Abstract
Volatile organic compounds (VOCs) play an important role in plant ecology and can be useful in pest management. This work characterises, for the first time, the VOC emissions of six industrial hemp (Cannabis sativa L.) cultivars grown in New Zealand: CFX-2, CRS-1, Ferimon 12, Katani, Futura 75, and Finola. Volatiles emitted from flowers and foliage of eight-week-old plants were collected using a dynamic headspace sampling method and analysed using gas chromatography coupled to mass spectrometry. We assessed the effect of cultivar, sex (monoecious, male, and female), and site (i.e., two sites differing in soil types, maintained under irrigation and rain-fed conditions) on VOC emissions. Thirty-five volatile compounds were tentatively identified from the headspace samples of hemp plants, but none of the cultivars emitted all 35 compounds. β-Myrcene was the most abundant compound in most cultivars. Overall, there was a significant effect of sex, and the interaction of sex and cultivar on the volatile profiles, but no effect of site. Female plants typically emitted more volatiles than their male counterparts and monoecious cultivars. The main compounds driving the difference between cultivars and sexes were (Z)- and (E)-β-ocimene. We hypothesize that differences in emission emerged as a defence strategy to protect costly female flowers from herbivores (since C. sativa is wind pollinated), but this hypothesis needs further testing. We recommend additional studies exploring how biotic and abiotic factors influence hemp VOC emissions, changes in VOCs throughout the crop cycle, the role of VOCs in plant-insect interactions and their use in pest management.
1. Introduction
All plants release secondary metabolites in the form of volatile organic compounds (VOCs) into the environment. These compounds mediate ecological interactions between plants and other organisms, e.g., attracting pollinators, acting as cues for foraging herbivores and their natural enemies, or warning neighbouring plants of potential herbivore attack [,,]. Volatile compounds are emitted from all plant organs (leaves, inflorescences, roots, etc.) [], and their production and release are highly plastic, responding to biotic and abiotic factors allowing for considerable variation in the quantity and composition of VOC blends within and among plant taxa []. Understanding the VOC emissions of a crop and the factors affecting them can be useful for its management, e.g., to select cultivars that are less attractive to herbivores, enhance the attraction of biological control agents, or promote priming (i.e., enhanced defence state) [,].
Cannabis sativa L. produces a wide array of secondary metabolites. This plant is best-known for its production of cannabinoids such as tetrahydrocannabinol (THC), which has psychoactive properties, but it is also a prolific producer of VOCs. About 200 scent compounds have been reported to make up the plant’s complex aroma []. Some compounds that are typically found in high concentrations in Cannabis spp. plants include β-myrcene, limonene, α-pinene, β-pinene, and terpineol [,,,,]. The individual compounds and relative concentrations contributing to the plant’s scent are becoming increasingly diverse as different cultivars emerge and continue to be bred for different purposes. For example, one drug-type cannabis (marijuana) cultivar has elevated levels of terpinolene and δ-limonene, giving the plant a woody citrus aroma, while one hybrid C. sativa × C. indica cultivar has a floral citrus aroma due to high amounts of linalool and limonene [,,,,].
C. sativa is an annual plant originating from central Asia that has been bred and cultivated for thousands of years as a valuable source of food, fibre and medicine, for cultural rituals and ceremonies, and for recreational use []. Industrial hemp (hereafter hemp) comprises a wide range of C. sativa cultivars, typically with low THC content, bred for numerous markets, including textiles, construction composite, nutrition and functional foods, cosmetics, nutraceuticals, and medicine []. The growing, processing and trading of seeds and stalks to produce foods and fibre from hemp in New Zealand has been legal under licence since 2006 with the Misuse of Drugs Act Amendment regulations of 2006 and 2018 [].
The Ministry of Health has approved 20 hemp cultivars for cultivation in New Zealand, including five New Zealand-bred varieties and 15 offshore-bred cultivars []. However, growing hemp in New Zealand is not without its challenges and, as for most new crops, there is limited genetics knowledge and a relatively low level of plant biology and agronomic understanding. Information on the imported varieties including their adaptation to local climatic conditions, yield potential, seed and fibre quality characteristics, and biological and ecological aspects must be investigated to inform agronomic practices, breeding programmes and financial decisions.
Massey University initiated a research project in 2019 to address these gaps, particularly in the lower North Island of New Zealand, which is a potential region for hemp production, using six offshore cultivars bred for seed and fibre use (Table 1). This study contributes to this research and aims to characterize the volatile profiles of the six hemp- cultivars and to explore the effect of cultivar, sex (monoecious, male, and female), and site (i.e., two sites differing in soil types, one maintained under supplementary irrigation and another under rain-fed conditions) on VOC emissions. This information is relevant to the exploration of other potential uses of these hemp varieties (e.g., in food and fragrance) and could be implemented in pest management practices.

Table 1.
