Polyarenes Distribution in the Soil-Plant System of Reindeer Pastures in the Polar Urals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Chemical Analysis of Soils
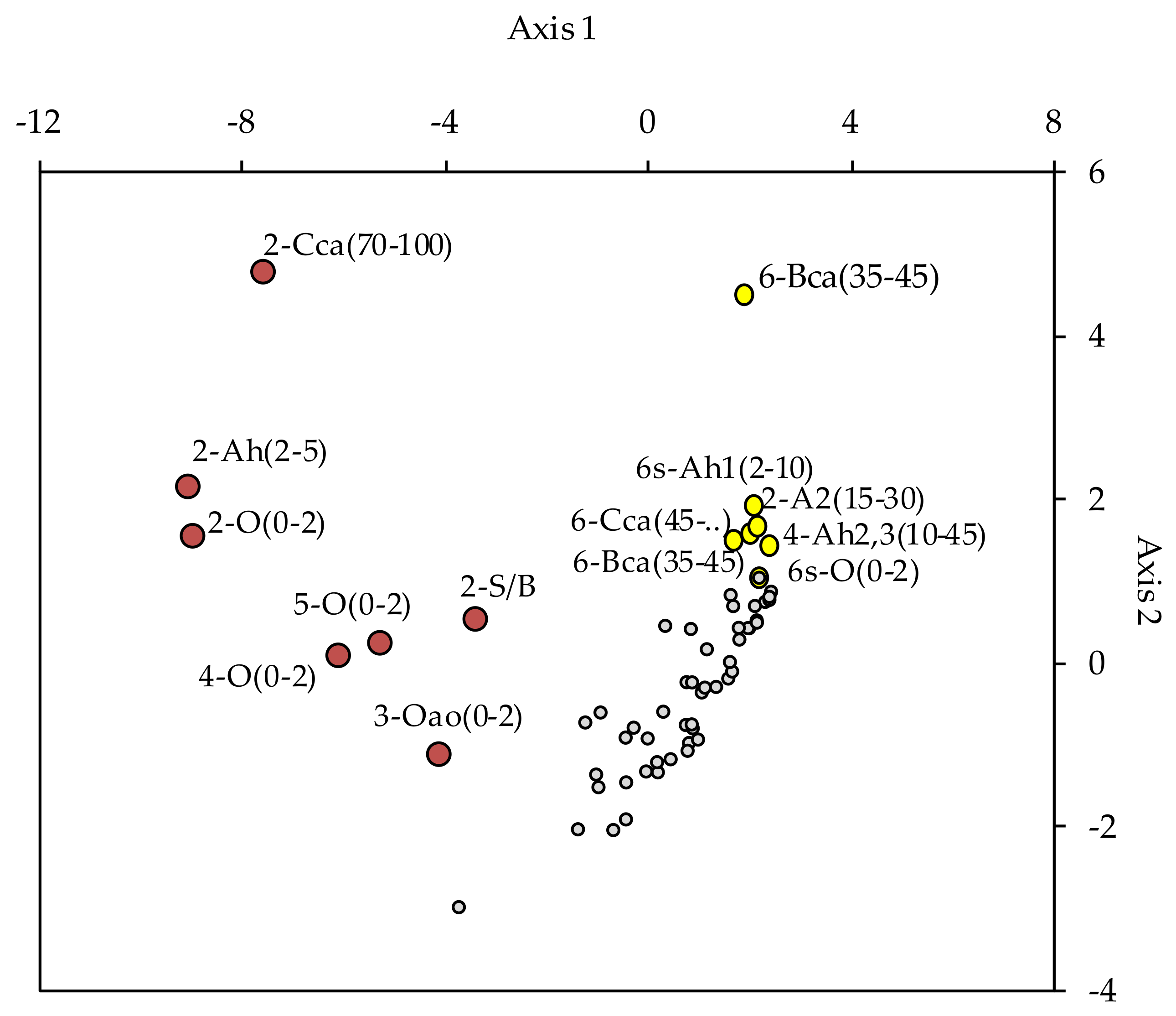
3. Results
4. Discussion
4.1. Standing Biomass
4.2. Soils
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shamrikova, E.V.; Zhangurov, E.V.; Kulugina, E.E.; Korolev, M.A.; Kubik, O.S.; Tumanova, E.A. Soils and soil cover of mountain and tundra landscapes of the Polar Urals on carbonate rocks: Diversity, classification, nitrogen and carbon distribution. Eurasian Soil Sci. 2020, 53, 1206–1221. [Google Scholar] [CrossRef]
- On detection, 2021; Appendix 3 to the Technical Regulations [in Russian]. Available online: https://docs.cntd.ru/document/902320560 (accessed on 13 December 2021).
