Combined Abiotic Stresses: Challenges and Potential for Crop Improvement
Abstract
1. Introduction
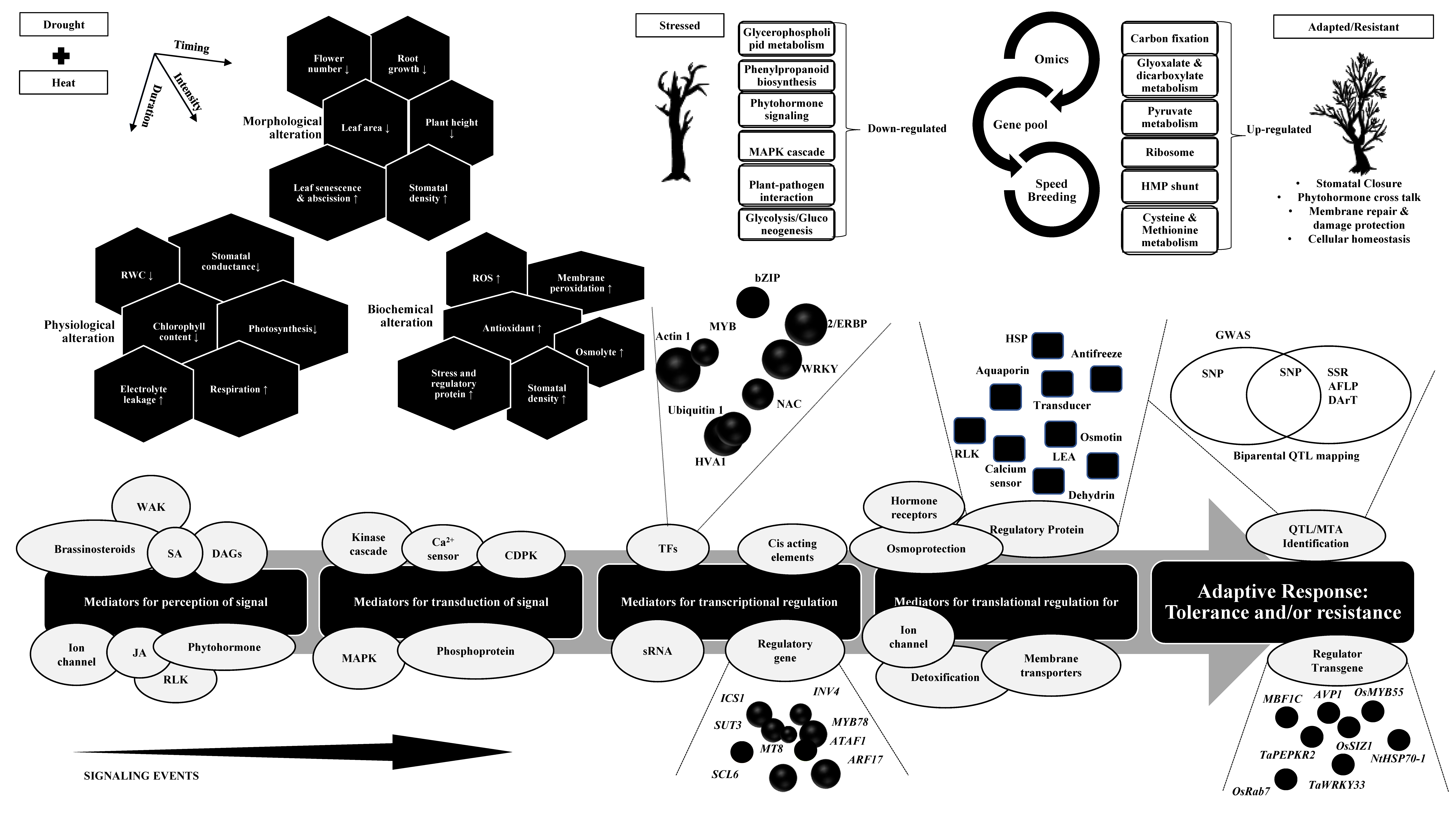
2. Implications of Combined Abiotic Stresses in Plants
2.1. Combined Abiotic Stresses and Growth and Development Implications
2.2. Combined Abiotic Stresses and Physiological Implications
2.3. Combined Abiotic Stresses and Molecular Implications
3. Effect of Combined Stress at Different Growth Stages and Crucial Processes
3.1. Antioxidant Defense
3.2. Mineral Transport Mechanism
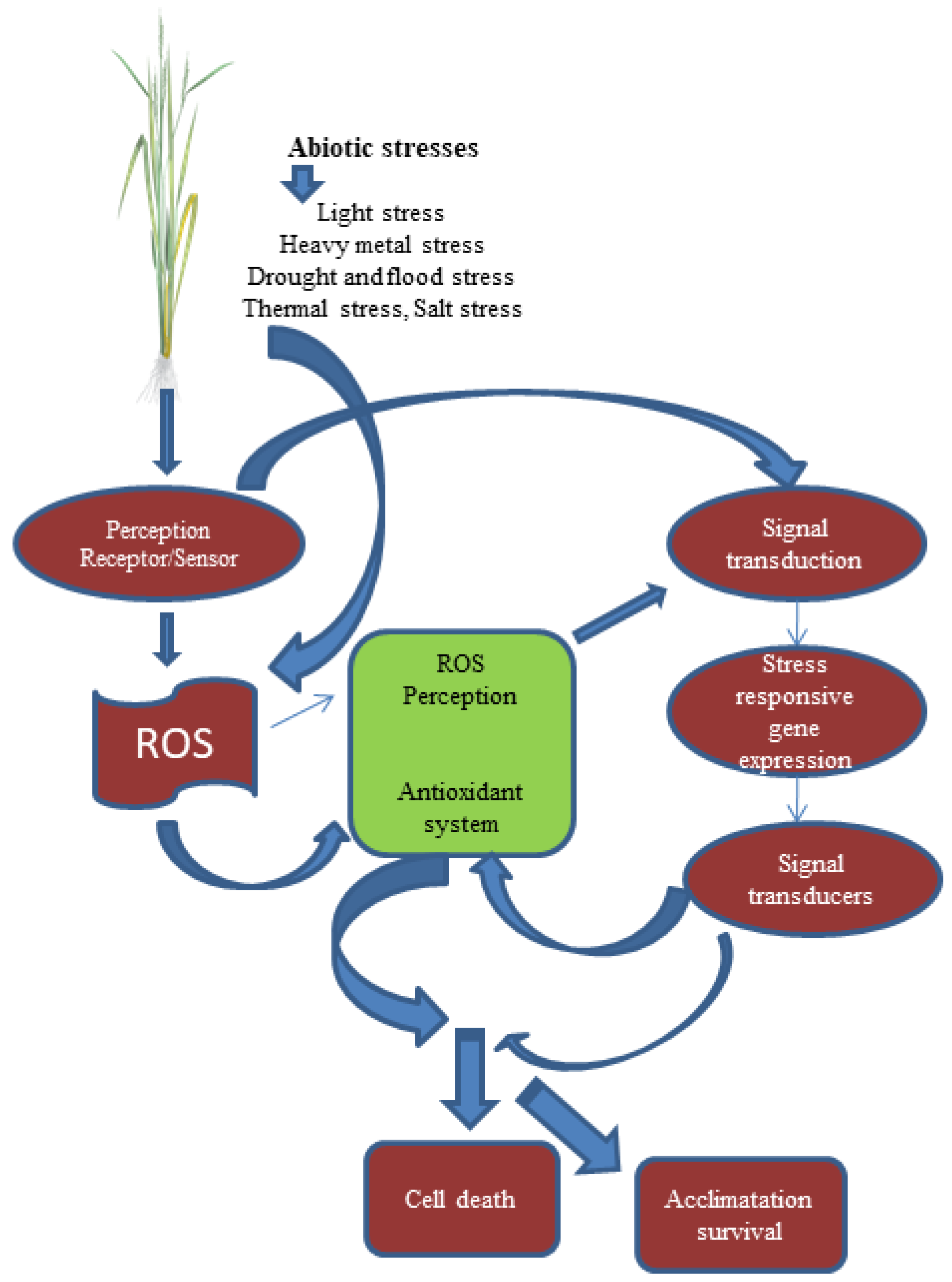
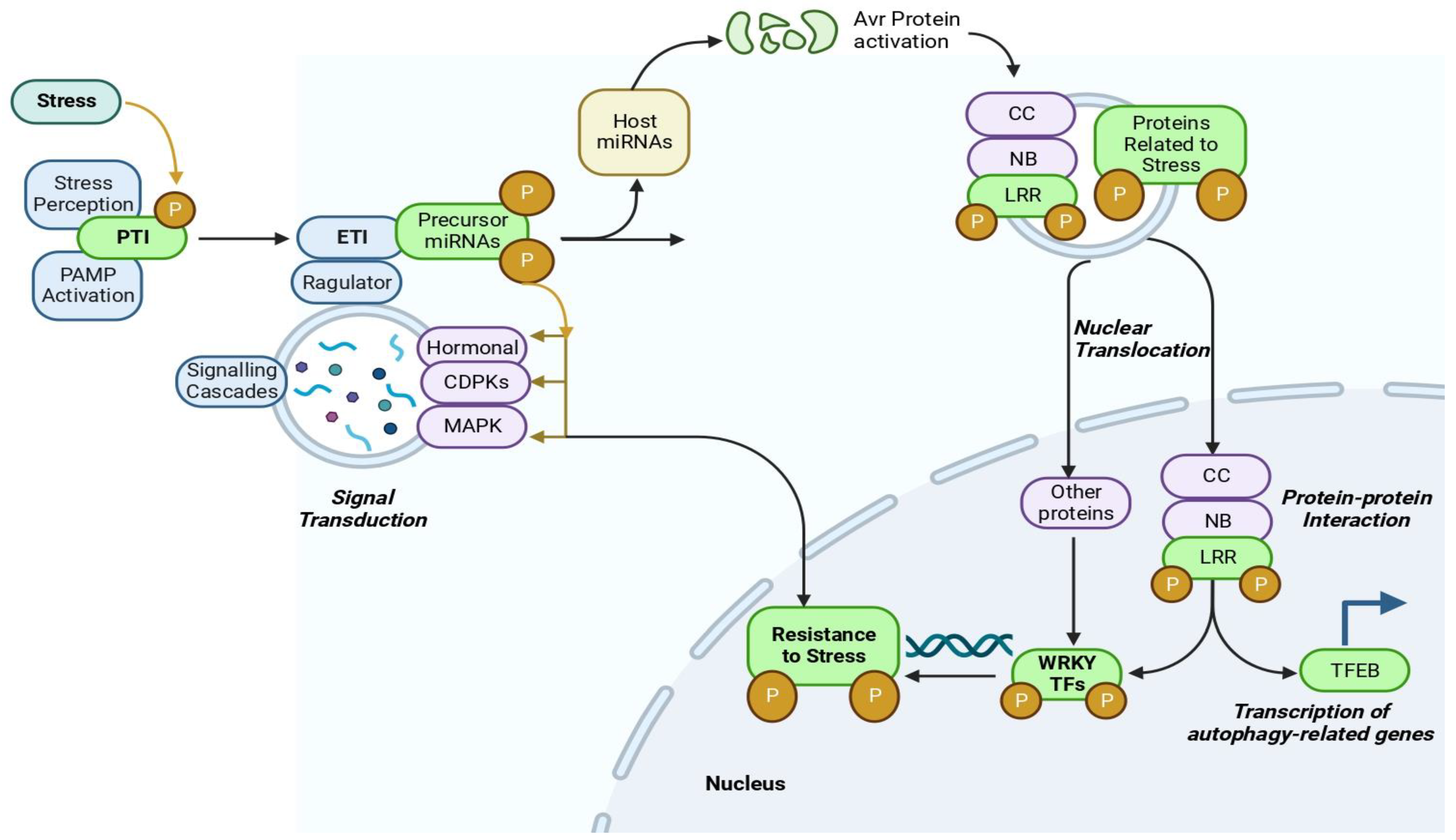
3.3. Signaling Mechanism
4. Molecular Mechanism and Signal Transduction Cascades under Combined Abiotic Stresses
5. Recent Advances in Biotechnological Approaches
6. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shabbir, R.; Javed, T.; Afzal, I.; Sabagh, A.E.; Ali, A.; Vicente, O.; Chen, P. Modern biotechnologies: Innovative and sustainable approaches for the improvement of sugarcane tolerance to environmental stresses. Agronomy 2021, 11, 1042. [Google Scholar] [CrossRef]
- Javed, T.; Shabbir, R.; Ali, A.; Afzal, I.; Zaheer, U.; Gao, S.J. Transcription factors in plant stress responses: Challenges and potential for sugarcane improvement. Plants 2020, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Pauk, J.; Kondic-Spika, A.; Grausgruber, H.; Allahverdiyev, T.; Sass, L.; Vass, I. Co-occurrence of mild salinity and drought synergistically enhances biomass and grain retardation in wheat. Front. Plant Sci. 2019, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, U.; Hussain, S.; Hussain, S.; Ali, B.; Ashraf, U.; Zamir, S.; Al-Robai, S.A.; Alzahrani, F.O.; Hano, C.; El-Esawi, M.A. Morpho-physio-biochemical and molecular responses of maize hybrids to salinity and waterlogging during stress and recovery phase. Plants 2021, 10, 1345. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Mehmood, U.; Ashraf, U.; Naseer, M.A. Combined salinity and waterlogging stress in plants: Limitations and tolerance mechanisms. In Climate Change and Crop Stress; Academic Press: Cambridge, MA, USA, 2022; pp. 95–112. [Google Scholar]
- Nasser, L.M.; Badu-Apraku, B.; Gracen, V.E.; Mafouasson, H.N. Combining ability of early-maturing yellow maize inbreds under combined drought and heat stress and well-watered environments. Agronomy 2020, 10, 1585. [Google Scholar] [CrossRef]
- Duan, H.; Ma, Y.; Liu, R.; Li, Q.; Yang, Y.; Song, J. Effect of combined waterlogging and salinity stresses on euhalophyte Suaeda glauca. Plant Physiol. Biochem. 2018, 127, 231–237. [Google Scholar] [CrossRef]
- Shahid, S.; Shahbaz, M.; Maqsood, M.F.; Farhat, F.; Zulfiqar, U.; Javed, T.; Fraz, A.M.; Alhomrani, M.; Alamri, A.S. Proline-Induced Modifications in Morpho-Physiological, Biochemical and Yield Attributes of Pea (Pisum sativum L.) Cultivars under Salt Stress. Sustainability 2022, 14, 13579. [Google Scholar] [CrossRef]
- Abobatta, W.F. Plant responses and tolerance to combined salt and drought stress. In Salt and Drought Stress Tolerance in Plants; Springer: Cham, Switzerland, 2020; pp. 17–52. [Google Scholar]
- Wichelns, D.; Qadir, M. Achieving sustainable irrigation requires effective management of salts, soil salinity, and shallow groundwater. Agric. Water Manag. 2015, 157, 31–38. [Google Scholar] [CrossRef]
- Lawas, L.M.F.; Zuther, E.; Jagadish, S.K.; Hincha, D.K. Molecular mechanisms of combined heat and drought stress resilience in cereals. Curr. Opin. Plant Biol. 2018, 45, 212–217. [Google Scholar] [CrossRef]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and drought stresses in crops and approaches for their mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef]
- Duhan, S.; Kumari, A.; Lal, M.; Sheokand, S. Oxidative stress and antioxidant defense under combined waterlogging and salinity stresses. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; John Wiley and Sons: Hoboken, NJ, USA, 2019; pp. 113–142. [Google Scholar]
- Haddadi, B.S.; Hassanpour, H.; Niknam, V. Effect of salinity and waterlogging on growth, anatomical and antioxidative responses in Mentha aquatica L. Acta Physiol. Plant. 2016, 38, 1–11. [Google Scholar] [CrossRef]
- Sekmen, A.H.; Ozgur, R.; Uzilday, B.; Turkan, I. Reactive oxygen species scavenging capacities of cotton (Gossypium hirsutum) cultivars under combined drought and heat induced oxidative stress. Environ. Exp. Bot. 2014, 99, 141–149. [Google Scholar] [CrossRef]
- Alam, I.; Sharmin, S.A.; Kim, K.H.; Kim, Y.G.; Lee, J.J.; Bahk, J.D.; Lee, B.H. Comparative proteomic approach to identify proteins involved in flooding combined with salinity stress in soybean. Plant Soil 2011, 346, 45–62. [Google Scholar] [CrossRef]
- Ahuja, I.; de Vos, R.C.; Bones, A.M.; Hall, R.D. Plant molecular stress responses face climate change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef]
- Hu, X.; Wu, L.; Zhao, F.; Zhang, D.; Li, N.; Zhu, G.; Li, C.; Wang, W. Phosphoproteomic analysis of the response of maize leaves to drought, heat and their combination stress. Front. Plant Sci. 2015, 6, 298. [Google Scholar] [CrossRef]
- Shahzad, K.; Hussain, S.; Arfan, M.; Hussain, S.; Waraich, E.A.; Zamir, S.; Saddique, M.; Rauf, A.; Kamal, K.Y.; Hano, C.; et al. Exogenously Applied Gibberellic Acid Enhances Growth and Salinity Stress Tolerance of Maize through Modulating the Morpho-Physiological, Biochemical and Molecular Attributes. Biomolecules 2021, 11, 1005. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef]
