Plasma-Treated Nitrogen-Enriched Manure Does Not Impose Adverse Effects on Soil Fauna Feeding Activity or Springtails and Earthworms Abundance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Fauna Feeding Activity
2.1.1. Experimental Design
2.1.2. Evaluating Feeding Activity
2.2. The Abundance of Springtails (Collembola)
2.2.1. Experimental Design
2.2.2. Evaluating the Abundance of Springtails
2.3. The Fate of Earthworms (Lumbricidae)
2.3.1. Experimental Design
2.3.2. Changes in Abundance and Weight of Earthworms
- Holes with 30 cm diameter and 20 cm are dug out in the field. The soil from the holes was visually inspected to exclude present earthworms.
- Earthworm-proof but water-permeable textile (tested before the experiment) is inserted into the hole.
- Earthworms (Lumbricidae) used in the experiment were excavated from the same experimental farm two days before and stored in a pile of soil pending the experiment. On the day of starting the experiment, the earthworms were detached from the soil pile, sorted, weighed, and an equal number of worms (11 in the 2021 trial and 13 in the 2022 trial) making up a similar total weight were deposited in separate containers and marked. The worms were not rinsed before weighing. The worms were handled cautiously and remained detached from the soil for the shortest possible period. After counting and weighing, earthworms were transferred to other containers with soil.