Description of the cultivars in which volatile emissions were investigated.
2. Materials and Methods
2.1. Plant Material
For this study, we used six hemp cultivars: CFX-2, CRS-1, Futura 75, Katani, Finola, and Ferimon 12. The selected cultivars have different breeding origins, plant reproduction types, and end uses (Table 1). Four of these cultivars are dioecious (develop male and female flowers on separate plants) and two cultivars are monoecious (male and female flowers develop on the same plant).
2.2. Study Site and Trial Design
We measured the above-ground volatile emissions of six eight-week-old hemp cultivars at two different outdoor plantation sites at Massey University, Palmerston North, New Zealand: site one at the Plant Growth Unit (PGU; 40°22′41.0″ S 175°36′37.1″ E) and site two at the Pasture and Crop Research Unit (PCRU; 40°23′11.2″ S 175°36′27.4″ E) (Figure 1). Each site followed a randomized complete block design, which contained four replicates. Each replicate consisted of six plots with six cultivars planted individually in each plot (i.e., cultivars did not mix and had their own allocated space). The experimental design and field layout were similar for both sites, with different randomization of the entries. The total plantation area for both sites was 41 m × 15 m with each cultivar grown in 6 m × 3 m plots with 15 cm of constant distance between the rows.

Figure 1.
Eight-week-old hemp plants used for volatile collection: (a) Female hemp plants; (b) Male hemp plants.
The soil types at both sites differed in their properties. The PCRU site contains Tokomaru silt loam, which is characterized by a silt profile with low root penetration. There is limited aeration in the root zone, and it is poorly drained, making it more prone to flooding during rainfall. The PGU site contains Manawatu silt loam, which is characterized by a loam profile with no significant root barrier within the first metre of soil. The soil is imperfectly drained with moderately limited aeration in the root zone. These characteristics were collated from the latest Manaaki Whenua (Landcare Research) Soil Report (2022) []. Soil moisture was maintained at the PGU site via sprinkler irrigation, while the PCRU site was not irrigated, but was rainfed. Weekly weather data (maximum and minimum temperature, rainfall, and relative humidity) from sowing until data collection, was obtained from a climate station from the National Institute of Water and Atmospheric Research (NIWA) located between the two sites (Table S1). Due to their proximity, weather data is assumed to be the same for both sites.
2.3. Volatile Collection and Analysis
Volatile compounds were collected from eight-week-old plants in February 2021 using a dynamic headspace sampling method []. For the dioecious cultivars, the emissions of male and female plants were measured separately. For each sample, a plant was randomly selected from each plot and the most apical portion of the stem containing the flowers and upper leaves was bagged using heat-resistant oven bags (GLAD 500 mm × 500 mm, ~20 L capacity) with both ends fastened. A portable PVAS22 pump (Volatile Assay Systems, Rensselaer, NY, USA) was used to simultaneously push (1.0 L/min) and pull (0.9 L/min) carbon-filtered air through the enclosed system using PTFE tubes (Figure 2). In the ‘pull’ tube, a collection filter containing 30 mg HayeSep Q adsorbent was inserted to collect the volatiles. Volatile compounds were collected for two hours per plant under similar weather conditions (dry and sunny) between the 8th and 12th of February 2021. After sampling, the plant material enclosed in the bags was removed and dried at 60 °C for 96 h and used to calculate the volatiles emitted per gram of dry weight (ng g DW−1 h−1).

Figure 2.
The volatile collection procedure: (a) Portable PVAS22 pump; (b) plant material bagged and fastened to create an enclosed system for volatile collection.
Collection filters were eluted using 200 µL of 95% hexane with 10 ng/µL nonyl acetate (C11H22O2) as an internal standard. Samples were stored at −80 °C. These samples were analysed using gas chromatography coupled to mass spectrometry (GC-MS QP2010S Shimadzu technologies) with a 30 m × 250 µm × 0.25 µm TG-5MS capillary column. Helium was the carrier gas, which was supplied at: 53.5 kPa pressure, 3.0 mL/min flow rate, 14.0 mL/min total flow, and 36.3 cm/s linear velocity. Operating conditions were as follows: injector temperature 250 °C; split ratio of 10; initial oven temperature was 50 °C held for 3 min, then increased to 200 °C at a rate of 9 °C/min. Tentative identification of compounds was achieved by comparing compounds of interest to target spectra in the mass spectra library from the National Institute of Standards and Technology (NIST).
2.4. Data Analysis
Statistical analyses were completed using IBM SPSS statistics version 28.0.1.1 [] and RStudio, Version 2022.02.2 []. For all analyses, a small constant (0.001) was used to replace zero values (compounds not detected in a sample). A general linear model (GLM) was used to explore the effect of the cultivar, sex, and site on the total VOC emissions, and pairwise comparisons were conducted to identify differences between treatments. Since no site effect was observed in the GLM, the data from both sites were pooled (for VOC emissions by site please refer to Table S2 and Figure S1 in Supplementary Material).