- Kul, R.; Ekinci, M.; Turan, M.; Ors, S.; Yildirim, E. How abiotic stress conditions affects plant roots. In Plant Roots; Book and Demand: Norderstedt, Germany, 2020. [Google Scholar]
- Yadav, S.; Modi, P.; Dave, A.; Vijapura, A.; Patel, D.; Patel, M. Effect of abiotic stress on crops. In Sustainable Crop Production; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Abdelraheem, A.; Esmaeili, N.; O’Connell, M.; Zhang, J. Progress and perspective on drought and salt stress tolerance in cotton. Ind. Crops Prod. 2019, 130, 118–129. [Google Scholar] [CrossRef]
- Javed, T.; Shabbir, R.; Hussain, S.; Naseer, M.A.; Ejaz, I.; Ali, M.M.; Ahmar, S.; Yousef, A.F. Nanotechnology for endorsing abiotic stresses: A review on the role of nanoparticles and nanocompositions. Func. Plant Biol. 2022. [Google Scholar] [CrossRef]
- Javaid, M.M.; Florentine, S.; Mahmood, A.; Wasaya, A.; Javed, T.; Sattar, A.; Sarwar, N.; Kalaji, H.M.; Ahmad, H.B.; Worbel, J.; et al. Interactive effect of elevated CO2 and drought on physiological traits of Datura stramonium. Front. Plant Sci. 2022, 13, 929378. [Google Scholar] [CrossRef]
- Islam, M.R.; Sarker, B.C.; Alam, M.A.; Javed, T.; Alam, M.J.; Zaman, M.S.U.; Azam, M.G.; Shabbir, R.; Raza, A.; Habib-ur-Rahman, M.; et al. Yield Stability and Genotype Environment Interaction of Water Deficit Stress Tolerant Mung Bean (Vigna radiata L. Wilczak) Genotypes of Bangladesh. Agronomy 2021, 11, 2136. [Google Scholar] [CrossRef]
- Singhal, R.K.; Fahad, S.; Kumar, P.; Choyal, P.; Javed, T.; Jinger, D.; Singh, P.; Saha, D.; Bose, B.; Akash, H.; et al. Beneficial elements: New Players in improving nutrient use efficiency and abiotic stress tolerance. Plant Growth Regul. 2022, 1–29. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wakeel, A. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Fghire, R.; Anaya, F.; Ali, O.I.; Benlhabib, O.; Ragab, R.; Wahbi, S. Physiological and photosynthetic response of quinoa to drought stress. Chil. J. Agric. Res. 2015, 75, 174–183. [Google Scholar] [CrossRef]
- Gull, A.; Lone, A.A.; Wani, N.I. Biotic and abiotic stresses in plants. In Abiotic and Biotic Stress in Plants; De Oliveira, A.B., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Bahar, A.A.; Faried, H.N.; Razzaq, K.; Ullah, S.; Akhtar, G.; Amin, M.; Bashir, M.; Ahmed, N.; Wattoo, F.M.; Ahmar, S.; et al. Potassium-induced drought tolerance of potato by improving morpho-physiological and biochemical attributes. Agronomy 2021, 11, 2573. [Google Scholar] [CrossRef]
- Hussain, S.; Khaliq, A.; Ali, B.; Hussain, H.A.; Qadir, T.; Hussain, S. Temperature extremes: Impact on rice growth and development. In Plant Abiotic Stress Tolerance; Springer: Cham, Switzerland, 2019; pp. 153–171. [Google Scholar]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van, B.F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Sardhara, K.; Mehta, K. Effects of Abiotic and Biotic Stress on the Plant. Acad. J. Bot. Sci. 2018, 1, 5–9. [Google Scholar]
- Goda, H.; Sasaki, E.; Akiyama, K.; Maruyama-Nakashita, A.; Nakabayashi, K.; Li, W.; Ogawa, M.; Yamauchi, Y.; Preston, J.; Aoki, K.; et al. The AtGenExpress hormone and chemical treatment data set: Experimental design, data evaluation, model data analysis and data access. Plant J. 2008, 55, 526–542. [Google Scholar] [CrossRef]
- Yoo, S.D.; Cho, Y.; Sheen, J. Emerging connections in the ethylene signaling network. Trends Plant Sci. 2009, 14, 270–279. [Google Scholar] [CrossRef]
- Kochhar, S.; Kochhar, V.K. Expression of antioxidant enzymes and heat shock proteins in relation to combined stress of cadmium and heat in Vigna mungo seedlings. Plant Sci. 2005, 168, 921–929. [Google Scholar] [CrossRef]
- Javed, T.; Afzal, I.; Shabbir, R.; Ikram, K.; Zaheer, M.S.; Faheem, M.; Ali, H.H.; Iqbal, J. Seed coating technology: An innovative and sustainable approach for improving seed quality and crop performance. J. Saudi Society Agri. Sci. 2022. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Q.; Xie, B.; Wang, Z.; Cui, J.; Hu, T. Effects of drought and salt stress on seed germination of three leguminous species. Afr. J. Biotechnol. 2011, 10, 17954–17961. [Google Scholar]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Mestre, T.C.; Mittler RO, N.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Zhou, G.S. Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta 2006, 224, 1080–1090. [Google Scholar] [CrossRef]
- Islam, F.; Ali, B.; Wang, J.; Farooq, M.A.; Gill, R.A.; Ali, S.; Wang, D.; Zhou, W. Combined herbicide and saline stress differentially modulates hormonal regulation and antioxidant defense system in Oryza sativa cultivars. Plant Physiol. Biochem. 2016, 107, 82–95. [Google Scholar] [CrossRef]
- Dobra, J.; Motyka, V.; Dobrev, P.; Malbeck, J.; Prasil, I.T.; Haisel, D.; Gaudinova, A.; Havlova, M.; Gubis, J.; Vankova, R. Comparison of hormonal responses to heat, drought and combined stress in tobacco plants with elevated proline content. J. Plant Physiol. 2010, 167, 1360–1370. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.-O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar] [CrossRef]
