Effect of Carbohydrate Nutrition on Egg Load and Population Parameters of Four Trichogramma Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasitoids
2.2. Hosts
2.3. Egg Load and Mature Eggs
2.4. Population Parameters
2.5. Data Analysis
3. Result
3.1. Egg Load
3.2. Mature Eggs
3.3. Population Parameters
3.4. Age-Specific Fecundity and Cumulative Fecundity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heimpel, G.E.; Mills, N. Biological Control: Ecology and Applications; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Zang, L.S.; Wang, S.; Zhang, F.; Desneux, N. Biological control with Trichogramma in China: History, present status, and perspectives. Annu. Rev. Entomol. 2021, 66, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M. Biological control with Trichogramma: Advances, successes, and potential of their use. Annu. Rev. Entomol. 1996, 41, 375–406. [Google Scholar] [CrossRef] [PubMed]
- Tabone, E.; Bardon, C.; Desneux, N.; Wajnberg, E. Parasitism of different Trichogramma species and strains on Plutella xylostella L. on greenhouse cauliflower. J. Pest Sci. 2010, 83, 251–256. [Google Scholar] [CrossRef]
- Qu, Y.Y.; Chen, X.; Monticelli, L.S.; Zhang, F.; Desneux, N.; Dai, H.J.; Ricardo, R.R.; Wang, S. Parasitism performance of the parasitoid Trichogramma dendrolimi on the plum fruit moth Grapholitha funebrana. Entomol. Gen. 2020, 40, 385–395. [Google Scholar] [CrossRef]
- Wang, P.; Li, M.J.; Bai, Q.R.; Ali, A.; Desneux, N.; Dai, H.J.; Zang, L.S. Performance of Trichogramma japonicum as a vector of Beauveria bassiana for parasitizing eggs of rice striped stem borer, Chilo suppressalis. Entomol. Gen. 2021, 41, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Jaworski, C.C.; Desneux, N.; Zhang, F.; Yang, P.; Wang, S. Long-term and large-scale releases of Trichogramma promote pesticide decrease in maize in northeastern China. Entomol. Gen. 2020, 40, 331–335. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.C.; Du, W.M.; Zang, L.S.; Ruan, C.C.; Zhang, J.J.; Zou, Z. Multi-parasitism: A promising approach to simultaneously produce Trichogramma chilonis and T. dendrolimi on eggs of Antheraea pernyi. Entomol. Gen. 2021, 41, 627–636. [Google Scholar]
- Wang, Y.; Zou, Z.P.; Hou, Y.Y.; Yang, X.B.; Wang, S.; Dai, H.J.; Xu, Y.Y.; Zang, L.S. Manually-extracted unfertilized eggs of Chinese oak silkworm, Antheraea pernyi, enhance mass production of Trichogramma parasitoids. Entomol. Gen. 2020, 40, 397–406. [Google Scholar] [CrossRef]
- Li, Z.X.; Shen, Z.R. Application of rDNA-ITS2 sequences to the molecular identification of Trichogramma spp. Acta Entomol. Sin. 2002, 45, 59–66. [Google Scholar]
- Song, R.C.; Chi, Y.M.; Wang, H.P.; Lu, G.Y.; Wang, Q.X.; Zhang, Q.B. A study on setting equipment of manufactured reproduction of Trichogramma. Trans. Chin. Soc. Agric. Eng. 1995, 11, 48–52. [Google Scholar]
- Xu, D.P.; Bai, Y.; Gong, X.; Xu, Z.J. Design of Trichogramma delivering system based on Hex-Rotor UAV. Trans. Chin. Soc. Agric. Mach. 2016, 47, 1–7. [Google Scholar]
- Zhang, J.J.; Ruan, C.C.; Zang, L.S.; Shao, X.W.; Shi, S.S. Technological improvements for mass production of Trichogramma and current status of their applications for biological control on agricultural pests in China. Chin. J. Biol. Control 2015, 31, 638–646. [Google Scholar]
- Zhang, J.J.; Zhang, X.; Zang, L.S.; Du, W.M.; Hou, Y.Y. Advantages of diapause in Trichogramma dendrolimi mass production on eggs of the Chinese silkworm, Antheraea pernyi. Pest Manag. Sci. 2018, 74, 959–965. [Google Scholar] [CrossRef]