Following the results of the GLM, a principal component analysis (PCA) was performed using the identified compounds for all sex × cultivar interactions. For individual compounds having a high contribution to the separation along principal components, emissions were compared using either ANOVA or a Kruskal–Wallis test. We also conducted a PCA to explore differences in emission by sex for male and female plants of dioecious cultivars and followed up with a t-test or Mann–Whitney test for the main compounds contributing to the separation.
3. Results
Thirty-five compounds were tentatively identified in the headspace collections of hemp plants, which were further assigned to their respective chemical classes. Most of the compounds were monoterpenes (19), followed by sesquiterpenes and sesquiterpenoids (10), and green leaf volatiles (GLVs, 2). The remaining four compounds were grouped as ‘other’. No cultivar emitted all thirty-five compounds; however, all cultivars emitted (Z)-3-hexen-1-ol acetate, α-pinene, β-pinene, β-myrcene, limonene, (Z)-β-ocimene, γ-caryophyllene and humulene. β-Myrcene was the most abundant compound in all cultivars, except for Futura 75 (where it was the second most abundant). No males emitted the compounds α-phellandrene, 2-carene, and 3-carene; these compounds were only emitted by females and one monoecious cultivar, Ferimon 12. In addition, the compound L-β-pinene was unique to females. No compounds were unique to males of the dioecious cultivars or to the monoecious cultivars (Table 2).

Table 2.
Identification and quantification of compounds present (ng gDW−1 h−1) in the headspace samples of six hemp cultivars. Data are presented as mean ± SEM (n = 80). GLV = Green leaf volatiles, M = Monoterpene, S = Sesquiterpene/sesquiterpenoid, O = other, hyphen (-) = not detected. To view the individual compounds as a percentage of the total emission values, please visit Table S5 in the Supplementary Material.
3.1. General Linear Model
A general linear model was used to explore the effect of cultivar, sex, and site on VOC emissions. The model revealed a significant effect of sex (F = 34.7, p < 0.001), and the interaction between sex and cultivar (F = 2.86, p < 0.05), but not of the other variables, either alone or in interaction. Follow-up pairwise comparisons between all possible sex × cultivar interactions revealed that all monoecious and male plants from all cultivars were not significantly different from one another but were significantly different (p < 0.05) from the CRS-1, Katani and Finola females (Figure 3). The CFX-2 females were not significantly different from male and monoecious cultivars (Figure 3).
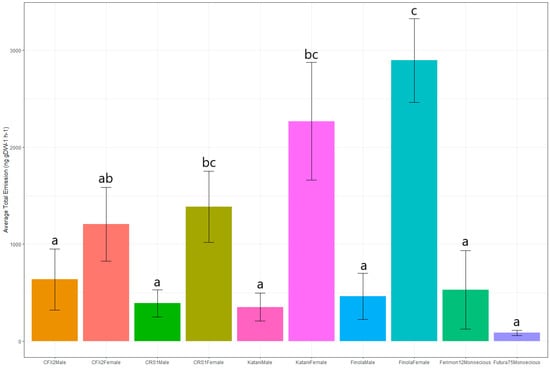
Figure 3.
Total VOC emissions (ng g DW−1 h−1) from six New Zealand grown hemp cultivars (four dioecious and two monoecious). Datasets for dioecious cultivars are separated by sex (male or female). Data are presented as mean ± SEM (n = 80). Different letters indicate a significant difference in emissions between the cultivar × sex interactions.
3.1.1. Principal Component Analysis for Sex × Cultivar Interactions
A principal component analysis (PCA) was used to further explore the differences in hemp VOC emissions between the sex × cultivar interactions. The PCA revealed a high overlap between the volatile profiles; however, the females of the Finola and Katani cultivars showed slight separation from the other sex × cultivar interactions along the first axis (Dim 1) (Figure 4). The first axis of the PCA (Dim1) explained 25.5.% of the total variance in emissions among the six hemp cultivars. This axis was mostly characterized by (Z)- β-ocimene and (E)-β-ocimene (ZbOci and EbOci in Figure 4).
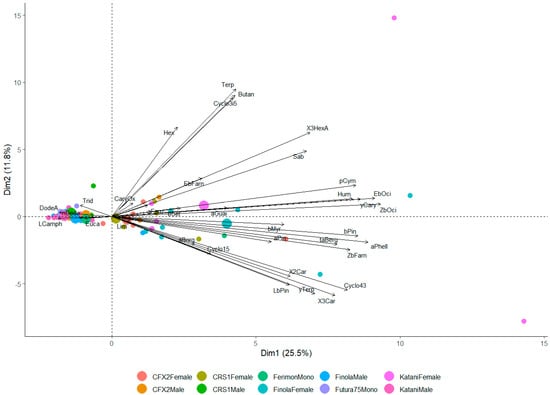
Figure 4.