- Li, X.; Lawas, L.M.F.; Malo, R.; Glaubitz, U.; Erban, A.; Mauleon, R.; Heuer, S.; Zuther, E.; Kopka, J.; Hincha, D.K.; et al. Metabolic and transcriptomic signatures of rice floral organs reveal sugar starvation as a factor in reproductive failure under heat and drought stress. Plant Cell Environ. 2015, 38, 2171–2192. [Google Scholar] [CrossRef]
- Rang, Z.W.; Jagadish, S.V.K.; Zhou, Q.M.; Craufurd, P.Q.; Heuer, S. Effect of high temperature and water stress on pollen germination and spikelet fertility in rice. Environ. Exp. Bot. 2011, 70, 58–65. [Google Scholar] [CrossRef]
- Sales, C.R.; Ribeiro, R.V.; Silveira, J.A.; Machado, E.C.; Martins, M.O.; Lagôa, A.M.M. Superoxide dismutase and ascorbate peroxidase improve the recovery of photosynthesis in sugarcane plants subjected to water deficit and low substrate temperature. Plant Physiol. Biochem. 2013, 73, 326–336. [Google Scholar] [CrossRef]
- Michael, P.I.; Krishnaswamy, M. The effect of zinc stress combined with high irradiance stress on membrane damage and antioxidative response in bean seedlings. Environ. Exp. Bot. 2011, 74, 171–177. [Google Scholar] [CrossRef]
- Baier, M.; Kandlbinder, A.; Golldack, D.; Dietz, K. Oxidative stress and ozone: Perception, signalling and response. Plant Cell Environ. 2005, 28, 1012–1020. [Google Scholar] [CrossRef]
- Hilker, M.; Schmülling, T. Stress priming, memory, and signalling in plants. Plant Cell Environ. 2019, 42, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, Z.H.; Liu, X.; Shabala, L.; Yu, M.; Zhou, M.; Salih, A.; Shabala, S. The loss of RBOHD function modulates root adaptive responses to combined hypoxia and salinity stress in Arabidopsis. Environ. Exp. Bot. 2019, 158, 125–135. [Google Scholar] [CrossRef]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef]
- Suzuki, N.; Bassil, E.; Hamilton, J.S.; Inupakutika, M.A.; Zandalinas, S.I.; Tripathy, D.; Luo, Y.; Dion, E.; Fukui, G.; Kumazaki, A.; et al. ABA is required for plant acclimation to a combination of salt and heat stress. PLoS ONE 2016, 11, e0147625. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Han, M.M.; Zhang, S.Y.; Ren, J.; Hu, F.; Wang, X. MAPKs as a cross point in H2O2 and auxin signaling under combined cadmium and zinc stress in rice roots. Russ. J. Plant Physiol. 2014, 61, 608–618. [Google Scholar] [CrossRef]
- Teige, M.; Scheikl, E.; Eulgem, T.; Dóczi, R.; Ichimura, K.; Shinozaki, K.; Dangl, J.L.; Hirt, H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol. Cell 2004, 15, 141–152. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Xu, B.; Bose, J.; Kaur, S.; Conn, V.; Domingos, P.; Ullah, S.; Wege, S.; Shabala, S.; et al. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 2015, 6, 7879. [Google Scholar] [CrossRef]
- Shabbir, R.; Javed, T.; Hussain, S.; Ahmar, S.; Naz, M.; Zafar, H.; Pandey, S.; Chauhan, J.; Siddiqui, M.H.; Pinghua, C. Calcium homeostasis and potential roles to combat environmental stresses in plants. South Afr. J. Bot. 2022, 148, 683–693. [Google Scholar] [CrossRef]
- Imam, J.; Mandal, N.P.; Variar, M.; Shukla, P. Advances in molecular mechanism toward understanding plantmicrobe interaction: A study of M. oryzae versus rice. In Frontier Discoveries and Innovations in Interdisciplinary Microbiology; Shukla, P., Ed.; Springer: New Delhi, India, 2016; pp. 79–96. [Google Scholar]
- Zhai, N.; Jia, H.; Liu, D.; Liu, S.; Ma, M.; Guo, X.; Li, H. GhMAP3K65, a cotton Raf-like MAP3K gene, enhances susceptibility to pathogen infection and heat stress by negatively modulating growth and development in transgenic Nicotiana benthamiana. Int. J. Mol. Sci. 2017, 18, 2462. [Google Scholar] [CrossRef]
- Nath, M.; Bhatt, D.; Prasad, R.; Tuteja, N. Reactive oxygen species (ros) metabolism and signaling in plant-mycorrhizal association under biotic and abiotic stress conditions. In Mycorrhiza—Eco-Physiology, Secondary Metabolites, Nanomaterials; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Gupta, A.; Senthil-Kumar, M. Concurrent stresses are perceived as new state of stress by the plants: Overview of impact of abiotic and biotic stress combinations. In Plant Tolerance to Individual and Concurrent Stresses; Senthil-Kumar, M., Ed.; Springer: New Delhi, India, 2017; pp. 1–15. [Google Scholar]
- Rejeb, I.B.; Pastor, V.; Mauch-Mani, B. Plant responses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef]
- Choudhary, P.; Mushtaq, M.; Singh, A.K.; Mukhtar, S.; Shah, A.A.; Mehta, G.; Bakshi, P. Genome Editing Using Crispr/Cas System: New Era Genetic Technology in Agriculture to Boost Crop Output. Euro. J. Exp. Biol. 2017, 7. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef]
- McGrann, G.R.; Steed, A.; Burt, C.; Goddard, R.; Lachaux, C.; Bansal, A.; Corbitt, M.; Gorniak, K.; Nicholson, P.; Brown, J.K. Contribution of the drought tolerance related stress-responsive NAC1 transcription factor to resistance of barley to Ramularia leaf spot. Mol. Plant Pathol. 2015, 16, 201–209. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, S.; Ma, X.; Wang, Y.; Kong, F.; Meng, Q. A stress-associated NAC transcription factor (SlNAC35) from tomato plays a positive role in biotic and abiotic stresses. Physiol. Plant. 2016, 158, 45–64. [Google Scholar] [CrossRef]
- Phukan, U.J.; Jeena, G.S.; Shukla, R.K. WRKY transcription factors: Molecular regulation and stress responses in plants. Front. Plant Sci. 2016, 7, 760. [Google Scholar] [CrossRef]
- Dangi, A.K.; Sharma, B.; Khangwal, I.; Shukla, P. Combinatorial interactions of biotic and abiotic stresses in plants and their molecular mechanisms: Systems biology approach. Mol. Biotechnol. 2018, 60, 636–650. [Google Scholar] [CrossRef]