- Wan, F.H.; Wang, R.; Ye, Z.C. Prospects of industrialization of natural enemy insect productions in China. Chin. J. Biol. Control 1999, 15, 135–138. [Google Scholar]
- Bell, G. Measuring the cost of reproduction. Oecologia 1984, 60, 378–383. [Google Scholar] [CrossRef]
- Roitberg, B.D. The cost of reproduction in rosehip flies, Rhagoletis basiola: Eggs are time. Evol. Ecol. 1989, 3, 183–188. [Google Scholar] [CrossRef]
- Bell, W.J.; Bohm, M.K. Oosorption in insects. Biol. Rev. 1975, 50, 373–396. [Google Scholar] [CrossRef]
- Chapman, R.F. The Insects: Structure and Function, 4th ed.; Cambridge University Press: New York, NY, USA, 1998; pp. 313–343. [Google Scholar]
- King, P.E.; Richards, J.G. Osorption in Nasonia vitripennis (Hymenoptera: Pteromalidae). J. Zool. 1968, 154, 495–516. [Google Scholar] [CrossRef]
- Collier, T.R. Host feeding, egg maturation, resorption, and longevity in the parasitoid Aphytis melinus (Hymenoptera: Aphelinidae). Ann. Entomol. Soc. Am. 1995, 88, 206–214. [Google Scholar] [CrossRef]
- Keinan, Y.; Keasar, T. Evidence for trans-generational effects on egg maturation schedules in a syn-ovigenic parasitoid. J. Insect Physiol. 2019, 117, 103910. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Biondi, A.; Nance, A.H.; Zappalà, L.; Hoelmer, K.A.; Daane, K.M. Assessment of Asobara japonica as a potential biological control agent for the spotted wing drosophila, Drosophila suzukii. Entomol. Gen. 2020, 41, 1–12. [Google Scholar]
- Ellers, J.; Jervis, M.A. Why are so few parasitoid wasp species pro-ovigenic? Evol. Ecol. Res. 2004, 6, 993–1002. [Google Scholar]
- Jervis, M.A.; Ferns, P.N. The timing of egg maturation in insects: Ovigeny index and initial egg load as measures of fitness and of resource allocation. Oikos 2004, 107, 449–460. [Google Scholar] [CrossRef]
- Boivin, G. Reproduction and immature development of egg parasitoids. In Egg Parasitoids in Agro-Ecosystems with Emphasis on Trichogramma; Springer: Dordrecht, The Netherlands, 2009; pp. 1–23. [Google Scholar]
- Jervis, M.A.; Heimpel, G.E.; Ferns, P.N.; Harvey, J.A.; Kidd, N.A.C. Life-history strategies in parasitoid wasps: A comparative analysis of ‘ovigeny’. J. Anim. Ecol. 2001, 70, 442–458. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, B.; Zhang, F.; Li, Y.X. Effects of host breeding on individual size and egg holding capacity of three Trichogramma species. Acta Entomol. Sin. 2015, 58, 1098–1107. [Google Scholar]
- Heimpel, G.E.; Collier, T.R. The evolution of host-feeding behaviour in insect parasitoids. Biol. Rev. Camb. Philos. Soc. 1996, 71, 373–400. [Google Scholar] [CrossRef]
- Bernstein, C.; Jervis, M. Food-searching in parasitoids: The dilemma of choosing between ‘immediate’ or future fitness gains. Incollection 2008, 7, 129–171. [Google Scholar]
- Jervis, M.A.; Kidd, N.A.C. Host-feeding strategies in hymenopteran parasitoids. Biol. Rev. 1986, 61, 395–434. [Google Scholar] [CrossRef]
- Kidd, N.A.C.; Jervis, M.A. Host-feeding and oviposition by parasitoids in relation to host stage: Consequences for parasitoid-host population dynamics. Popul. Ecol. 1991, 33, 87–99. [Google Scholar] [CrossRef]
- Bodin, A.; Vinauger, C.; Lazzari, C.R. Behavioural and physiological state dependency of host seeking in the blood-sucking insect Rhodnius prolixus. J. Exp. Biol. 2009, 212, 2386–2393. [Google Scholar] [CrossRef] [PubMed]