- Makarov, D.A.; Komarov, A.A.; Ovcharenko, V.V.; Nebera, E.A.; Kozhushkevich, A.I.; Kalantaenko, A.M.; Afanasieva, E.L.; Demidova, S.V. Dioxon and heavy metals contamination of reindeer offal from Russian far north regions. Agric. Biol. 2018, 53, 364–373. [Google Scholar] [CrossRef]
- Ophof, A.A.; Oldeboer, K.W.; Kumpula, J. Intake and chemical composition of winter and spring forage plants consumed by semi-domesticated reindeer (Rangifer tarandus tarandus) in Northern Finland. Anim. Feed. Sci.Technol. 2013, 185, 190–195. [Google Scholar] [CrossRef]
- Han, Z.; Li, J.; Gu, T.; Yang, R.; Fu, Z.; Yan, B.; Chen, G. Effects of torrefaction on the formation and distribution of dioxins during wood and PVC pyrolysis: An experimental and mechanistic study. J. Anal. Appl. Pyrolysis 2021, 157, 105240. [Google Scholar] [CrossRef]
- Lehto, K.-M.; Puhakka, J.A.; Lemmetyien, H. Biodegradation of selected UV-irradiated and non-irradiated polycyclic aromatic hydrocarbons (PAHs). Biodegradation 2003, 14, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Hamid, N.; Syed, J.H.; Junaid, M.; Zhang, G.; Malik, R.N. Elucidating the urban levels, sources and health risks of polycyclic aromatic hydrocarbons (PAHs) in Pakistan: Implications for changing energy demand. Sci. Total Environ. 2017, 619–620, 165–175. [Google Scholar] [CrossRef]
- Shamilishvily, G.; Abakumov, E.; Gabov, D. Polycyclic aromatic hydrocarbon in urban soils of an Eastern European megalopolis: Distribution, source identification and cancer risk evaluation. Solid Earth. 2018, 9, 669–682. [Google Scholar] [CrossRef] [Green Version]
- Balbino, S.; Repajic, M.; Solaric, T.; Hunjek, D.D.; Skevin, D.; Kraljic, K.; Obranovic, M.; Levaj, B. Oil Uptake and Polycyclic Aromatic Hydrocarbons (PAH) in Fried Fresh-Cut Potato: Effect of Cultivar, Anti-Browning Treatment and Storage Conditions. Agronomy 2020, 10, 1773. [Google Scholar] [CrossRef]
- McGrath, T.E.; Wooten, J.B.; Chan, W.G.; Hajaligol, M.R. Formation of polycyclic aromatic hydrocarbons from tobacco: The link between low temperature residual solid (char) and PAH formation. Food Chem. Toxicol. 2007, 45, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Vane, C.H.; Rawlins, B.G.; Kim, A.W.; Moss-Hayes, V.; Kendrick, C.P.; Leng, M.J. Sedimentary transport and fate of polycyclic aromatic hydrocarbons (PAH) from managed burning of moorland vegetation on a blanket peat, South Yorkshire, UK. Sci. Total Environ. 2013, 449, 81–94. [Google Scholar] [CrossRef]