- Lopes-Caitar, V.S.; Silva SM, H.; Marcelino-Guimaraes, F.C. Plant small heat shock proteins and its interactions with biotic stress. In Heat Shock Proteins and Plants; Asea, A., Kaur, P., Calderwood, S., Eds.; Springer: Cham, Switzerland, 2016; Volume 10, pp. 19–39. [Google Scholar]
- Taxak, P.C.; Khanna, S.M.; Bharadwaj, C.; Kishor, G.; Sukhdeep, K.; Meenu, C.; Gitanjali, T.; Sarika, J.; Iquebal, M.A.; Anil, R.; et al. Transcriptomic signature of Fusarium toxin in chickpea unveiling wilt pathogenicity pathways and marker discovery. PMPP Physiol. Mol. Plant Pathol. 2017, 100, 163–177. [Google Scholar] [CrossRef]
- Tran, N.P.; Park, J.K.; Lee, C.G. Proteomics analysis of proteins in green alga Haematococcus lacustris (Chlorophyceae) expressed under combined stress of nitrogen starvation and high irradiance. Enzym. Microb. Technol. 2009, 45, 241–246. [Google Scholar] [CrossRef]
- Rampino, P.; Mita, G.; Fasano, P.; Borrelli, G.M.; Aprile, A.; Dalessandro, G.; De Bellis, L.; Perrotta, C. Novel durum wheat genes up-regulated in response to a combination of heat and drought stress. Plant Physiol. Biochem. 2012, 56, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhang, D.; Zhao, Y.; Wang, W.; Yang, H.; Tai, F.; Li, C.; Hu, X. The difference of physiological and proteomic changes in maize leaves adaptation to drought, heat, and combined both stresses. Front. Plant Sci. 2016, 7, 1471. [Google Scholar] [CrossRef] [PubMed]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Priya, M.; Dhanker, O.P.; Siddique, K.H.M.; HanumanthaRao, B.; Nair, R.M.; Pandey, S.; Singh, S.; Varshney, R.K.; Prasad, P.V.V.; Nayyar, H. Drought and heat stress-related proteins: An update about their functional relevance in imparting stress tolerance in agricultural crops. Theor. Appl. Genet. 2019, 132, 1607–1638. [Google Scholar] [CrossRef]
- Yuan, Y.; Cairns, J.E.; Babu, R.; Gowda, M.; Makumbi, D.; Magorokosho, C.; Zhang, A.; Liu, Y.; Wang, N.; Hao, Z.; et al. Genome-wide association mapping and genomic prediction analyses reveal the genetic architecture of grain yield and flowering time under drought and heat stress conditions in maize. Front. Plant Sci. 2019, 9, 1919. [Google Scholar] [CrossRef]
- Templer, S.E.; Ammon, A.; Pscheidt, D.; Ciobotea, O.; Schuy, C.; McCollum, C.; Sonnewald, U.; Hanemann, A.; Forster, J.; Ordon, F.; et al. Metabolite profiling of barley flag leaves under drought and combined heat and drought stress reveals metabolic QTLs for metabolites associated with antioxidant defense. J. Exp. Bot. 2017, 68, 1697–1713. [Google Scholar] [CrossRef]
- Bohra, A.; Chand Jha, U.; Godwin, I.D.; Kumar Varshney, R. Genomic interventions for sustainable agriculture. Plant Biotechnol. J. 2020, 18, 2388–2405. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, B. Comparative transcriptomic analysis reveals common molecular factors responsive to heat and drought stress in Agrostis stolonifera. Sci. Rep. 2018, 8, 15181. [Google Scholar] [CrossRef]
- Obata, T.; Witt, S.; Lisec, J.; Palacios-Rojas, N.; Florez-Sarasa, I.; Yousfi, S.; Araus, J.L.; Cairns, J.E.; Fernie, A.R. Metabolite profiles of maize leaves in drought, heat, and combined stress field trials reveal the relationship between metabolism and grain yield. Plant Physiol. 2015, 169, 2665–2683. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Q.; Chen, K.; Zhao, S.; Zhang, C.; Pan, R.; Zhuang, W. Integrated microRNA and transcriptome profiling reveals a miRNA-mediated regulatory network of embryo abortion under calcium deficiency in peanut (Arachis hypogaea L.). BMC Genom. 2019, 20, 1–17. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Integrated analysis of small RNA, transcriptome, and degradome sequencing reveals the water-deficit and heat stress response network in durum wheat. Int. J. Mol. Sci. 2020, 21, 6017. [Google Scholar] [CrossRef]
- Katam, R.; Shokri, S.; Murthy, N.; Singh, S.K.; Suravajhala, P.; Khan, M.N.; Bahmani, M.; Sakata, K.; Reddy, K.R. Proteomics, physiological, and biochemical analysis of cross tolerance mechanisms in response to heat and water stresses in soybean. PLoS ONE 2020, 15, e0233905. [Google Scholar] [CrossRef]
- Dugasa, M.T.; Cao, F.; Ibrahim, W.; Wu, F. Differences in physiological and biochemical characteristics in response to single and combined drought and salinity stresses between wheat genotypes differing in salt tolerance. Physiol. Plant. 2019, 165, 134–143. [Google Scholar] [CrossRef]
- Li, P.-C.; Yang, X.-Y.; Wang, H.-M.; Pan, T.; Yang, J.-Y.; Wang, Y.-Y.; Xu, Y.; Yang, Z.-F.; Xu, C.-W. Metabolic responses to combined water deficit and salt stress in maize primary roots. J. Integr. Agric. 2021, 20, 109–119. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef]
- Prasad PV, V.; Pisipati, S.R.; Momčilović, I.; Ristic, Z. Independent and combined effects of high temperature and drought stress during grain filling on plant yield and chloroplast EF-Tu expression in spring wheat. J. Agron. Crop Sci. 2011, 197, 430–441. [Google Scholar] [CrossRef]
- Vile, D.; Pervent, M.; Belluau, M.; Vasseur, F.; Bresson, J.; Muller, B.; Granier, C.; Simonneau, T. Arabidopsis growth under prolonged high temperature and water deficit: Independent or interactive effects? Plant Cell Environ. 2012, 35, 702–718. [Google Scholar] [CrossRef]