- Lenteren, J.; Bigler, F. Quality Control of Mass Reared Egg Parasitoids. In Egg Parasitoids in Agro-Ecosystems with Emphasis on Trichogramma; Springer: Dordrecht, The Netherlands, 2009; pp. 315–340. [Google Scholar]
- Zhang, G.; Zimmermann, O.; Hassan, S.A. Pollen as a source of food for egg parasitoids of the genus Trichogramma (Hymenoptera: Trichogrammatidae). Biocontrol. Sci. Technol. 2004, 14, 201–209. [Google Scholar] [CrossRef]
- Wäckers, F.L. Suitability of (extra-) floral nectar, pollen and honeydew as insect food sources. In Plant-Provided Food for Carnivorous Insects: A Protective Mutualism and Its Applications; Cambridge University Press: Cambridge, UK, 2005; pp. 17–74. [Google Scholar]
- Witting-Bissinger, B.E.; Orr, D.B.; Linker, H.M. Effects of floral resources on fitness of the parasitoids Trichogramma exiguum (Hymenoptera: Trichogrammatidae) and Cotesia congregata (Hymenoptera: Braconidae). Biol. Control 2008, 47, 180–186. [Google Scholar] [CrossRef]
- Jervis, M.A.; Kidd, N.A.C.; Fitton, M.G.; Huddleston, T.; Dawah, H.A. Flower-visiting by hymenopteran parasitoids. J. Nat. Hist. 1993, 27, 67–105. [Google Scholar] [CrossRef]
- Jervis, M.A.; Kidd, N.A.C.; Heimpel, G.E. Parasitoid adult feeding behavior and biological control-a review. Biocontrol. News Inf. 1996, 17, 11–16. [Google Scholar]
- Damien, M.; Llopis, S.; Desneux, N.; Baaren, J.V.; Lann, C.L. How does floral nectar quality affect life history strategies in parasitic wasps? Entomol. Gen. 2019, 40, 147–156. [Google Scholar] [CrossRef]
- Fuchsberg, J.R.; Yong, T.H.; Losey, J.E.; Carter, M.E.; Hoffmann, M.P. Evaluation of corn leaf aphid (Rhopalosiphum maidis; Homoptera: Aphididae) honeydew as a food source for the egg parasitoid Trichogramma ostriniae (Hymenoptera: Trichogrammatidae). Biol. Control 2006, 40, 230–236. [Google Scholar] [CrossRef]
- Wu, H.P.; Meng, L.; Li, B.P. Effects of feeding frequency and sugar concentrations on lifetime reproductive success of Meteorus pulchricornis (Hymenoptera: Braconidae). Biol. Control 2008, 45, 353–359. [Google Scholar]
- Benelli, G.; Giunti, G.; Tena, A.; Desneux, N.; Caselli, A.; Canale, A. The impact of adult diet on parasitoid reproductive performance. J. Pest Sci. 2017, 90, 807–823. [Google Scholar] [CrossRef]
- Monticelli, L.S.; Tena, A.; Idier, M.; Amiens-Desneux, E.; Desneux, N. Quality of aphid honeydew for a parasitoid varies as a function of both aphid species and host plant. Biol. Control 2020, 140, 104099. [Google Scholar] [CrossRef]
- Tunbilek, A.S.; Bilbil, H.; Bakir, S.; Silici, S. The performance of Trichogramma (Hymenoptera: Trichogrammatidae) Parasitoids feeding on honey sources. Tarim Bilim. Derg. 2021, 27, 400–406. [Google Scholar] [CrossRef]
- Tian, J.C.; Wang, G.W.; Romeis, J.; Zheng, X.S.; Xu, H.X.; Zang, L.S.; Lu, Z.X. Different performance of two Trichogramma (Hymenoptera: Trichogrammatidae) species feeding on sugars. Environ. Entomol. 2016, 45, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Bugg, R.L.; Ellis, R.T.; Carlson, R.W. Ichneumonidae (Hymenoptera) Using Extrafloral Nectar of Faba Bean (Vicia faba L. Fabaceae) in Massachusetts. Biolog. Agric. Horticult. Int. J. Sustain. Prod. Syst. 1989, 6, 107–114. [Google Scholar] [CrossRef]
- Karen, I.; David, N.F. Aphid honeydew as a carbohydrate source for Edovum puttleri (Hymenoptera: Eulophidae). Environ. Entomol. 1988, 17, 941–944. [Google Scholar]