- Gennadiev, A.N.; Pikovskii, Y.I.; Tsibart, A.S.; Smirnova, M.A. Hydrocarbons in Soils: Origin, Composition, and Behavior (Review). Eurasian Soil Sci. 2015, 48, 1076–1089. [Google Scholar] [CrossRef]
- Bandowe, B.A.M.; Ruckamp, D.; Braganca, M.A.L.; Laabs, V.; Amelung, W.; Martius, C.; Wilcke, W. Naphthalene production by microorganisms associated with termites: Evidence from a microcosm experiment. Soil Biol. Biochem. 2009, 41, 630–639. [Google Scholar] [CrossRef]
- Belis, C.A.; Offenthaler, I.; Weiss, P. Semivolatiles in the forest environment: The case of PAHs. Plant Ecophysiol. 2001, 8, 47–73. [Google Scholar] [CrossRef]
- Labana, S.; Kapur, M.; Malik, D.; Prakash, D.; Jain, R. Diversity, Biodegradation and bioremediation of polycyclic aromatic hydrocarbons. Environ. Bioremediation Technol. 2007, 409–443. [Google Scholar] [CrossRef]
- Krauss, M.; Wilcke, W.; Martius, C.; Bandeira, A.G.; Garrcia, M.V.B.; Amelung, W. Atmospheric versus biological sources of polycyclic aromatic hydrocarbons (PAHs) in a tropical rain forest environment. Environ. Pollut. 2005, 135, 143–154. [Google Scholar] [CrossRef] [PubMed]
- García-Falcón, M.S.; Soto-González, B.; Simal-Gándara, J. Evolution of the Concentrations of Polycyclic Aromatic Hydrocarbons in Burnt Woodland Soils. Environ. Sci. Technol. 2006, 40, 759–763. [Google Scholar] [CrossRef]
- Gennadiev, A.N.; Tsibart, A.S. Pyrogenic polycyclic aromatic hydrocarbons in soils of reserved and anthropogenically modified areas: Factors and features of accumulation. Eurasian Soil Sci. 2013, 46, 28–36. [Google Scholar] [CrossRef]
- Rey-Salgueiro, L.; Martínez-Carballo, E.; Merino, A.; Vega, J.A.; Fonturbel, M.T.; Simal-Gandara, J. Polycyclic Aromatic Hydrocarbons in Soil Organic Horizons Depending on the Soil Burn Severity and Type of Ecosystem. Land Degrad. Dev. 2018, 29, 2112–2123. [Google Scholar] [CrossRef]
- Campos, I.; Abrantes, N.; Pereira, P.; Micaelo, A.C.; Vale, C.; Keizer, J.J. Forest fires as potential triggers for production and mobilization of polycyclic aromatic hydrocarbons to the terrestrial ecosystem. Land Degrad. Dev. 2019, 30, 2360–2370. [Google Scholar] [CrossRef]
- Mergelov, N.; Petrov, D.; Zazovskaya, E.; Dolgikh, A.; Golyeva, A.; Matskovsky, V.; Bichurin, R.; Turchinskaya, S.; Belyaev, V.; Goryachkin, S. Soils in Karst Sinkholes Record the Holocene History of Local Forest Fires at the North of European Russia. Forests 2020, 11, 1268. [Google Scholar] [CrossRef]