- Jiang, L.; Yoshida, T.; Stiegert, S.; Jing, Y.; Alseekh, S.; Lenhard, M.; Pérez-Alfocea, F.; Fernie, A.R. Multi-omics approach reveals the contribution of KLU to leaf longevity and drought tolerance. Plant Physiol. 2021, 185, 352–368. [Google Scholar] [CrossRef]
- Giraud, E.; Ho, L.H.; Clifton, R.; Carroll, A.; Estavillo, G.; Tan, Y.-F.; Howell, K.; Ivanova, A.; Pogson, B.; Millar, A.H.; et al. The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol. 2008, 147, 595–610. [Google Scholar] [CrossRef]
- Mahmoud, D.; Pandey, R.; Sathee, L.; Dalal, M.; Singh, M.P.; Chinnusamy, V. Regulation of expression of genes associated with nitrate response by osmotic stress and combined osmotic and nitrogen deficiency stress in bread wheat (Triticum aestivum L.). Plant Physiol. Rep. 2020, 25, 200–215. [Google Scholar] [CrossRef]
- Meena, S.K.; Pandey, R.; Sharma, S.; Kumar, T.; Singh, M.P.; Dikshit, H.K. Physiological basis of combined stress tolerance to low phosphorus and drought in a diverse set of mungbean germplasm. Agronomy 2021, 11, 99. [Google Scholar] [CrossRef]
- Brouder, S.M.; Volenec, J.J. Impact of climate change on crop nutrient and water use efficiencies. Physiol. Plant. 2008, 133, 705–724. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, C.; Lu, Q.; Wen, X.; Lu, C. The combined effect of salt stress and heat shock on proteome profiling in Suaeda salsa. J. Plant Physiol. 2011, 168, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ballesta MD, C.; Bastías, E.; Zhu, C.; Schäffner, A.R.; González-Moro, B.; González-Murua, C.; Carvajal, M. Boric acid and salinity effects on maize roots. Response of aquaporins ZmPIP1 and ZmPIP2, and plasma membrane H+-ATPase, in relation to water and nutrient uptake. Physiol. Plant. 2008, 132, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Quiñones Martorello, A.S.; Fernandez, M.E.; Monterubbianesi, M.G.; Colabelli, M.N.; Laclau, P.; Gyenge, J.E. Effect of combined stress (salinity+ hypoxia) and auxin rooting hormone addition on morphology and growth traits in six Salix spp. clones. New For. 2020, 51, 61–80. [Google Scholar] [CrossRef]
- Kanani, H.; Dutta, B.; Klapa, M.I. Individual vs. combinatorial effect of elevated CO2 conditions and salinity stress on Arabidopsis thaliana liquid cultures: Comparing the early molecular response using time-series transcriptomic and metabolomic analyses. BMC Systems Biol. 2010, 4, 177. [Google Scholar] [CrossRef]
- Kaplan, F.; Zhao, W.; Richards, J.T.; Wheeler, R.M.; Guy, C.L.; Levine, L.H. Transcriptional and metabolic insights into the differential physiological responses of Arabidopsis to optimal and supraoptimal atmospheric CO2. PLoS ONE 2012, 7, e43583. [Google Scholar] [CrossRef]
- Yu, J.; Li, R.; Fan, N.; Yang, Z.; Huang, B. Metabolic pathways involved in carbon dioxide enhanced heat tolerance in Bermudagrass. Front. Plant Sci. 2017, 8, 1506. [Google Scholar] [CrossRef]
- Pérez-López, U.; Miranda-Apodaca, J.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. Growth and nutritional quality improvement in two differently pigmented lettuce cultivars grown under elevated CO2 and/or salinity. Sci. Hortic. 2015, 195, 56–66. [Google Scholar] [CrossRef]
- Conesa, A.; Madrigal, P.; Tarazona, S.; Gomez-Cabrero, D.; Cervera, A.; McPherson, A.; Szcześniak, M.W.; Gaffney, D.J.; Elo, L.L.; Zhang, X.; et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016, 17, 13. [Google Scholar] [CrossRef]
- Zargar, S.M.; Mahajan, R.; Nazir, M.; Nagar, P.; Kim, S.T.; Rai, V.; Masi, A.; Ahmad, S.M.; Shah, R.A.; Ganai, N.A.; et al. Common bean proteomics: Present status and future strategies. J. Proteom. 2017, 169, 239–248. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.; Yan, C.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Ionomic and metabolic responses to neutral salt or alkaline salt stresses in maize (Zea mays L.) seedlings. BMC Plant Biol. 2017, 17, 41. [Google Scholar] [CrossRef]
- Qiu, G.Y.; Omasa, K.; Sase, S. An infrared-based coefficient to screen plant environmental stress: Concept, test and applications. Funct. Plant Biol. 2009, 36, 990–997. [Google Scholar] [CrossRef]
- Wedeking, R.; Mahlein, A.K.; Steiner, U.; Oerke, E.C.; Goldbach, H.E.; Wimmer, M.A. Osmotic adjustment of young sugar beets (Beta vulgaris) under progressive drought stress and subsequent rewatering assessed by metabolite analysis and infrared thermography. Funct. Plant Biol. 2016, 44, 119–133. [Google Scholar] [CrossRef]
- Altangerel, N.; Ariunbold, G.O.; Gorman, C.; Alkahtani, M.H.; Borrego, E.J.; Bohlmeyer, D.; Scully, M.O. In vivo diagnostics of early abiotic plant stress response via Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2017, 114, 3393–3396. [Google Scholar] [CrossRef]
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 2020, 175, 104040. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Papakyriakou, E.; Petropoulos, S.A.; Tzortzakis, N. The combined and single effect of salinity and copper stress on growth and quality of Mentha spicata plants. J. Hazard. Mater. 2019, 368, 584–593. [Google Scholar] [CrossRef]
- Weber, M.B.; Schat, H.; Bookum-Van Der Maarel, T. The effect of copper toxicity on the contents of nitrogen compounds in Silene vulgaris (Moench) Garcke. Plant Soil 1991, 133, 101–109. [Google Scholar] [CrossRef]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop Improvement by exploiting physio-morphological traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef]