- Hogervorst, P.A.M.; W€ackers, F.L.; Romeis, J. Effects of honeydew sugar composition on the longevity of Aphidius ervi. Entomol. Exp. Appl. 2007, 122, 223–232. [Google Scholar] [CrossRef]
- Lundgren, J.G. Relationships of Natural Enemies and Non-Prey foods. In Progress in Biological Control; Springer Science & Business Media: Berlin, Germany, 2009; Volume 7, pp. 279–329. [Google Scholar]
- Olson, D.M.; Fadamiro, H.Y.; Lundgren, J.O.; Heimpel, G.E. Effects of sugar feeding on carbohydrate and lipid metabolism in a parasitoid wasp. Physiol. Entomol. 2000, 25, 17–26. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; Strange-George, J.E.; Kulhanek, C.A.; Wäckers, F.L.; Heimpel, G.E. Sugar feeding by the aphid parasitoid Binodoxys communis: How does honeydew compare with other sugar sources? J. Insect Physiol. 2008, 54, 481–491. [Google Scholar] [CrossRef]
- Tompkins, J.M.L.; Wratten, S.D.; Wäckers, F.L. Nectar to improve parasitoid fitness in biological control: Does the sucrose:hexose ratio matter? Basic Appl. Ecol. 2009, 11, 264–271. [Google Scholar] [CrossRef]
- Leatemia, J.A.; Laing, J.; Corrigan, J.E. Effects of adult nutrition on longevity, fecundity, and offspring sex ratio of Trichogramma minutum Riley (Hymenoptera: Trichogrammatidae). Can. Entomol. 1995, 127, 245–254. [Google Scholar] [CrossRef]
- Tylianakis, J.M.; Didham, R.K.; Wratten, S.D. Improved fitness of aphid parasitoids receiving resource subsidies. Ecology 2004, 85, 658–666. [Google Scholar] [CrossRef]
- Liang, H.Y.; Yang, X.M.; Sun, L.J.; Zhao, C.D.; Chi, H.; Zheng, C.Y. Sublethal effect of spirotetramat on the life table and population growth of Frankliniella occidentalis (Thysanoptera: Thripidae). Entomol. Gen. 2020, 41, 219–231. [Google Scholar] [CrossRef]
- Ding, H.Y.; Lin, Y.Y.; Tuan, S.J.; Tang, L.C.; Chi, H.; Atlıhan, R.; Ozgokce, M.S.; Guncan, A. Integrating demography, predation rate, and computer simulation for evaluation of Orius strigicollis as biological control agent against Frankliniella intonsa. Entomol. Gen. 2021, 41, 179–196. [Google Scholar] [CrossRef]
- Chi, H.; You, M.S.; Atlihan, R.; Smith, C.L.; Kavousi, A.; Ozgokce, M.S.; Guncan, A.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y.; et al. Age-Stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2019, 40, 103–124. [Google Scholar] [CrossRef]
- Lin, N.Q. Classification of Trichogramma from China (Hymenoptera: ApicOidea); Fujian Science and Technology Press: Fuzhou, China, 1994; pp. 42–63. [Google Scholar]
- Yuan, X.; Feng, X.X.; Li; Dun, S. Effects of ultraviolet radiation on Trichogramma reproduction. Guangdong Agric. Sci. 2012, 39, 91–94. [Google Scholar]
- Riddick, E.W. Influence of honey and maternal age on egg load of lab-cultured Cotesia marginiventris. Biocontrol 2007, 52, 613–618. [Google Scholar] [CrossRef]
- Ozkan, C. Effect of food, light and host instar on the egg load of the synovigenic endoparasitoid Venturia canescens (Hymenoptera: Ichneumonidae). J. Pest Sci. 2007, 80, 79–83. [Google Scholar] [CrossRef]
- Zhang, C.R. Autonomous Regulation of Egg Development in Whitefly Parasitoids; Jilin Agricultural University: Changchun, China, 2016. [Google Scholar]
- Wang, Y. Factors Affecting Follows Eggs Develop Key Research; Jilin Agricultural University: Changchun, China, 2021. [Google Scholar]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis [Online]. 2021. Available online: http://140.120.197.173/Ecology/prod02.htm (accessed on 9 February 2022).