Principal component analysis (PCA) biplot showing scores of individual plants for each cultivar × sex interaction. The PCA was based on the 35 compounds tentatively identified from six hemp cultivars; small circles correspond to individual samples and larger circles correspond to the treatment average.
(Z)-β-Ocimene and (E)-β-ocimene emissions, were further compared between sex × cultivar interactions. There was a significant difference in the emission of both (Z)-β-ocimene (χ2 = 40.17, p < 0.001) and (E)-β-ocimene (χ2 = 28.88, p < 0.001). Follow up pair-wise comparisons of (Z)-β-ocimene revealed that the Futura 75 monoecious cultivar emitted significantly lower quantities of (Z)-β-ocimene when compared to female Finola plants (Z = 5.05, p < 0.001), female Katani plants (Z = 3.71, p < 0.001), and female CFX-2 plants (Z = 3.27, p = 0.04). Additionally, female Finola plants emitted significantly higher amounts of this compound when compared to male CRS-1 plants (Z = 3.94, p = 0.003), male Katani plants (Z = 3.71, p = 0.009), and the monoecious Ferimon 12 (Z = 3.46, p = 0.02). Pair-wise comparisons for (E)-β-ocimene revealed that the Futura 75 monoecious cultivar emitted significantly lower quantities of this compound when compared to female Finola (Z = 4.40, p < 0.001) and female Katani (Z = 3.59, p = 0.01).
3.1.2. Principal Component Analysis for Sex (Male vs. Female)
A PCA was used to explore differences in hemp volatile emissions between male and female plants for the dioecious cultivars only. Male and female plants were clearly separated based on volatile emissions along the first PCA axis (Dim1, Figure 5). The first axis of the PCA explained 25.2% of the total variance in emissions among the six hemp cultivars. As in the previous analysis, the main compounds contributing to the separation were (Z)-β-ocimene and (E)-β-ocimene (ZbOci and EbOci on Figure 5). Female plants emitted higher amounts of both (Z)-β-ocimene (W = 868, p < 0.001) and (E)-β-ocimene (W = 777, p < 0.001) than their male counterparts.
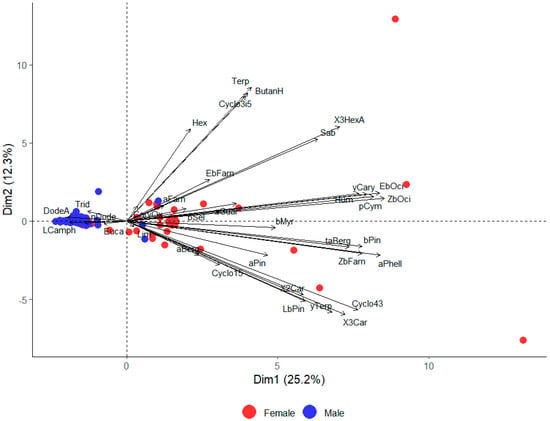
Figure 5.
Principal component analysis (PCA) biplot showing scores of individual plants from different sexes (only for male and female plants of dioecious cultivars). The PCA was based on 35 compounds tentatively identified from the four dioecicous hemp cultivars (CFX-2, CRS-1, Katani, and Finola). Small circles correspond to individual samples and larger circles correspond to the treatment average.
There was a significant difference in volatile emissions between male and female plants for all compound groups. Females emitted more monoterpenes (W = 982, p = 0.03), sesquiterpenes (W = 1053, p = 0.005), and GLVs (W = 1170, p < 0.001). However, males emitted a higher quantity of the ‘other’ compounds (W = 507, p = 0.002). When the compounds comprising this group (dodecane, tridecane, dodecanoic acid, and butyric acid) were analysed individually, it was found that, on average, dodecane was emitted in higher amounts by male than female plants (W = 629, p = 0.01), contributing to this group difference (Table 2).
4. Discussion
4.1. Key VOCs in the Hemp Headspace and Their Potential Use for Pest Management
This study presents the first analysis of VOCs emitted from hemp cultivars grown in New Zealand. In this study, we tentatively identified and quantified thirty-five volatile organic compounds from the headspace of hemp plants, with most of these compounds being monoterpenes and sesquiterpenes. Common compounds to all tested cultivars include β-myrcene, γ-caryophyllene, α-pinene, β-pinene and limonene, reflecting species-specificity in the emission of these compounds []. This is consistent with previous studies, where these compounds were also identified in the leaf [] and fresh bud samples [] of C. sativa (marijuana type) plants.
β-Myrcene was the most abundant compound in most cultivars. Myrcene is a plant-produced monoterpene, released by multiple species such as bay leaf, juniper, lemongrass, and thyme. It has multiple ecological roles and industrial applications, with antibiotic and insect repellent properties, and is used for flavours, fragrances, and polymer production []. Therefore, high myrcene-producing plants may provide improved defence against pathogens and herbivores; and could be used to produce additional products beyond fibre and seeds.