- Vaughan, M.M.; Block, A.; Christensen, S.A.; Allen, L.H.; Schmelz, E.A. The effects of climate change associated abiotic stresses on maize phytochemical defenses. Phytochem. Rev. 2018, 17, 37–49. [Google Scholar] [CrossRef]
- Bhat, J.A.; Ali, S.; Salgotra, R.K.; Mir, Z.A.; Dutta, S.; Jadon, V.; Tyagi, A.; Mushtaq, M.; Jain, N.; Singh, P.K.; et al. Genomic selection in the era of next generation sequencing for complex traits in plant breeding. Front. Genet. 2016, 7, 221. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, P.; Dave, W.; Robert, J.; Bensen, D.C.; Anstrom, J.H.; Stoecker, M.; Abad, M.; Kumar, G.; Salvador, S.; DÓrdine, R.; et al. Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in maize under water-limited conditions. Plant Physio. 2008, 147, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Nemudryi, A.A.; Valetdinova, K.R.; Medvedev, S.P.; Zakian, S.M. Talen and CRISPR/Cas genome editing systems: Tools of discovery. Acta Nat. 2014, 6, 19–40. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K. Genome editing with engineered nucleases in plants. Plant Cell Physiol. 2014, 56, 389–400. [Google Scholar] [CrossRef]
- Matres, J.M.; Hilscher, J.; Datta, A.; Armario-Nájera, V.; Baysal, C.; He, W.; Huang, X.; Zhu, C.; Valizadeh-Kamran, R.; Trijatmiko, K.R.; et al. Genome editing in cereal crops: An overview. Transgenic. Res. 2021, 30, 461–498. [Google Scholar] [CrossRef]
- Mushtaq, M.; Ahmad Dar, A.; Skalicky, M.; Tyagi, A.; Bhagat, N.; Basu, U.; Bhat, B.A.; Zaid, A.; Ali, S.; Dar, T.U.H.; et al. CRISPR-based genome editing tools: Insights into technological breakthroughs and future challenges. Genes 2021, 12, 797. [Google Scholar] [CrossRef]
- Ceasar, S.A.; Rajan, V.; Prykhozhij, S.V.; Berman, J.N.; Ignacimuthu, S. Insert, remove or replace: A highly advanced genome editing system using CRISPR/Cas9. BBA-Mol. Cell Res. 2016, 1863, 2333–2344. [Google Scholar] [CrossRef]
- Bortesi, L.; Zhu, C.; Zischewski, J.; Perez, L.; Bassié, L.; Nadi, R.; Forni, G.; Lade, S.B.; Soto, E.; Jin, X.; et al. Patterns of CRISPR/Cas9 activity in plants, animals and microbes. Plant Biotechnol. J. 2016, 14, 2203–2216. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K. Genome editing to improve abiotic stress responses in plants. Progress Mol. Biol. Trans. Sci. 2017, 149, 99–109. [Google Scholar]
- Hyun, T.K. CRISPR/Cas-based genome editing to improve abiotic stress tolerance in plants. Bot. Serbica 2020, 44, 121–127. [Google Scholar] [CrossRef]
- Shi, J.; Gao, H.; Wang, H.; Lafitte, H.R.; Archibald, R.L.; Yang, M.; Hakimi, S.M.; Mo, H.; Habben, J.E. ARGOS 8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol. J. 2017, 15, 207–216. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, N.; Zhou, G.; Hussain, S.; Ahmed, S.; Tian, H.; Wang, S. Knockout of the entire family of AITR genes in Arabidopsis leads to enhanced drought and salinity tolerance without fitness costs. BMC Plant Biol. 2021, 21, 137. [Google Scholar] [CrossRef]
- Osakabe, Y.; Watanabe, T.; Sugano, S.S.; Ueta, R.; Ishihara, R.; Shinozaki, K.; Osakabe, K. Optimization of CRISPR/Cas9 genome editing to modify abiotic stress responses in plants. Sci. Rep. 2016, 6, 26685. [Google Scholar] [CrossRef]
- Roca Paixão, J.F.; Gillet, F.X.; Ribeiro, T.P.; Bournaud, C.; Lourenço-Tessutti, I.T.; Noriega, D.D.; Melo BP de Almeida-Engler, J.; Grossi-de-Sa, M.F. Improved drought stress tolerance in Arabidopsis by CRISPR/dCas9 fusion with a histone acetyltransferase. Sci. Rep. 2019, 9, 8080. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, X.; Li, J.; Zhao, D. Construction and analysis of tify1a and tify1b mutants in rice (Oryza sativa) based on CRISPR/Cas9 technology. J. Agric. Biotech. 2017, 25, 1003–1012. [Google Scholar]
- Zeng, Y.; Wen, J.; Zhao, W.; Wang, Q.; Huang, W. Rational improvement of rice yield and cold tolerance by editing the three genes OsPIN5b, GS3, and OsMYB30 with the CRISPR-Cas9 System. Front. Plant Sci. 2020, 10, 1663. [Google Scholar] [CrossRef]
- Lou, D.; Wang, H.; Liang, G.; Yu, D. OsSAPK2 confers abscisic acid sensitivity and tolerance to drought stress in rice. Front. Plant Sci. 2017, 8, 993. [Google Scholar] [CrossRef]
- Farhat, S.; Jain, N.; Singh, N.; Sreevathsa, R.; Dash, P.K.; Rai, R.; Yadav, S.; Kumar, P.; Sarkar, A.K.; Jain, A.; et al. CRISPR-Cas9 directed genome engineering for enhancing salt stress tolerance in rice. Semin. Cell Dev. Biol. 2019, 96, 91–99. [Google Scholar] [CrossRef]
- Yin, Y.; Qin, K.; Song, X.; Zhang, Q.; Zhou, Y.; Xia, X.; Yu, J. BZR1 transcription factor regulates heat stress tolerance through FERONIA receptor-like kinase-mediated reactive oxygen species signaling in tomato. Plant Cell Physiol. 2018, 59, 2239–2254. [Google Scholar] [CrossRef]
- Tang, X.; Lowder, L.G.; Zhang, T.; Malzahn, A.A.; Zheng, X.; Voytas, D.F.; Zhong, Z.; Chen, Y.; Ren, Q.; Li, Q.; et al. A CRISPR-Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat. Plants 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, Y.; Du, J.; Zhao, Y.; Xia, L. Generation of targeted point mutations in rice by a modified CRISPR/Cas9 system. Mol. Plant. 2017, 10, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhu, J.K. Precise editing of a target base in the rice genome using a modified CRISPR/Cas9 system. Mol. Plant. 2017, 10, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Wei, P.; Zhang, B.; Gou, F.; Feng, Z.; Mao, Y.; Yang, L.; Zhang, H.; Xu, N.; et al. The CRISPR/C as9 system produces specific and homozygous targeted gene editing in rice in one generation. Plant Biotechnol. J. 2014, 12, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.F.; Li, H.; Qin, R.Y.; Li, J.; Qiu, C.H.; Yang, Y.C.; Ma, H.; Li, L.; Wei, P.C.; Yang, J.B. Generation of inheritable and “transgene clean” targeted genome-modified rice in later generations using the CRISPR/Cas9 system. Sci. Rep. 2015, 5, 11491. [Google Scholar] [CrossRef]