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Monographs on Statistics and Applied Probability, No. 57; Chapman and Hall: London, UK, 1993; p. 436. [Google Scholar]
- Wei, M.F.; Chi, H.; Guo, Y.F.; Li, X.W.; Zhao, L.L.; Ma, R.Y. Demography of Cacopsylla chinensis (Hemiptera: Psyllidae) reared on four cultivars of Pyrus bretschneideri and P. communis (Rosales: Rosaceae) pears with estimations of confidence intervals of specific life table statistics. J. Econ. Entomol. 2020, 113, 2343–2353. [Google Scholar] [CrossRef]
- Smucker, M.D.; Allan, J.; Carterette, B. A comparison of statistical significance tests for information retrieval evaluation. In Proceedings of the Sixteenth ACM Conference on Information and Knowledge Management, CIKM, Lisbon, Portugal, 6–10 November 2007; pp. 623–632. [Google Scholar]
- BAI, B.; Smith, S.M. Effect of host availability on reproduction and survival of the parasitoid wasp Trichogramma minutum. Ecol. Entomol. 1993, 18, 279–286. [Google Scholar] [CrossRef]
- Chen, K.W.; Liu, H.Z.; He, Y.R. Relationship between fecundity and age of Trichogramma ostrinalis. Acta Entomol. Sin. 2005, 48, 712–717. [Google Scholar]
- Pak, G.A.; Oatman, E.R. Comparative life table, behavior and competition studies of Trichogramma brevicapillum and T. pretiosum. Entomol. Exp. Appl. 1982, 32, 68–79. [Google Scholar] [CrossRef]
- Bai, B.; Mackauer, M. Influence of superparasitism on development rate and adult size in a solitary parasitoid wasp, Aphidius ervi. Funct. Ecol. 1992, 6, 302–307. [Google Scholar] [CrossRef]
- Mills, N.J.; Kuhlmann, U. The relationship between egg load and fecundity among Trichogramma parasitoids. Ecol. Entomol. 2000, 25, 315–324. [Google Scholar] [CrossRef]
- Bjorksten, T.A.; Hoffmann, A.A. Plant cues influence searching behaviour and parasitism in the egg parasitoid Trichogramma nr. brassicae. Ecol. Entomol. 1998, 23, 355–362. [Google Scholar] [CrossRef]
- Carrière, Y.; Roitberg, B.D. Optimality modelling and quantitative genetics as alternatives to study the evolution of foraging behaviours in insect herbivores. Evol. Ecol. 1996, 10, 289–305. [Google Scholar] [CrossRef]
- Sirot, E.; Krivon, V. Adaptive superparasitism and host-parasitoid dynamics. Bull. Math. Biol. 1997, 59, 23–42. [Google Scholar] [CrossRef]