- Wilcke, W. SYNOPSIS Polycyclic Aromatic Hydrocarbons (PAHs) in Soil—A Review. J. Plant Nutr. Soil Sci. 2000, 163, 229–248. [Google Scholar] [CrossRef]
- Yakovleva, E.V.; Gabov, D.N.; Vasilevich, R.S.; Goncharova, N.N. Participation of Plants in the Formation of Polycyclic Aromatic Hydrocarbons in Peatlands. Eurasian Soil Sci. 2020, 53, 317–329. [Google Scholar] [CrossRef]
- Zhangurov, E.V.; Startsev, V.V.; Dubrovskiy, Y.A.; Degteva, S.V.; Dumov, A.A. Morphogenetic Features of Soils under Mountainous Larch Forests and Woodlands in the Subpolar Urals. Eurasian Soil Sci. 2019, 52, 1463–1476. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. 2015. World Reference Base for Soil Resources International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports; FAO: Rome, Italy, 2014; p. 106. [Google Scholar]
- Shamrikova, E.V.; Zhangurov, E.V.; Kulugina, E.E.; Kubik, O.S.; Korolev, M.A. Composition of water extracts from vegetation, soils on carbonate rocks, and surface water in the northern part of the Polar Urals. Eurasian Soil Sci. 2021, 54, 1161–1175. [Google Scholar] [CrossRef]
- ISO. Soil Quality-Determination of Carbonate Content Volumetric Method; ISO: Geneva, Switzerland, 1995. [Google Scholar]
- Wacker, L.; Němec, M.; Bourquin, J. A revolutionary graphitisation system: Fully automated, compact and simple. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 931–934. [Google Scholar] [CrossRef]
- Petrov, D.G. The paths of migration of charcoal particles in the post-pyrogenic soils of the taiga and tundra depending on features of fire and environmental factors. Dokuchaev Soil Bull. 2020, 105, 109–145. [Google Scholar] [CrossRef]
- Surova, T.; Troitski, L.; Punning, I. Holozene paleogeography and absolute chronology of the Polar Urals. Eest. NSV Tead. Akad. Toimetised. Geol. 1975, 2, 152–159. [Google Scholar]
- Nikiforova, L.D. Izmeneniye prirodnoy sredy v golotsene na severo-vostoke yevropeyskoy SSSR. Avtoref. Dis. Kand. Geograf. Nauk. 1980, 25. (in Russian). [Google Scholar]
- Masclet, P.; Hoyau, V.; Jaffrezo, J.L.; Cachier, H. Polycyclic aromatic hydrocarbon deposition on the ice sheet of Greenland Part I: Superficial snow. Atmos. Environ. 2000, 34, 3195–3207. [Google Scholar] [CrossRef]