This study showed that male plants from dioecious cultivars and monoecious cultivars emit lower amounts of volatiles and do not differ significantly in their volatile emissions. In contrast, female plants from dioecious cultivars typically emit significantly more volatiles than their male counterparts or monoecious plants (excluding CFX-2). The main compounds contributing to this separation were (Z)-β-ocimene and (E)-β-ocimene, which were emitted in higher amounts by female plants of dioecious cultivars. In contrast, in the monoecious Futura 75 cultivar, (Z)-β-ocimene was emitted in extremely low quantities, while (E)-β-ocimene was not detected in the GC-MS analysis (Table 2).
β-Ocimene is a ubiquitous monoterpene volatile compound with multiple biological roles. It is thought to be relevant in pollinator attraction and to play a defensive role by being toxic to herbivores or attracting natural enemies when emitted by vegetative tissue upon herbivore damage []. For instance, an electrophysiological study of the parasitoid wasp Cotesia sesamiae (Hymenoptera: Braconidae), showed sensitivity to quantitative changes in several minor compounds collected from a headspace sample of African forage grass (Brachiaria brizantha) exposed to an ovipositing pest species, including (E)-β-ocimene []. An electroantennography and behavioural study showed that the braconid parasitoid Glyptapanteles liparidis can detect this compound and is attracted to black poplar (Populus nigra) plants that emit it following herbivore damage by its host Lymantria dispar []. (Z)-β-Ocimene is a major constituent of the essential oil of clove basil (Ocimum gratissimum) and has been shown to have a toxicity effect on five insect pests of stored products []. These examples suggest a direct and indirect role of β-ocimene isomers in plant defence.
Plant derived volatile compounds play an important role in insect host finding and acceptance []. Therefore, identifying key compounds or blends influencing the behaviour of insects could assist in the development of pest protection strategies, either by selecting cultivars that are less attractive or deterrent to pest species or those that are more attractive to their natural enemies (predators or parasitoids) [,]. Given the abundance of myrcene and the importance of ocimene in characterising male vs. female hemp cultivars, these compounds are good candidates to assess their effect on the behaviour of insects associated with hemp in New Zealand, using similar approaches as those in other systems e.g., [,,,].
4.2. Differences in VOC Emission between Sexes
We found that female hemp plants produce more volatiles than their male counterparts (and monoecious cultivars); this is likely due to the presence of stalked glandular trichomes in the female flowers. These trichomes produce resin that contains cannabinoids and other volatile and non-volatile secondary metabolites []. Trichomes are typically absent from male flowers and leaves, and their abundance and content can be influenced by genetics and the environment []; therefore, it is not surprising to find differences in volatile emission among female plants of different cultivars (especially in hemp plants that are selected for their low cannabinoid content).
Sexual dimorphism of floral and inflorescence traits is common among many dioecious plant groups. For example, a review of thirty-three sexually dimorphic species found that the scent composition between sexes differed for all species and that male plants typically emitted more volatiles than females []. However, in this study, females emitted more volatiles than males, which may be a point of interest and could be explored further.
Sexual differences in plant scents have the potential to alter the preference and behaviour of some insect species. For instance, male and female flowers across several Phyllantheae plant tribes had major qualitative differences in their volatile blends, with their pollinators (Epicephala, Lepidoptera) showing a preference for the males over the females []. This was shown again by Waelti et al. [], who investigated the floral signals of Silene latifolia (white champion) and the behaviour of their moth pollinators, Hadena bicruris, to these signals. It was found that although males produced smaller flowers, they produced more in number and emitted significantly more scent compounds than female flowers. It was also found that the naïve pollinating moths showed a higher preference for male flowers, although this was only true for male moths.
Since C. sativa plants do not rely on insects for pollination, differences in male and female volatile profiles may reflect their evolutionary history with other insects such as herbivores. In this scenario, higher emissions could be a defensive strategy to repel/deter herbivores or recruit their natural enemies to protect costly female flowers. Monoecious plants may invest less in volatile production, due to the cost associated with the production of both male and female reproductive organs (which is supported by their delayed flowering time when compared to other cultivars; see Table S3).
A pioneer study on box elder maple (Acer negundo) suggested that female plants are chemically better defended than males []. A meta-analysis by Cornelissen and Stiling [] strengthens this claim by revealing that male plants are often visited by higher numbers of herbivores and suffer more damage as a result of herbivory than female plants. Therefore, it is thought that dimorphism in the form of chemical defences can affect herbivory rates between sexes. Since monoterpenes and sesquiterpenes make up many of the compounds released after tissue damage from herbivores [], it is possible that they play a defensive role in C. sativa plants, especially in hemp cultivars, where other defensive metabolites such as THC are present in lower amounts. However, further studies must be conducted to explore this theory.