- Hughes, J.P.; Harvey, I.F.; Hubbard, S.F. Host-searching behavior of Venturia canescens (Grav.) (Hymenoptera: Ichneumonidae): Interference—The effect of mature egg load and prior behavior. J. Insect Behav. 1994, 7, 433–454. [Google Scholar] [CrossRef]
- Boot, W.J.; Minkenberg, O.P.J.M.; Rabbinge, R.; Moed, G.H. Biological control of the leafminer Liriomyza bryoniae by seasonal inoculative releases of Diglyphus isaea: Simulation of a parasitoid-host system. Neth. J. Plant Pathol. 1992, 98, 203–212. [Google Scholar] [CrossRef]
- Krivan, V.; Sirot, E. Searching for Food or Hosts: The influence of parasitoids behavior on host-parasitoid dynamics. Theor. Popul. Biol. 1997, 51, 201–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prokopy, R.J.; Cooley, S.S.; Prokopy, J.J.; Quan, Q.; Buonaccorsi, J.P. Interactive effects of resource abundance and state of adults on residence of Apple Maggot (Diptera: Tephritidae) flies in host tree patches. Environ. Entomol. 1994, 23, 304–315. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Coli, W.M. Influence of understory cover and surrounding habitat on interactions between beneficial arthropods and pests in orchards. Agric. Ecosyst. Environ. 1994, 50, R7. [Google Scholar]
- Mayhew, P.J. Daily activity rhythms in adult odonata examined with a dynamic programming model. Neth. J. Zool. 1997, 48, 101–119. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Mangel, M.; Rosenheim, J.A. Effects of time limitation and egg limitation on lifetime reproductive success of a parasitoid in the field. Am. Nat. 1998, 152, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Rosenheim, J.A. An evolutionary argument for egg limitation. Evolution 1996, 50, 2089–2094. [Google Scholar] [PubMed]
- Sevenster, J.G.; Ellers, J.; Driessen, G. An evolutionary argument for time limitation. Evolution 1998, 52, 1241–1244. [Google Scholar]
- Mackauer, M.; Sullivan, D.J. A new species of praon (hymenoptera: Aphidiidae), parasitic on the dusky-veined walnut aphid, Callaphis juglandis (homoptera: Aphididae). Can. Entomol. 1982, 114, 1159–1161. [Google Scholar] [CrossRef]