- Nawaz GHan, Y.; Usman, B.; Liu, F.; Qin, B.; Li, R. Knockout of OsPRP1, a gene encoding proline-rich protein, confers enhanced cold sensitivity in rice (Oryza sativa L.) at the seedling stage. 3 Biotech. 2019, 9, 254. [Google Scholar] [CrossRef]
- Shen, C.; Que, Z.; Lu, Q.; Liu, T.; Li, S.; Zou, J.; Chen, G. The Rice Annexin Gene OsAnn5 Is a Positive Regulator of Cold Stress Tolerance at the Seedling Stage; Research Square: Durham, NC, USA, 2020. [Google Scholar]
- Ogata, T.; Ishizaki, T.; Fujita, M.; Fujita, Y. CRISPR/Cas9-targeted mutagenesis of OsERA1 confers enhanced responses to abscisic acid and drought stress and increased primary root growth under nonstressed conditions in rice. PLoS ONE 2020, 15, e0243376. [Google Scholar] [CrossRef]
- Liao, S.; Qin, X.; Luo, L.; Han, Y.; Wang, X.; Usman, B.; Nawaz, G.; Zhao, N.; Liu, Y.; Li, R. CRISPR/Cas9-induced mutagenesis of Semi-Rolled Leaf 1,2 confers curled leaf phenotype and drought tolerance by influencing protein expression patterns and ROS scavenging in rice (Oryza sativa L.). Agronomy 2019, 9, 728. [Google Scholar] [CrossRef]
- Wang, T.; Xun, H.; Wang, W.; Ding, X.; Tian, H.; Hussain, S.; Dong, Q.; Li, Y.; Cheng, Y.; Wang, C.; et al. Mutation of GmAITR genes by CRISPR/Cas9 genome editing results in enhanced salinity stress tolerance in soybean. Front. Plant Sci. 2021, 12, 779598. [Google Scholar] [CrossRef]
- Kumar, S.V.V.; Verma, R.K.; Yadav, S.K.; Yadav, P.; Watts, A.; Rao MVChinnusamy, V. CRISPR-Cas9 mediated genome editing of drought and salt tolerance (OsDST) gene in indica mega rice cultivar MTU1010. Physiol. Mol. Biol. Plants 2020, 26, 1099–1110. [Google Scholar] [CrossRef]
- Kim, D.; Alptekin, B.; Budak, H. CRISPR/Cas9 genome editing in wheat. Funct. Integr. Genom. 2018, 18, 31–41. [Google Scholar] [CrossRef]
- Bertier, L.D.; Ron, M.; Huo, H.; Bradford, K.J.; Britt, A.B.; Michelmore, R.W. High-resolution analysis of the efficiency, heritability, and editing outcomes of CRISPR/Cas9- induced modifications of NCED4 in lettuce (Lactuca sativa). G3: Genes Genomes Genet. 2018, 8, 1513–1521. [Google Scholar] [CrossRef]
- Tran, M.T.; Doan, D.T.H.; Kim, J.; Song, Y.J.; Sung, Y.W.; Das, S.; Kim, E.J.; Son, G.H.; Kim, S.H.; Van Vu, T.; et al. CRISPR/Cas9- based precise excision of SlHyPRP1 domain(s) to obtain salt stress-tolerant tomato. Plant Cell Rep. 2021, 40, 999–1011. [Google Scholar] [CrossRef]
- Bouzroud, S.; Gasparini, K.; Hu, G.; Barbosa, M.A.M.; Rosa, B.L.; Fahr, M.; Bendaou, N.; Bouzayen, M.; Zsögön, A.; Smouni, A.; et al. Down regulation and loss of Auxin Response Factor 4 function using CRISPR/Cas9 alters plant growth, stomatal function and improves tomato tolerance to salinity and osmotic stress. Genes 2020, 11, 272. [Google Scholar] [CrossRef]
- Makhotenko, A.V.; Khromov, A.V.; Snigir, E.A.; Makarova, S.S.; Makarov, V.V.; Suprunova, T.P.; Kalinina, N.O.; Taliansky, M.E. Functional analysis of coilin in virus resistance and stress tolerance of potato Solanum tuberosum using CRISPR-Cas9 editing. Dokl. Biochem. Biophys. 2019, 484, 88–91. [Google Scholar] [CrossRef]
- Javed, T.; Zhou, J.R.; Li, J.; Hu, Z.T.; Wang, Q.N.; Gao, S.J. Identification and expression profiling of WRKY family genes in sugarcane in response to bacterial pathogen infection and nitrogen implantation dosage. Front. Plant Sci. 2022, 13, 917953. [Google Scholar] [CrossRef]
- Chowdhury, M.K.; Hasan, M.A.; Bahadur, M.M.; Islam, M.R.; Hakim, M.A.; Iqbal, M.A.; Javed, T.; Raza, A.; Shabbir, R.; Sorour, S.; et al. Evaluation of drought tolerance of some wheat (Triticum aestivum L.) genotypes through phenology, growth, and physiological indices. Agronomy 2021, 11, 1792. [Google Scholar] [CrossRef]
- Javed, T.; Ali, M.M.; Shabbir, R.; Anwar, R.; Afzal, I.; Mauro, R.P. Alleviation of copper-induced stress in pea (Pisum sativum L.) through foliar application of gibberellic acid. Biology 2021, 10, 120. [Google Scholar] [CrossRef]
- Mahmood, T.; Wang, X.; Ahmar, S.; Abdullah, M.; Iqbal, M.S.; Rana, R.M.; Yasir, M.; Khalid, S.; Javed, T.; Mora-Poblete, F.; et al. Genetic potential and inheritance pattern of phenological growth and drought tolerance in cotton (Gossypium hirsutum L.). Front. Plan Sci. 2021, 12, 705392. [Google Scholar] [CrossRef]
- Afzal, I.; Imran, S.; Javed, T.; Basra, S.M.A. Evaluating the integrative response of moringa leaf extract with synthetic growth promoting substances in maize under early spring conditions. South Afr. J. Bot. 2020, 132, 378–387. [Google Scholar] [CrossRef]
- Abbas, A.; Shah, A.N.; Shah, A.A.; Nadeem, M.A.; Alsaleh, A.; Javed, T.; Alotaibi, S.S.; Abdelsalam, N.R. Genome-Wide Analysis of Invertase Gene Family, and Expression Profiling under Abiotic Stress Conditions in Potato. Biology 2022, 11, 539. [Google Scholar] [CrossRef] [PubMed]