4.3. Factors Influencing VOC Emission
The VOC emissions of plants can be influenced by environmental conditions. For example, temperature, UV-radiation, and soil nutrients have been shown to affect the VOC emissions of different plant species (native and introduced) occurring in New Zealand [,,,]. Soil, in particular, is a key factor driving volatile compounds’ emissions in different systems because soil nutrients and soil water availability influence plant metabolism, and because soil structure (e.g., porosity) and associated biota can impact nutrient cycling, bioavailability, and root penetrability, among other factors [].
In our system, we compared two sites with different soil types (differing in porosity, water logging and root penetrability), which could influence water moisture levels and nutrient uptake by plants. Soil moisture data recorded during the season confirmed the differences of soil characteristics regarding water retention and water logging between the two sites (Table S4). However, our results did not indicate differences in volatile emission between sites (Table S2 and Figure S1), perhaps due to similar climatic conditions (Table S1). However, considering the current and potential geographic variations in hemp cultivation in New Zealand, further studies comparing hemp volatiles under different environmental conditions and studies using controlled settings can help elucidate the impact of single and combined environmental factors on the plants’ chemistry and ecology. Studies exploring the influence of biotic agents on volatile emissions (e.g., herbivores and other plant visitors, rhizosphere and phyllosphere microorganisms) are also encouraged.
There are 14 additional hemp cultivars (not included in this study) approved by the Ministry of Health [], as well as different agronomic practices that could be used to produce hemp (e.g., hydroponics under greenhouse conditions). Extending this study to include the remaining cultivars would strengthen our knowledge of hemp volatile emissions under New Zealand conditions. This could include the incorporation of other analyses, such as the influence of biotic and abiotic factors, and different cultivation methods.
The data collected in this study only captured a snapshot of volatile emissions at one stage of the plant’s developmental cycle during flowering (eight weeks after sowing). Collecting volatile emissions at different sampling points over the cultivation cycle could provide more insight into the temporal or phenological variations in hemp volatiles, such as differences between vegetative and flowering stages of growth. This information could be useful to explore plant-insect interactions throughout the crop cycle as in Proffit et al. [].
5. Conclusions
This study characterized the VOC profiles of six hemp cultivars, and explored the influence of cultivar, sex, and site on VOC emissions. We found that all cultivars are prolific emitters of terpenoids. Abundant compounds such as β-myrcene could have important applications in pest management and the production of other products beyond seeds and fibre (e.g., fragrances and flavours). Our results show a marked difference in the volatile emissions between female plants and their male counterparts or monoecious cultivars, with female plants emitting more VOCs. We hypothesize that this is a defensive mechanism to protect costly female flowers from herbivores, mediated by compounds such as (E)- and (Z)-β-ocimene, but this hypothesis remains to be tested. There was no difference between the two hemp plantation sites used in this study, probably due to similar climatic conditions; however, biotic and abiotic factors are known to influence VOC emissions, and their effect on hemp plants must be explored further under diverse climatic conditions. We recommend additional research including other cultivars and exploring VOCs at different stages of the crop cycle, alongside studies on the role of hemp VOCs on plant–insect interactions to inform pest management practices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12112651/s1, Figure S1: Principal component analysis (PCA) biplot showing scores of individual plants grown at two different sites; Table S1: Weather data from sowing to end of data collection obtained from a climate station located between the two sites as reported by The National Climate Database from the National Institute of Water and table Atmospheric Research (NIWA); Table S2: Identification and quantification of compounds present (ng g DW−1 h−1) in the headspace samples between the two sampled sites; Table S3: Flowering date (beginning of flowering) of the six hemp cultivars at both sites; Table S4: Field capacity measured in both sites from sowing to end of data collection; Table S5: Percentage contribution of different volatile compounds to the volatile blend of the six hemp cultivars.
Author Contributions
Conceptualization and methodology, F.K., S.K., A.C.M. and S.S.-B.; fieldwork, F.K. and S.K.; data analysis F.K. and A.C.M.; F.K. led the writing of the manuscript. S.K., A.C.M. and S.S.-B. were involved in editing and providing comments.; supervision, A.C.M. and S.S.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Massey University Research Fund grant number “1000022961”.
Data Availability Statement
Data will be made available upon request.