- Nemirovskaya, I.A.; Novigatskii, A.N. Hydrocarbons in the snow and ice cover and waters of the Arctic Ocean. Geochem. Int. 2003, 41, 585–594. [Google Scholar]
- Schneidemesser, E.; Schauer, J.J.; Shafer, M.M.; Hagler, G.S.; Bergin, M.H.; Steig, E.J. A method for the analysis of ultra-trace levels of semi-volatile and non-volatile organic compounds in snow and application to a Greenland snow pit. Polar Sci. 2008, 2, 251–266. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yao, T.; Cong, Z.; Yan, X.; Kang, S.; Zhang, Y. Gradient distribution of persistent organic contaminants along northern slope of central-Himalayas, China. Sci. Total Env. 2006, 372, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, E.; Gabov, D. Polyarenes accumulation in tundra ecosystem influenced by coal industry of Vorkuta. Pol. Polar Res. 2020, 41, 237–268. [Google Scholar] [CrossRef]
- Demin, B.N.; Graevskii, A.P.; Demeshkin, A.S.; Vlasov, S.V. Pollution of soil-vegetation complex around Barentsburg Mine by polycyclic aromatic hydrohydrocarbons. Arkt. Ekol. Ekon. 2012, 3, 62–73. [Google Scholar]
- Wang, J.; Bao, H.; Zhang, H.; Jiao, L.; Hong, H.; Wu, F. Effects of cuticular wax content and specific leaf area on accumulation and partition of PAHs in different tissues of wheat leaf. Environ. Sci. and Pollut. Res. 2020, 27, 18793–18802. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.L.; Maguire, K.L.; Anderson, D.R.; McGrath, S.P. Enhanced dissipation of chrysene in planted soil: The impact of a rhizobial inoculums. Soil Biol. Biochem. 2004, 36, 33–38. [Google Scholar] [CrossRef]
- Wilcke, W.; Amelung, W. Persistent organic pollutants in native grassland soils along a climosequence in North America. Soil Sci. Soc. Am. J. 2000, 64, 2140–2148. [Google Scholar] [CrossRef]
- Gabov, D.N.; Beznosikov, V.A. Polycyclic Aromatic Hydrocarbons in Tundra Soils of the Komi Republic. Eurasian Soil Sci. 2014, 47, 18–25. [Google Scholar] [CrossRef]
- Orlov, D.S. Humic Substances of Soils and General Theory of Humification; CRC Press: Boca Raton, FL, USA, 1995; Volume 266. [Google Scholar] [CrossRef]
- Yakovleva, E.V.; Gabov, D.N.; Beznosikov, V.A. Effect of Different Doses of Benz[A]Pyrene on Composition of Policiclic Aromatic Hydrocarbons in Sand Culture. Agrokhimia 2015, 6, 90–96. [Google Scholar]
- Thormann, M.N.; Szumigalski, A.R.; Bayley, S.E. Aboveground peat and carbon accumulation potentials along a bog-fen-marsh wetland gradient in southern boreal Alberta, Canada. Wetlands 1999, 19, 305–317. [Google Scholar] [CrossRef]