- Kopelman, A.H.; Chabora, P.C. Resource variability and life history parameters of Leptopilina boulardi (Hymenoptera: Eucoilidae). Ann. Entomol. Soc. Am. 1992, 85, 195–199. [Google Scholar] [CrossRef]
- Jervis, M.; Ferns, P. Towards a general perspective on life-history evolution and diversification in parasitoid wasps. Biol. J. Linn. Soc. 2011, 104, 443–461. [Google Scholar] [CrossRef]
- Noldus, L.; Rumei, X.; Lenteren, J.C.V. The parasite-host relationship between Encarsia formosa gahan (hymenoptera, aphelinidae) and Trialeurodes vaporariorum (Westwood) (Homoptera, Aleyrodidae). J. Appl. Entomol. 1986, 101, 159–176. [Google Scholar] [CrossRef]
- Liu, Y.H.; Yan, X.F.; Ye, G.Y.; Li, X.H. Effects of different diets on the development of egg nests and oogenesis of Telenomus theophilae. Chin. J. Biosaf. 2006, 15, 112–115. [Google Scholar]
- Song, N.; Luo, M.H.; Yuan, G.H. Effects of feeding on parasitoids. Chin. J. Environ. Entomol. 2006, 28, 132–138. [Google Scholar]
- Narvaez, A.; Cancino, J.; Nelson, C.D.; Wyckhuys, K.A.G. Effect of different dietary resources on longevity, carbohydrate metabolism, and ovarian dynamics in two fruit fly parasitoids. Arthropod-Plant Interact. 2012, 6, 361–374. [Google Scholar] [CrossRef]
- Hu, H.; Liu, Y.H. Effects of different sugars on longevity, fecundity and nutrient storage of Chrysophorus cerevisiae. Chin. J. Biol. Control 2014, 30, 165–170. [Google Scholar]
- Zhang, Y.B.; Yang, N.W.; Wang, J.J.; Wan, F.H. Effect of six carbohydrate sources on the longevity of a whitefly parasitoid Eretmocerus hayati (Hymenoptera:Aphelinidae). J. Asia-Pac. Entomol. 2014, 17, 723–728. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Tian, J.C.; Zheng, X.S.; Xu, H.X.; Lu, Y.H.; Yang, Y.J.; Zang, L.S.; Lv, Z.X. Feasibility analysis of Oxalis corniculata and Galium odoratum as honey source plants of Trichogramma chilonis. Acta Agric. Zhejiangensis 2017, 29, 106–112. [Google Scholar]

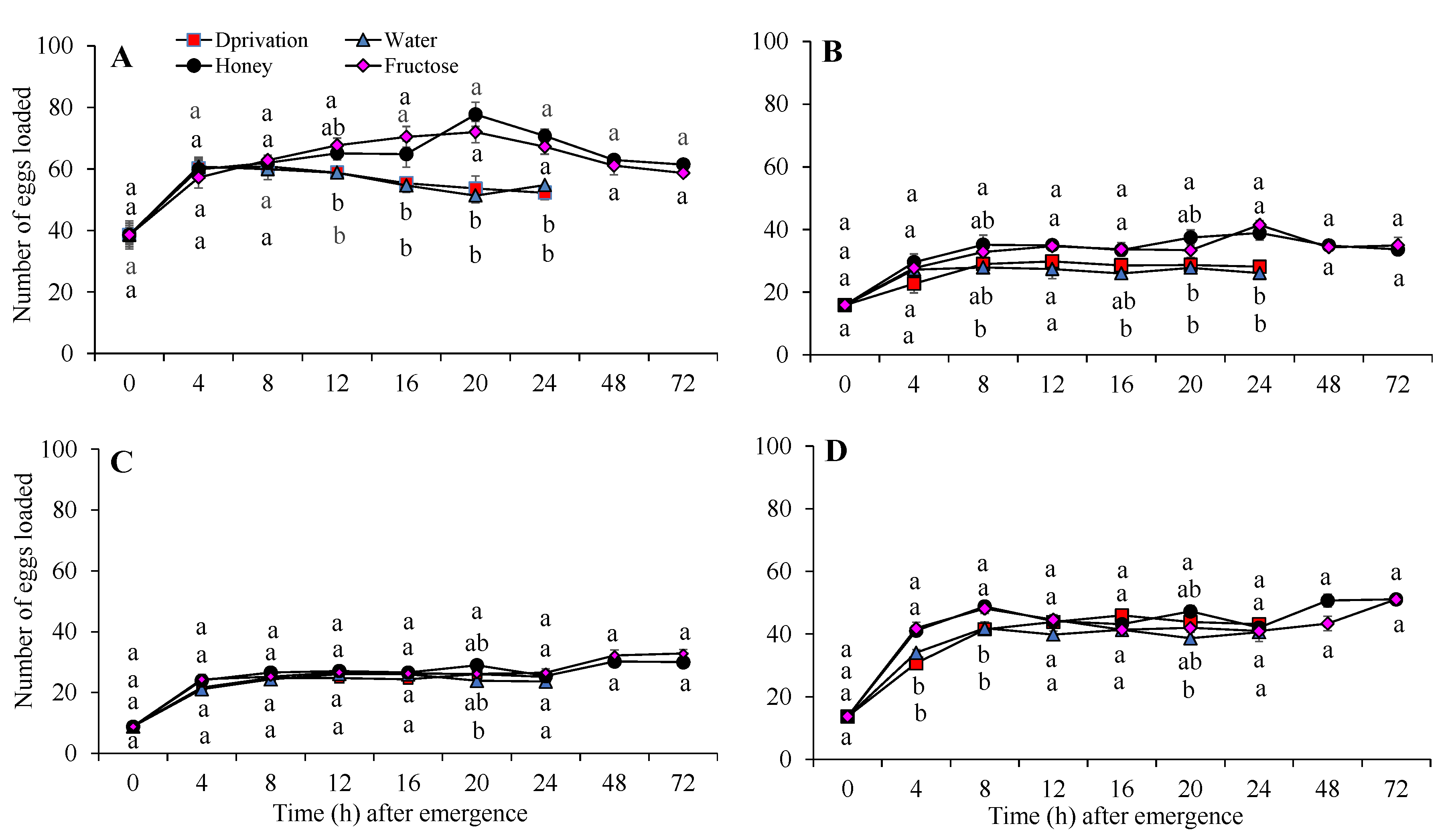
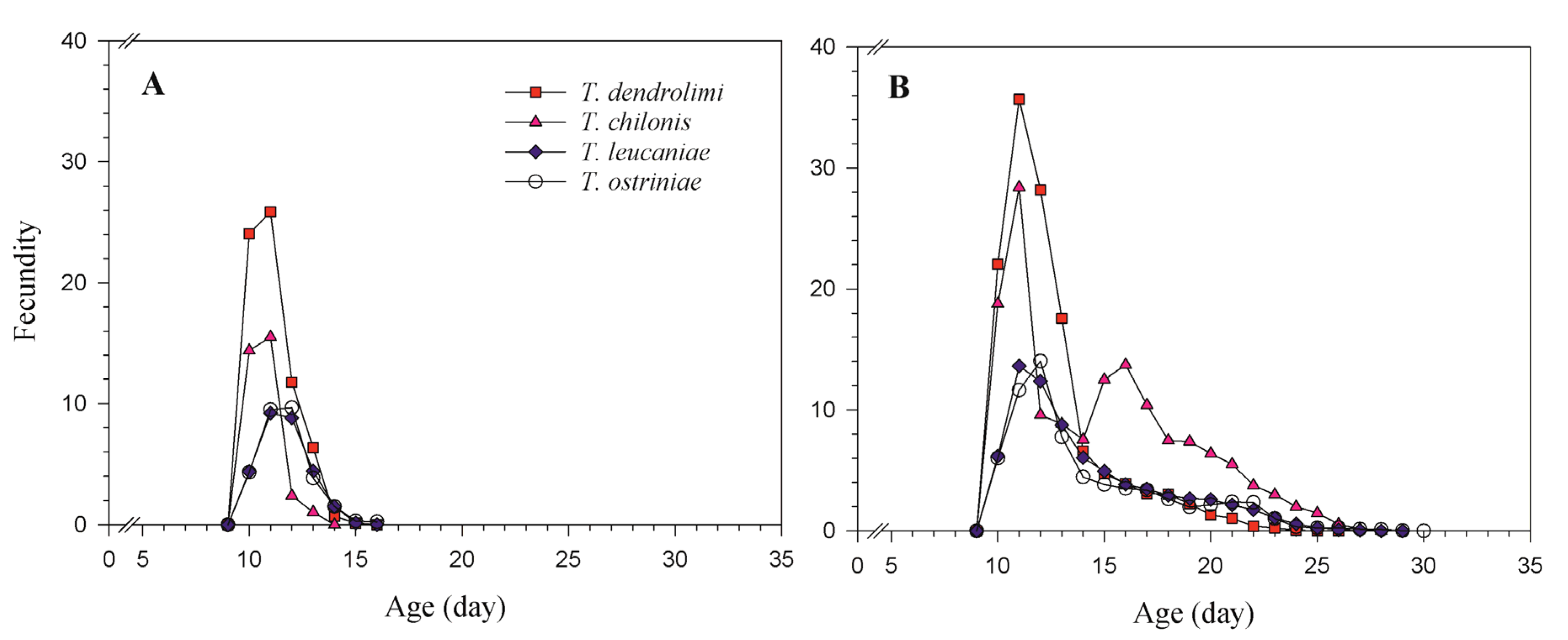

| Parameter | T. dendrolimi | T. chilonis | T. leucaniae | T. ostriniae | ||||
|---|---|---|---|---|---|---|---|---|
| Honey | Water | Honey | Water | Honey | Water | Honey | Water | |