Acknowledgments
We wish to thank the Plant Horticultural Unit at Massey University for access to the hemp plantations for data collection. We are also thankful to Evans Effah and Mari Nakano for their assistance during fieldwork and GC-MS analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Effah, E.; Holopainen, J.K.; McCormick, A.C. Potential roles of volatile organic compounds in plant competition. Perspect. Plant Ecol. Evol. Syst. 2019, 38, 58–63. [Google Scholar] [CrossRef]
- Clavijo McCormick, A.; Unsicker, S.B.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Effah, E.; Barrett, D.P.; Peterson, P.G.; Potter, M.A.; Holopainen, J.K.; Clavijo McCormick, A. Seasonal and environmental variation in volatile emissions of the New Zealand native plant Leptospermum scoparium in weed-invaded and non-invaded sites. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Sanchez-Moreiras, A.M.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193, 997–1008. [Google Scholar] [CrossRef]
- Shrivastava, G.; Rogers, M.; Wszelaki, A.; Panthee, D.R.; Chen, F. Plant volatiles-based insect pest management in organic farming. Crit. Rev. Plant Sci. 2010, 29, 123–133. [Google Scholar] [CrossRef]
- War, A.R.; Sharma, H.C.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Herbivore induced plant volatiles: Their role in plant defense for pest management. Plant Signal. Behav. 2011, 6, 1973–1978. [Google Scholar] [CrossRef]
- Oswald, I.W.; Ojeda, M.A.; Pobanz, R.J.; Koby, K.A.; Buchanan, A.J.; Del Rosso, J.; Guzman, M.A.; Martin, T.J. Identification of a new family of prenylated volatile sulfur compounds in cannabis revealed by comprehensive two-dimensional gas chromatography. ACS Omega 2021, 6, 31667–31676. [Google Scholar] [CrossRef]
- Hood, L.; Dames, M.; Barry, G. Headspace volatiles of marijuana. Nature 1973, 242, 402–403. [Google Scholar] [CrossRef]
- Rice, S.; Koziel, J.A. Characterizing the smell of marijuana by odor impact of volatile compounds: An application of simultaneous chemical and sensory analysis. PLoS ONE 2015, 10, e0144160. [Google Scholar] [CrossRef]
- Samburova, V.; McDaniel, M.; Campbell, D.; Wolf, M.; Stockwell, W.R.; Khlystov, A. Dominant volatile organic compounds (VOCs) measured at four cannabis growing facilities: Pilot study results. J. Air Waste Manag. Assoc. 2019, 69, 1267–1276. [Google Scholar] [CrossRef]
- Wang, C.-T.; Wiedinmyer, C.; Ashworth, K.; Harley, P.C.; Ortega, J.; Vizuete, W. Leaf enclosure measurements for determining volatile organic compound emission capacity from Cannabis spp. Atmos. Environ. 2019, 199, 80–87. [Google Scholar] [CrossRef]
- Tang, C.-H.; Ten, Z.; Wang, X.-S.; Yang, X.-Q. Physicochemical and functional properties of hemp (Cannabis sativa L.) protein isolate. J. Agric. Food Chem. 2006, 54, 8945–8950. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Misuse of Drugs. (Industrial Hemp) Amendment Regulations 2018. 2018. Available online: https://www.legislation.govt.nz/regulation/public/2018/0217/latest/LMS117577.html (accessed on 29 August 2022).
- NZ Ministry of Health. Hemp (Industrial Hemp). 2006. Available online: www.health.govt.nz (accessed on 29 August 2022).
- Horizons Regional Council. Manaaki Whenua (Landcare Research). Soil Report. 2022. Available online: https://smap.landcareresearch.co.nz/maps-and-tools/factsheets/ (accessed on 31 August 2022).
- Tholl, D.; Boland, W.; Hansel, A.; Loreto, F.; Röse, U.S.; Schnitzler, J.P. Practical approaches to plant volatile analysis. Plant J. 2006, 45, 540–560. [Google Scholar] [CrossRef] [PubMed]
- IBM Corp. IBM SPSS Statistics for Windows; Version 28.0.1.1; IBM Corp.: Armonk, NY, USA, 2021. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio: Boston, MA, USA, 2022; Available online: http://www.rstudio.com/ (accessed on 11 June 2022).