- Golovatskaya, E.A.; Nikonova, L.G. Decomposition of the plant remains in peat soils of oligotrophic mires. Vestn. Tomsk. Gos. Univ. Biol. 2013, 3, 137–151. [Google Scholar] [CrossRef]
- Gabov, D.N.; Vasilevich, R.S.; Yakovleva, E.V.; Zueva, O.M. Aromatic Compounds in Tuberous Peatlands of the Permafrost Area. Geoekol. Inzh. Geol. Gidrogeol. Geokriol. 2017, 6, 15–29. (In Russian) [Google Scholar]
- Mackay, D.; Shiu, W.Y. Aqueous solubility of polynuclear aromatic hydrocarbons. J. Chem. Eng. Data. 1977, 22, 399–402. [Google Scholar] [CrossRef]
- Krauss, M.; Wilcke, W. Sorption Strength of Persistent Organic Pollutants in Particle-size Fractions of Urban Soils. Soil Sci. Soc. Am. J. 2002, 66, 430–437. [Google Scholar] [CrossRef]
- Cai, T.; Ding, Y.; Zhang, Z.; Wang, X.; Wang, T.; Ren, Y.; Dong, Y. Effects of total organic carbon content and leaching water volume on migration behavior of polycyclic aromatic hydrocarbons in soils by column leaching tests. Environ. Pollut. 2019, 254, 112981. [Google Scholar] [CrossRef]
- Daso, A.P.; Akortia, E.; Okonkwo, J.O. Concentration profiles, source apportionment and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in dumpsite soils from Agbogbloshie e-waste dismantling site, Accra, Chana. Environ. Sci. Pollut. Res. 2016, 23, 10883–10894. [Google Scholar] [CrossRef]
- Adam, G.; Gamoh, K.; Morris, D.G.; Duncan, H. Effect of alcohol addition on the movement of petroleum hydrocarbon fuels in soil. Sci. Total Environ. 2002, 286, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Vasilevich, R.S.; Beznosikov, V.A. Effect of climate changes in the holocene on the distribution of humic substances in the profile of forest-tundra peat mounds. Eurasian Soil Sci. 2017, 50, 1271–1282. [Google Scholar] [CrossRef]
- Vasilevich, R.S.; Beznosikov, V.A.; Lodygin, E.D. Molecular structure of humus substances in permafrost peat mounds in forest-tundra. Eurasian Soil Sci. 2019, 52, 283–295. [Google Scholar] [CrossRef]
- Di Donato, N.; Chen, H.; Waggoner, D.; Hatcher, P.G. Potential origin and formation for molecular components of humic acids in soils. Geochim. Cosmochim. Acta. 2016, 178, 210–222. [Google Scholar] [CrossRef] [Green Version]