| Female adult longevity (days) | 10.18 ± 0.23 c | 1.61 ± 0.08 e | 15.33 ± 0.23 a | 1.33 ± 0.34 f | 13.05 ± 0.25 b | 1.79 ± 0.07 e | 15.48 ± 0.30 a | 2.05 ± 0.08 d |
| Fecundity (F) | 146.64 ± 1.89 b | 78.49 ± 1.60 c | 173.53 ± 5.26 a | 40.15 ± 0.39 d | 83.69 ± 3.43 c | 32.45 ± 1.33 f | 80.45 ± 3.32 c | 35.47 ± 0.73 e |
| Oviposition days | 9.39 ± 0.22 c | 1.61 ± 0.08 e | 13.88 ± 0.21 a | 1.33 ± 0.34 f | 10.18 ± 0.37 c | 1.79 ± 0.07 e | 11.33 ± 0.28 b | 2.05 ± 0.08 d |
| Net reproduction rate (R0) (offspring) | 130.0 ± 4.8 b | 68.9 ± 2.7 c | 147.4 ± 7.3 a | 33.3 ± 1.5 d | 73.2 ± 4.0 c | 28.5 ± 1.1 e | 68.1 ± 3.6 c | 29.4 ± 1.4 de |
| Intrinsic rate of increase r (day−1) | 0.4178 ± 0.0048 a | 0.3895 ± 0.0049 b | 0.3933 ± 0.0048 b | 0.3301 ± 0.0048 c | 0.3373 ± 0.0049 c | 0.2918 ± 0.0045 d | 0.3317 ± 0.0053 c | 0.2940 ± 0.0047 d |
| Finite rate of increase (λ) (day−1) | 1.5186 ± 0.0133 a | 1.4761 ± 0.0070 b | 1.4818 ± 0.0070 b | 1.3911 ± 0.0067 c | 1.4011 ± 0.0068 c | 1.3388 ± 0.0060 d | 1.3934 ± 0.0073 c | 1.3417 ± 0.0063 d |
| Mean generation time (T) (days) | 11.65 ± 0.10 b | 10.87 ± 0.09 c | 12.69 ± 0.14 a | 10.62 ± 0.07 d | 12.73 ± 0.12 a | 11.46 ± 0.11 b | 12.72 ± 0.13 a | 11.49 ± 0.10 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Iqbal, A.; Mu, M.-Y.; Zang, Z.-Y.; Hou, Y.-Y.; Zang, L.-S. Effect of Carbohydrate Nutrition on Egg Load and Population Parameters of Four Trichogramma Species. Agronomy 2022, 12, 3143. https://doi.org/10.3390/agronomy12123143
Wang Y, Iqbal A, Mu M-Y, Zang Z-Y, Hou Y-Y, Zang L-S. Effect of Carbohydrate Nutrition on Egg Load and Population Parameters of Four Trichogramma Species. Agronomy. 2022; 12(12):3143. https://doi.org/10.3390/agronomy12123143
Chicago/Turabian StyleWang, Yong, Asim Iqbal, Ming-Yue Mu, Zhuo-Yi Zang, Yang-Yang Hou, and Lian-Sheng Zang. 2022. "Effect of Carbohydrate Nutrition on Egg Load and Population Parameters of Four Trichogramma Species" Agronomy 12, no. 12: 3143. https://doi.org/10.3390/agronomy12123143
APA StyleWang, Y., Iqbal, A., Mu, M.-Y., Zang, Z.-Y., Hou, Y.-Y., & Zang, L.-S. (2022). Effect of Carbohydrate Nutrition on Egg Load and Population Parameters of Four Trichogramma Species. Agronomy, 12(12), 3143. https://doi.org/10.3390/agronomy12123143