- Effah, E.; Min Tun, K.; Rangiwananga, N.; Clavijo McCormick, A. Mānuka clones differ in their volatile profiles: Potential implications for plant defence, pollinator attraction and bee products. Agronomy 2022, 12, 169. [Google Scholar] [CrossRef]
- Wiebelhaus, N.; Kreitals, N.M.; Almirall, J.R. Differentiation of marijuana headspace volatiles from other plants and hemp products using capillary microextraction of volatiles (CMV) coupled to gas-chromatography–mass spectrometry (GC–MS). Forensic Chem. 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Ross, S.A.; ElSohly, M.A. The volatile oil composition of fresh and air-dried buds of Cannabis sativa. J. Nat. Prod. 1996, 59, 49–51. [Google Scholar] [CrossRef]
- Behr, A.; Johnen, L. Myrcene as a natural base chemical in sustainable chemistry: A critical review. ChemSusChem Chem. Sustain. Energy Mater. 2009, 2, 1072–1095. [Google Scholar] [CrossRef]
- Farré-Armengol, G.; Filella, I.; Llusià, J.; Peñuelas, J. β-Ocimene, a key floral and foliar volatile involved in multiple interactions between plants and other organisms. Molecules 2017, 22, 1148. [Google Scholar] [CrossRef]
- Bruce, T.J.; Midega, C.A.; Birkett, M.A.; Pickett, J.A.; Khan, Z.R. Is quality more important than quantity? Insect behavioural responses to changes in a volatile blend after stemborer oviposition on an African grass. Biol. Lett. 2010, 6, 314–317. [Google Scholar] [CrossRef]
- Clavijo-McCormick, A.; Irmisch, S.; Reinecke, A.; Boeckler, G.A.; Veit, D.; Reichelt, M.; Hansson, B.S.; Gershenzon, J.; Köllner, T.G.; Unsicker, S.B. Herbivore-induced volatile emission in black poplar: Regulation and role in attracting herbivore enemies. Plant Cell Environ. 2014, 37, 1909–1923. [Google Scholar] [CrossRef] [PubMed]
- Ogendo, J.; Kostyukovsky, M.; Ravid, U.; Matasyoh, J.; Deng, A.; Omolo, E.; Kariuki, S.; Shaaya, E. Bioactivity of Ocimum gratissimum L. oil and two of its constituents against five insect pests attacking stored food products. J. Stored Prod. Res. 2008, 44, 328–334. [Google Scholar] [CrossRef]
- Effah, E.; Svendsen, L.; Barrett, D.P.; Clavijo McCormick, A. Exploring plant volatile-mediated interactions between native and introduced plants and insects. Sci. Rep. 2022, 12, 15450. [Google Scholar] [CrossRef]
- Tanney, C.A.; Backer, R.; Geitmann, A.; Smith, D.L. Cannabis glandular trichomes: A cellular metabolite factory. Front. Plant Sci. 2022, 12, 1923. [Google Scholar] [CrossRef] [PubMed]
- Ashman, T.L. Sniffing out patterns of sexual dimorphism in floral scent. Funct. Ecol. 2009, 23, 852–862. [Google Scholar] [CrossRef]
- Okamoto, T.; Kawakita, A.; Goto, R.; Svensson, G.P.; Kato, M. Active pollination favours sexual dimorphism in floral scent. Proc. R. Soc. B Biol. Sci. 2013, 280, 20132280. [Google Scholar] [CrossRef]
- Waelti, M.O.; Page, P.A.; Widmer, A.; Schiestl, F.P. How to be an attractive male: Floral dimorphism and attractiveness to pollinators in a dioecious plant. BMC Evol. Biol. 2009, 9, 1–7. [Google Scholar] [CrossRef]
- Jing, S.W.; Coley, P.D. Dioecy and herbivory: The effect of growth rate on plant defense in Acer negundo. Oikos 1990, 58, 369–377. [Google Scholar] [CrossRef]
- Cornelissen, T.; Stiling, P. Sex-biased herbivory: A meta-analysis of the effects of gender on plant-herbivore interactions. Oikos 2005, 111, 488–500. [Google Scholar] [CrossRef]
- Degenhardt, J.; Gershenzon, J.; Baldwin, I.T.; Kessler, A. Attracting friends to feast on foes: Engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr. Opin. Biotechnol. 2003, 14, 169–176. [Google Scholar] [CrossRef]
- Effah, E.; Barrett, D.P.; Peterson, P.G.; Potter, M.A.; Holopainen, J.K.; Clavijo McCormick, A. Seasonal volatile emission patterns of the endemic New Zealand shrub Dracophyllum subulatum on the North Island Central Plateau. Front. Plant Sci. 2021, 12, 734531. [Google Scholar] [CrossRef] [PubMed]
- Effah, E.; Barrett, D.P.; Peterson, P.G.; Wargent, J.J.; Potter, M.A.; Holopainen, J.K.; Clavijo McCormick, A. Herbivory and attenuated UV radiation affect volatile emissions of the invasive weed Calluna vulgaris. Molecules 2020, 25, 3200. [Google Scholar] [CrossRef] [PubMed]
- Effah, E.; Barrett, D.P.; Peterson, P.G.; Godfrey, A.J.R.; Potter, M.A.; Holopainen, J.K.; Clavijo McCormick, A. Natural variation in volatile emissions of the invasive weed Calluna vulgaris in New Zealand. Plants 2020, 9, 283. [Google Scholar] [CrossRef]
- Ormeño, E.; Fernandez, C. Effect of soil nutrient on production and diversity of volatile terpenoids from plants. Curr. Bioact. Compd. 2012, 8, 71–79. [Google Scholar] [PubMed]
- Proffit, M.; Schatz, B.; Bessière, J.-M.; Cheri, C.; Soler, C.; Hossaert-McKey, M. Signalling receptivity: Comparison of the emission of volatile compounds by figs of Ficus hispida before, during and after the phase of receptivity to pollinators. Symbiosis 2008, 45, 15–24. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).