- Smitha, M.J.; Flowersa, T.H.; Duncana, H.J.; Saitob, H. Study of PAH dissipation and phytoremediation in soils: Comparing freshly spiked with weathered soil from a former coking works. J. Hazard. Mater. 2011, 192, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
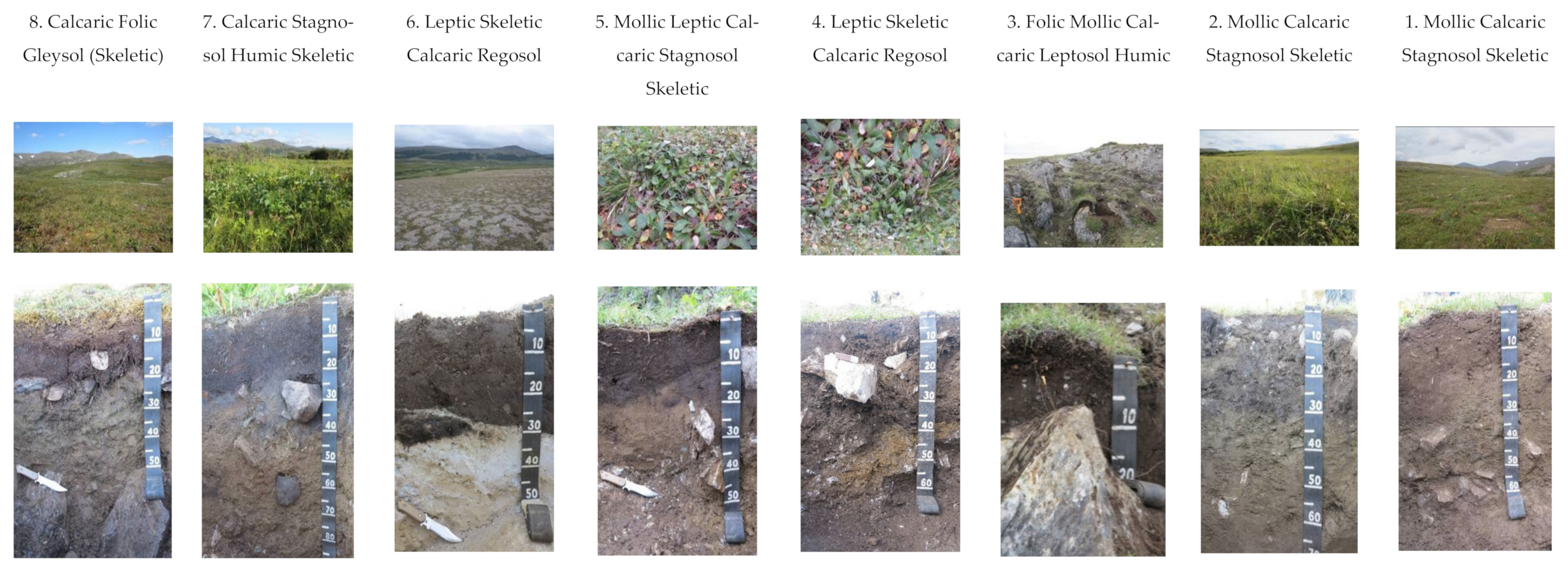


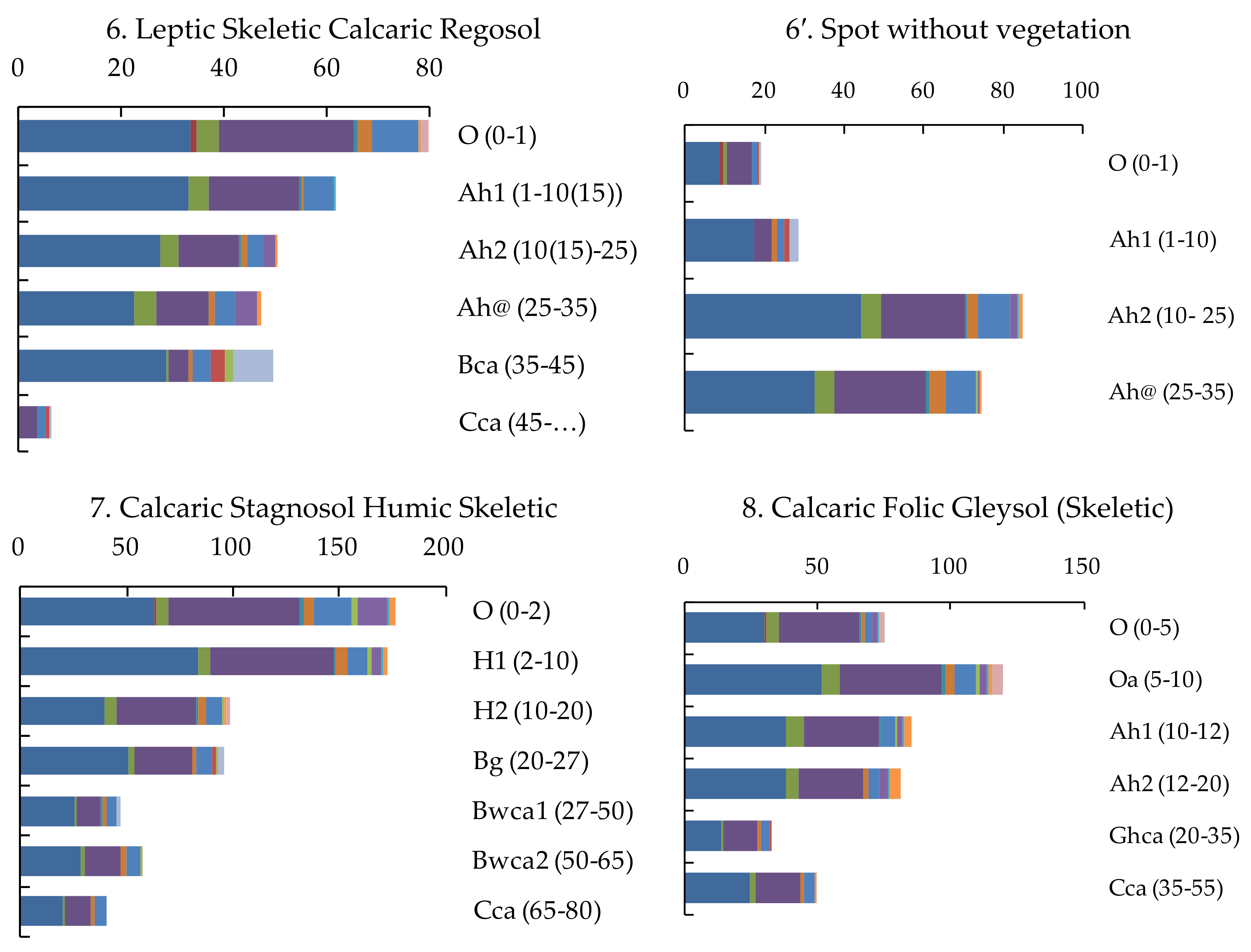
| Soil Type | Horizon | Depth | pHH2O | Ctot | Cinorg | Ntot | CPAH 1 |
|---|---|---|---|---|---|---|---|
| cm | g kg−1 | μg kg−1 | |||||
| 1. Mollic Calcaric Stagnosol Skeletic | Standing Biomass | – 2 | 443.0 | 0.0 3 | 11.8 | 105.4 | |
| O | 0–1 | 6.67 | 147.0 | 9.4 | 62.2 | ||
| Ah1 | 1–5 | 6.99 | 33.0 | 2.4 | 64.1 | ||
| Ah2 | 5–15 | 7.33 | 33.0 | 0.6 | 2.3 | 11.5 | |
| Bwca1 | 15–35 | 7.72 | 11.8 | 6.1 | 0.7 | 6.1 | |
| Bwca2 | 35–60 | 7.90 | 7.6 | 5.2 | 0.5 | 8.9 | |
| 1′. Spot without Vegetation | Cca | 0–1 | 7.84 | 1.4 | 0.2 | 0.1 | 34.8 |
| Ah1@ | 1-5 | 7.87 | 1.1 | 0.0 | 0.1 | 29.8 | |
| Ah2@ | 5–20 | 7.57 | 1.0 | 0.0 | 0.1 | 41.5 | |
| CRMca | 20–30 | 7.89 | 0.7 | 0.2 | 0.1 | 13.1 | |
| BCca | 30–60 | 8.05 | 0.9 | 0.6 | 0.0 | 20.3 | |
| 2. Mollic Calcaric Stagnosol Skeletic | Standing Biomass | – | 421.0 | 0.0 | 17.5 | 106.1 | |
| O | 0–2 | 7.00 | 243.0 | 17.2 | 179.6 | ||
| Ah | 2–5 | 6.71 | 132.0 | 0.8 | 10.9 | 174.7 | |
| A1 | 5–15 | 7.47 | 43.0 | 4.9 | 2.9 | 26.1 | |
| A2 | 15–30 | 7.62 | 44.0 | 9.1 | 3.1 | 26.6 | |
| Bwca1 | 30–55 | 7.92 | 9.2 | 6.5 | 0.4 | 7.9 | |
| Bwca2 | 55–70 | 7.95 | 3.5 | 1.1 | 0.4 | 25.2 | |
| Cca | 70–100 | 8.10 | 4.4 | 4.1 | 0.2 | 107.9 | |
| 3. Folic Mollic Calcaric Leptosol Humic | Standing Biomass | – | 485.0 | 0.0 | 19.8 | – | |
| O | 0–2 | 7.22 | 241.0 | 1.4 | 12.9 | 72.1 | |
| Oa | 2–4 | 7.40 | 188.0 | 2.5 | 11.7 | 151.1 | |
| Ah | 4–15 | 7.50 | 63.0 | 5.5 | 5.2 | 66.5 | |
| Ahca | 15–20 | 7.69 | 57.0 | 4.4 | 4.9 | 63.1 | |
| Blanket 4 | 7.70 | 67.0 | 10.6 | 5.3 | 56.0 | ||
| 4. Leptic Skeletic Calcaric Regosol | Standing Biomass | 6.1 | 42.0 | 0.0 | 1.9 | 40.2 | |
| O | 0–2 | 7.0 | 25.7 | 0.0 | 1.8 | 144.6 | |
| Ah1 | 2–10 | 7.3 | 8.9 | 0.0 | 0.9 | 104.3 | |
| Ah2 | 10–30 | 7.5 | 6.3 | 1.6 | 0.5 | 28.5 | |
| Ah3 | 30–45 | 7.6 | 5.7 | 1.5 | 0.4 | 50.7 | |
| Bwca | 45–55 | 7.8 | 6.7 | 6.1 | 0.1 | 20.1 | |
| Cca | 55–65 | 7.7 | 6.0 | 1.6 | 0.4 | 26.1 | |
| 5. Mollic Leptic Calcaric Stagnosol Skeletic | Standing Biomass | – | 440.0 | 0.0 | 18.9 | 67.8 | |
| O | 0–2 | 6.90 | 302.0 | 19.6 | 125.8 | ||
| Ah1 | 2–5 | 6.59 | 110.0 | 9.8 | 106.2 | ||
| Ah2 | 5–15(20) | 6.97 | 76.0 | 6.9 | 65.3 | ||