- Javad, S.; Shah, A.A.; Ramzan, M.; Sardar, R.; Javed, T.; Al-Huqail, A.A.; Ali, H.M.; Chaudhry, O.; Yasin, N.A.; Ahmed, S.; et al. Hydrogen sulphide alleviates cadmium stress in Trigonella foenum-graecum by modulating antioxidant enzymes and polyamine content. Plant Biol. 2022, 24, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Aslam, Z.; Hussain, S.; Javed, T.; Hussain, S.; Bashir, S.; Hussain, I.; Belliturk, K.; Adamski, R.; Siuta, D.; et al. Soil Application of Wheat Straw Vermicompost Enhances Morpho-Physiological Attributes and Antioxidant Defense in Wheat Under Drought Stress. Front. Environ. Sci. 2022, 387. [Google Scholar] [CrossRef]
- Ahmad, A.; Aslam, Z.; Hussain, S.; Javed, T.; Hussain, S.; Bashir, S.; Alotaibi, S.; Kalaji, H.M.; Telesiński, A. Rice straw vermicompost enriched with cellulolytic microbes ameliorate the negative effect of drought in wheat through modulating the morpho-physiological attributes. Front. Environ. Sci. 2022, 497. [Google Scholar] [CrossRef]
- Balbaa, M.G.; Osman, H.T.; Kandil, E.E.; Javed, T.; Lamlom, S.F.; Ali, H.M.; Kalaji, H.M.; Wróbel, J.; Telesiñski, A.; Brysiewicz, A.; et al. Determination of morpho-physiological and yield traits of maize inbred lines (Zea mays L.) under optimal and drought stress conditions. Front. Plant Sci. 2022, 13, 959203. [Google Scholar] [CrossRef]
- Bukhari, M.A.; Yousaf, M.; Ahmad, Z.; Rafay, M.; Shah, A.N.; Abbas, A.; Shah, A.A.; Javed, T.; Afzal, M.; Ali, S.; et al. Enhancing drought stress tolerance in Camelina (Camelina sativa L.) through exogenous application of potassium. Physiol. Plant. 2022, 174, e13779. [Google Scholar] [CrossRef]
- Faiz, S.; Shah, A.A.; Naveed, N.H.; Nijabat, A.; Yasin, N.A.; Batool, A.I.; Ali, H.M.; Javed, T.; Simon, P.W.; Ali, A. Synergistic application of silver nanoparticles and indole acetic acid alleviate cadmium induced stress and improve growth of Daucus carota L. Chemosphere 2022, 290, 133200. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; García-Caparrós, P.; Rahman, M.A.; Ogwugwa, V.H.; Saeed, F.; Jin, W. Melatonin-mediated temperature stress tolerance in plants. GM Crops Food 2022, 13, 196–217. [Google Scholar] [CrossRef]

| Sr. No. | Combined Stress Occurrences | Observed Plant Tolerance Strategy | Reference |
|---|---|---|---|
| 1 | Drought + Salinity |
| [85,86] |
| 2 | Drought + Heat |
| [87,88,89,90] |
| 3 | Drought + Cold |
| [48,91] |
| 4 | Drought + Light |
| |
| 5 | Drought + Nutrient |
| [92,93] |
| 6 | Drought + ↑ CO2 |
| [94] |
| 7 | Salt + Heat |
| [41,95] |
| 8 | Salt + Nutrient |
| [96] |
| 9 | Salt + high CO2 or absence of O2 |
| [97] |
| 10 | Heat + CO2 |
| [98,99,100] |
| 11 | Light + CO2 |
| [101] |
| Crop | Targeted Gene | Phenotype/Tolerance | Reference |
|---|---|---|---|
| Arabidopsis | AtAITRs | Drought and salt tolerance | [124] |
| AtOST2 | Drought tolerance | [125] | |
| AREB1 | Drought tolerance | [126] | |
| Rice | TIFY1a, TIFY1b | Cold tolerance | [127,128,129,130] |
| MYB30 | Cold tolerance | ||
| SAPK2 | Drought tolerance | ||
| OsRR22 | Salt tolerance | ||
| OsEPFL9 | Stomata density regulation | [131] | |
| OsNRAMP5 | Minimise the cadmium content | [132] | |
| OsPDS, OsSBEIIb | Nutrient improvement | [133] | |
| NRT1.1B | Enhance nitrogen use efficiency | [134] | |
| OsEPSPS OsDERF1, OsPMS3 OsMSH1, OsMYB5 | Drought tolerance | [135] | |
| OsAOX1a OsAOX1b, OsAOX1c OsBEL | Tolerance against various abiotic stress | [136] | |
| ABI4, GL1 OST2 | Role in stomata opening | [125] | |
| OsPRP1 | Cold sensitive | [137] | |
| OsAnn5 | Cold tolerance | [138] | |
| OsERA1 | Drought tolerance | [139] | |
| OsSRL1,2 | Drought tolerance | [140] | |
| OsVDE | Salinity tolerance | [141] | |
| OsDST | Salinity tolerance | [142] | |
| Maize | ARGOS8 | Drought tolerance and enhance yield | [123] |
| Wheat | TaDREB2, TaERF3 | Drought resistance | [143] |
| Lettuce | NCED4 | Heat tolerance | [144] |
| Tomato | SlMAPK3 | Heat tolerance | |
| SlBZR1 | Heat tolerance | [131] | |
| SlLBD40 | Drought tolerance | [83] | |
| SlHyPRP1 | Salinity tolerance | [145] | |
| SlARF4 | Salinity tolerance | [146] | |
| Potato | Coilin | Salinity tolerance | [147] |
| Sugarcane | WRKY49 | Nitrogen stress | [148] |
| Soybean | GmAITR | Salinity tolerance | [141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabbir, R.; Singhal, R.K.; Mishra, U.N.; Chauhan, J.; Javed, T.; Hussain, S.; Kumar, S.; Anuragi, H.; Lal, D.; Chen, P. Combined Abiotic Stresses: Challenges and Potential for Crop Improvement. Agronomy 2022, 12, 2795. https://doi.org/10.3390/agronomy12112795
Shabbir R, Singhal RK, Mishra UN, Chauhan J, Javed T, Hussain S, Kumar S, Anuragi H, Lal D, Chen P. Combined Abiotic Stresses: Challenges and Potential for Crop Improvement. Agronomy. 2022; 12(11):2795. https://doi.org/10.3390/agronomy12112795
Chicago/Turabian StyleShabbir, Rubab, Rajesh Kumar Singhal, Udit Nandan Mishra, Jyoti Chauhan, Talha Javed, Sadam Hussain, Sachin Kumar, Hirdayesh Anuragi, Dalpat Lal, and Pinghua Chen. 2022. "Combined Abiotic Stresses: Challenges and Potential for Crop Improvement" Agronomy 12, no. 11: 2795. https://doi.org/10.3390/agronomy12112795
APA StyleShabbir, R., Singhal, R. K., Mishra, U. N., Chauhan, J., Javed, T., Hussain, S., Kumar, S., Anuragi, H., Lal, D., & Chen, P. (2022). Combined Abiotic Stresses: Challenges and Potential for Crop Improvement. Agronomy, 12(11), 2795. https://doi.org/10.3390/agronomy12112795







