. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.; Liu, H.; Nie, Z.; Gao, W.; Li, C.; Lin, Y.; Zhao, P. AsA–GSH Cycle and Antioxidant Enzymes Play Important Roles in Cd Tolerance of Wheat. Bull. Environ. Contam. Toxicol. 2018, 101, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Yoshida, N.; Fujita, M. Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul. 2014, 73, 31–44. [Google Scholar] [CrossRef]
- Hernández, J.A.; Barba-Espín, G.; Diaz-Vivancos, P. Glutathione-Mediated Biotic Stress Tolerance in Plants. In Glutathione in Plant Growth, Development, and Stress Tolerance; Springer International Publishing: New York, NY, USA, 2017; pp. 309–329. [Google Scholar]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Valderrama, R.; Mata-Pérez, C.; Padilla, M.N.; Corpas, F.J.; Barroso, J.B. Antioxidant Systems are Regulated by Nitric Oxide-Mediated Post-translational Modifications (NO-PTMs). Front. Plant Sci. 2016, 7, 152. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.; Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Hao, S.; Cao, H.; Wang, H.; Pan, X. The physiological responses of tomato to water stress and re-water in different growth periods. Sci. Hortic. 2019, 249, 143–154. [Google Scholar] [CrossRef]
- Elmardy, N.A.; Yousef, A.F.; Lin, K.; Zhang, X.; Ali, M.M.; Lamlom, S.F.; Kalaji, H.M.; Kowalczyk, K.; Xu, Y. Photosynthetic performance of rocket (Eruca sativa. Mill.) grown under different regimes of light intensity, quality, and photoperiod. PLoS ONE 2021, 16, e0257745. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.A.; Yousef, A.F.; Ali, M.M.; Ahmed, A.I.; Lamlom, S.F.; Strobel, W.R.; Kalaji, H.M. Exogenously applied nitrogenous fertilizers and effective microorganisms improve plant growth of stevia (Stevia rebaudiana Bertoni) and soil fertility. AMB Express 2021, 11, 133. [Google Scholar] [CrossRef]
- Liang, D.; Yousef, A.F.; Wei, X.; Ali, M.M.; Yu, W.; Yang, L.; Oelmüller, R.; Chen, F. Increasing the performance of Passion fruit (Passiflora edulis) seedlings by LED light regimes. Sci. Rep. 2021, 11, 20967. [Google Scholar] [CrossRef]
- Wu, X.; Sun, T.; Xu, W.; Sun, Y.; Wang, B.; Wang, Y.; Li, Y.; Wang, J.; Wu, X.; Lu, Z.; et al. Unraveling the Genetic Architecture of Two Complex, Stomata-Related Drought-Responsive Traits by High-Throughput Physiological Phenotyping and GWAS in Cowpea (Vigna. Unguiculata L. Walp). Front. Genet. 2021, 12, 743758. [Google Scholar] [CrossRef]
- Filipović, A. Water Plant and Soil Relation under Stress Situations. In Soil Moisture Importance; IntechOpen: London, UK, 2021. [Google Scholar]
- Chen, J.; Wang, Q.; Li, M.; Liu, F.; Li, W. Does the different photosynthetic pathway of plants affect soil respiration in a subtropical wetland? Ecol. Evol. 2016, 6, 8010–8017. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, Y.; Lu, B.; Miao, Z. Response of Physiological Indicators to Environmental Factors under Water Level Regulation of Paddy Fields in Southern China. Water 2018, 10, 1772. [Google Scholar] [CrossRef] [Green Version]
- Kaushal, R.; Singh, I.; Thapliyal, S.D.; Gupta, A.K.; Mandal, D.; Tomar, J.M.S.; Kumar, A.; Alam, N.M.; Kadam, D.; Singh, D.V.; et al. Rooting behaviour and soil properties in different bamboo species of Western Himalayan Foothills, India. Sci. Rep. 2020, 10, 4966. [Google Scholar] [CrossRef]
- Pandey, V.; Swami, R.K.; Narula, A. Harnessing the Potential of Roots of Traditional Power Plant: Ocimum. Front. Plant Sci. 2021, 12, 2387. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, J.W.; Wang, K.J.; Liu, P.; Dong, S.T. Effects of drought stress on the grain yield and root physiological traits of maize varieties with different drought tolerance. Ying Yong Sheng Tai Xue Bao 2010, 21, 48–52. [Google Scholar] [PubMed]
- Ullah, H.; Gul, B.; Khan, H.; Zeb, U. Effect of salt stress on proximate composition of duckweed (Lemna minor L.). Heliyon 2021, 7, e07399. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Ali, M.M.; Pan, K.; Su, S.; Xu, J.; Chen, F. Ebb-and-Flow Subirrigation Improves Seedling Growth and Root Morphology of Tomato by Influencing Root-Softening Enzymes and Transcript Profiling of Related Genes. Agronomy 2022, 12, 494. https://doi.org/10.3390/agronomy12020494
Wang K, Ali MM, Pan K, Su S, Xu J, Chen F. Ebb-and-Flow Subirrigation Improves Seedling Growth and Root Morphology of Tomato by Influencing Root-Softening Enzymes and Transcript Profiling of Related Genes. Agronomy. 2022; 12(2):494. https://doi.org/10.3390/agronomy12020494
Chicago/Turabian StyleWang, Kelei, Muhammad Moaaz Ali, Keke Pan, Shiwen Su, Jian Xu, and Faxing Chen. 2022. "Ebb-and-Flow Subirrigation Improves Seedling Growth and Root Morphology of Tomato by Influencing Root-Softening Enzymes and Transcript Profiling of Related Genes" Agronomy 12, no. 2: 494. https://doi.org/10.3390/agronomy12020494