| Bwca1 | 15(20)–30 | 7.40 | 18.0 | 1.5 | 38.4 | ||
| Bwca2 | 30–45 | 7.73 | 16.0 | 6.6 | 1.3 | 43.3 | |
| 6. Leptic Skeletic Calcaric Regosol | Standing Biomass | – | 420.0 | 0.0 | 9.4 | 51.8 | |
| O | 0–1 | 7.57 | 236.0 | 70.3 | 8.7 | 74.1 | |
| Ah1 | 1–10(15) | 8.04 | 116.0 | 81.1 | 3.2 | 57.8 | |
| Ah2 | 10(15)–25 | 8.08 | 124.0 | 50.2 | 5.4 | 47.4 | |
| Ah@ | 25–35 | 7.98 | 121.0 | 44.0 | 6.2 | 44.3 | |
| Bca | 35–45 | 8.32 | 118.0 | 103.8 | 0.0 | 46.6 | |
| Cca | 45–… | 8.50 | 118.0 | 122.4 | 5.7 | ||
| 6′. Spot without Vegetation | O | 0–1 | 7.76 | 110.0 | 91.3 | 2.2 | 17.2 |
| Ah1 | 1–10 | 7.93 | 112.0 | 93.1 | 2.2 | 26.7 | |
| Ah2 | 10–25 | 7.89 | 115.0 | 77.4 | 4.0 | 79.7 | |
| Ah@ | 25–35 | 8.00 | 126.0 | 67.2 | 4.7 | 70.1 | |
| 7. Calcaric Stagnosol Humic Skeletic | Standing Biomass | – | 405.0 | 0.0 | 23.3 | 72.6 | |
| O | 0–2 | 5.58 | 331.0 | 25.4 | 164.9 | ||
| H1 | 2–10 | 6.30 | 243.0 | 17.1 | 162.1 | ||
| H2 | 10–20 | 6.49 | 167.0 | 12.0 | 92.5 | ||
| Bg | 20–27 | 6.61 | 21.0 | 1.6 | 90.1 | ||
| Bwca1 | 27–50 | 7.61 | 16.0 | 7.0 | 0.6 | 44.6 | |
| Bwca2 | 50–65 | 7.97 | 22.0 | 24.6 | 0.4 | 53.4 | |
| Cca | 65–80 | 8.04 | 21.0 | 24.4 | 0.4 | 37.9 | |
| 8. Calcaric Folic Gleysol (Skeletic) | Standing Biomass | – | 410.0 | 0.0 | 8.5 | 32.7 | |
| O | 0–5 | 6.41 | 384.0 | 17.3 | 69.8 | ||
| Oa | 5–10 | 6.77 | 345.0 | 16.0 | 112.4 | ||
| Ah1 | 10–12 | 6.91 | 198.0 | 12.9 | 80.2 | ||
| Ah2 | 12–20 | 6.95 | 156.0 | 10.3 | 76.4 | ||
| Ghca | 20–35 | 7.72 | 27.0 | 5.5 | 1.6 | 30.5 | |
| Cca | 35–55 | 7.99 | 16.0 | 7.6 | 0.8 | 46.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamrikova, E.; Yakovleva, E.; Gabov, D.; Zhangurov, E.; Korolev, M.; Zazovskaya, E. Polyarenes Distribution in the Soil-Plant System of Reindeer Pastures in the Polar Urals. Agronomy 2022, 12, 372. https://doi.org/10.3390/agronomy12020372
Shamrikova E, Yakovleva E, Gabov D, Zhangurov E, Korolev M, Zazovskaya E. Polyarenes Distribution in the Soil-Plant System of Reindeer Pastures in the Polar Urals. Agronomy. 2022; 12(2):372. https://doi.org/10.3390/agronomy12020372
Chicago/Turabian StyleShamrikova, Elena, Evgeniia Yakovleva, Dmitry Gabov, Egor Zhangurov, Michail Korolev, and Elya Zazovskaya. 2022. "Polyarenes Distribution in the Soil-Plant System of Reindeer Pastures in the Polar Urals" Agronomy 12, no. 2: 372. https://doi.org/10.3390/agronomy12020372