- Next, 10 cm of soil was filled back into the holes, and the earthworms were placed over the top.
- The next 5 cm of soil was carefully mixed with the fertilizer and filled back into the hole.
- As a supplementary food source for the worms, 100 g of grass was spread on this layer.
- Two cm loose soil scattered over.
- Finally, the last 3 cm of loose soil was scattered on the top.
- Outer edges of the textile were fetched together and closed over. At last, a heavy substance (a stone) was placed over the top to inhibit wind opening or bird feeding. Finally, the experimental units were covered with white plastic tarpaulin on days of intense sun to avoid excessive temperature caused by the sun and the black textile.
- At the end of the experiment (8 days), the soil bags were lifted out of the soil and dispersed over a flat surface. The living earthworms were carefully handpicked, counted, and weighed in less than five minutes to avoid desiccation.
2.4. Data Handling and Statistical Analyzes
3. Results
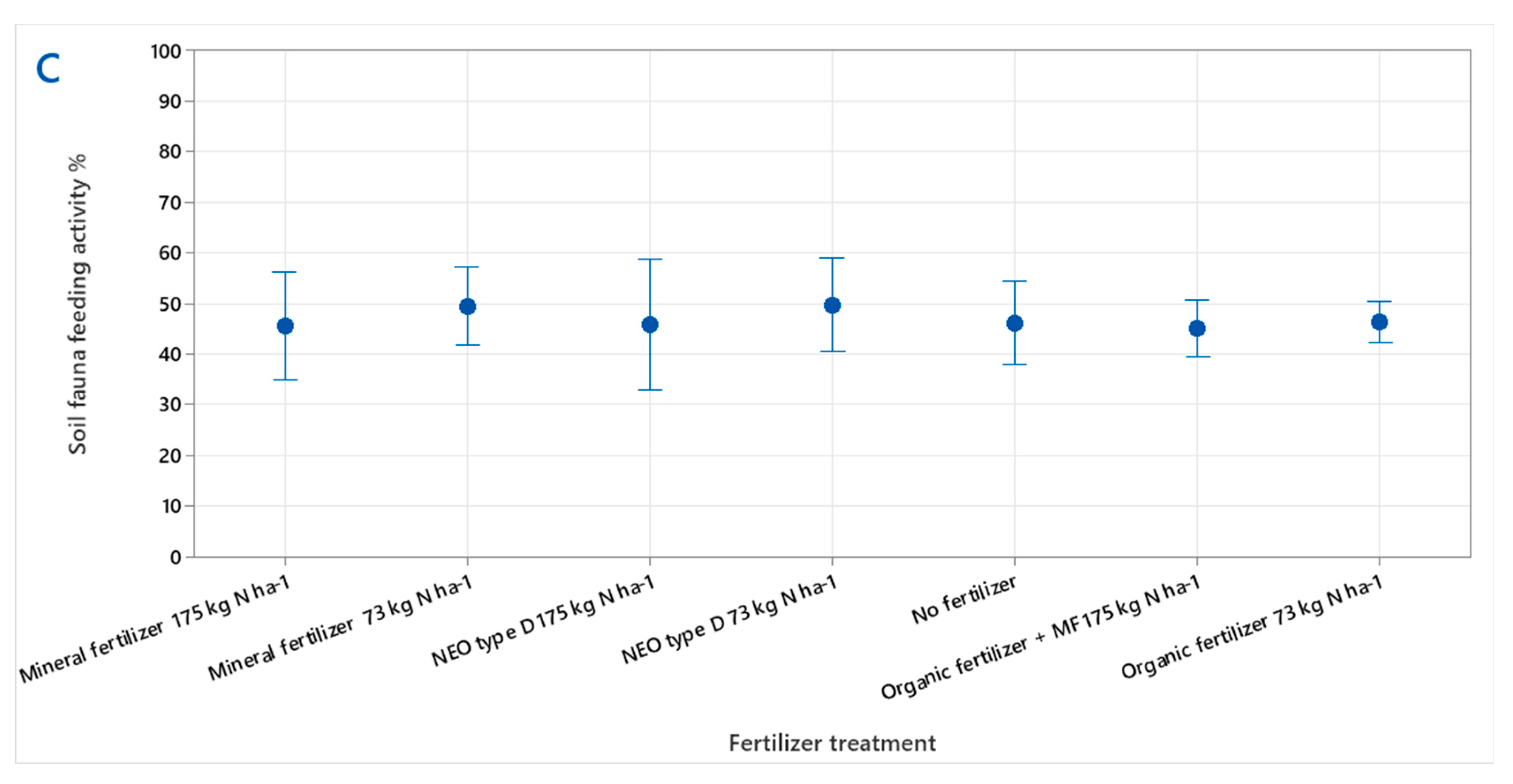
3.1. Soil Fauna Feeding Activity
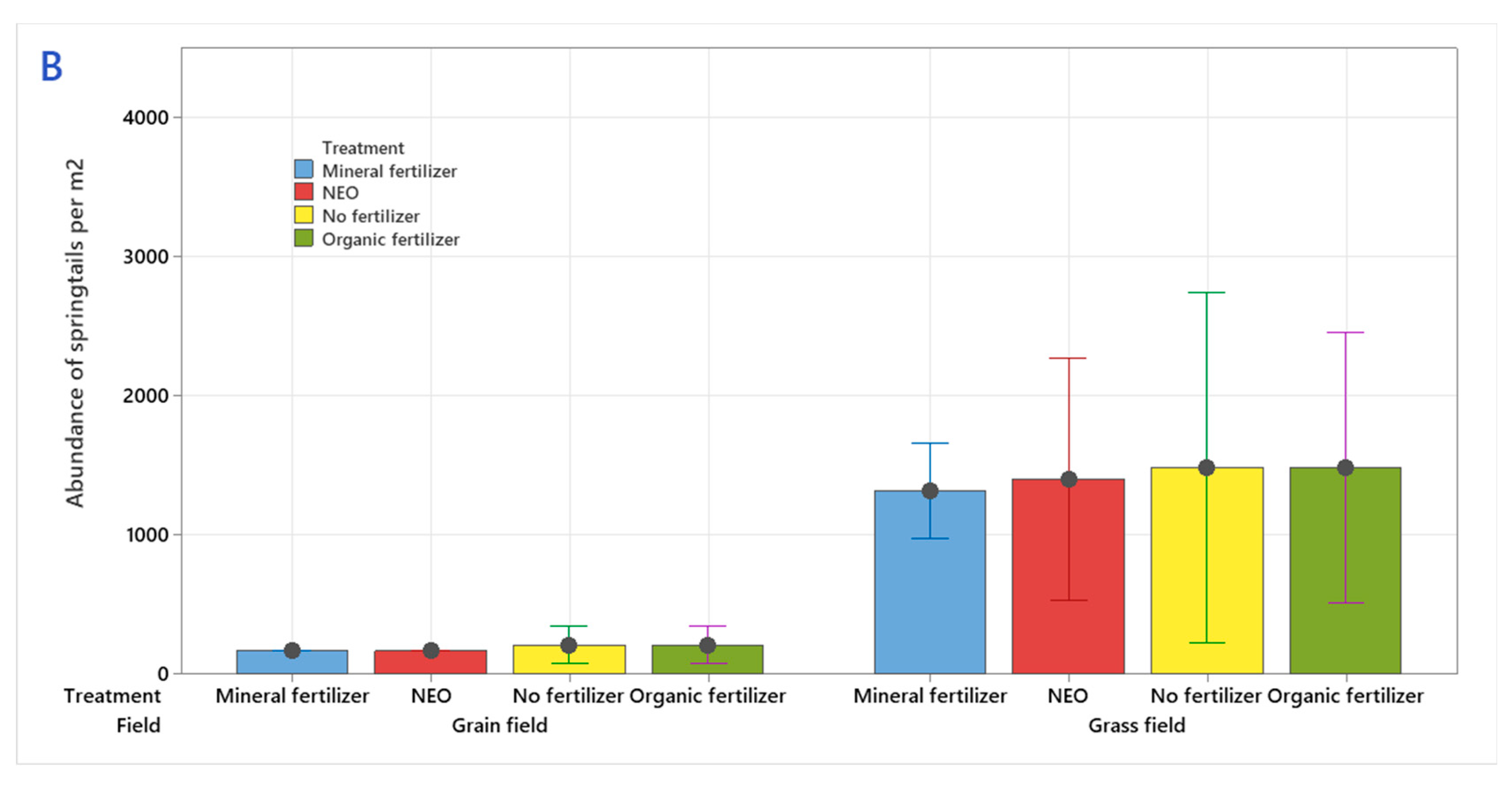
3.2. The Abundance of Springtails (Collembola)
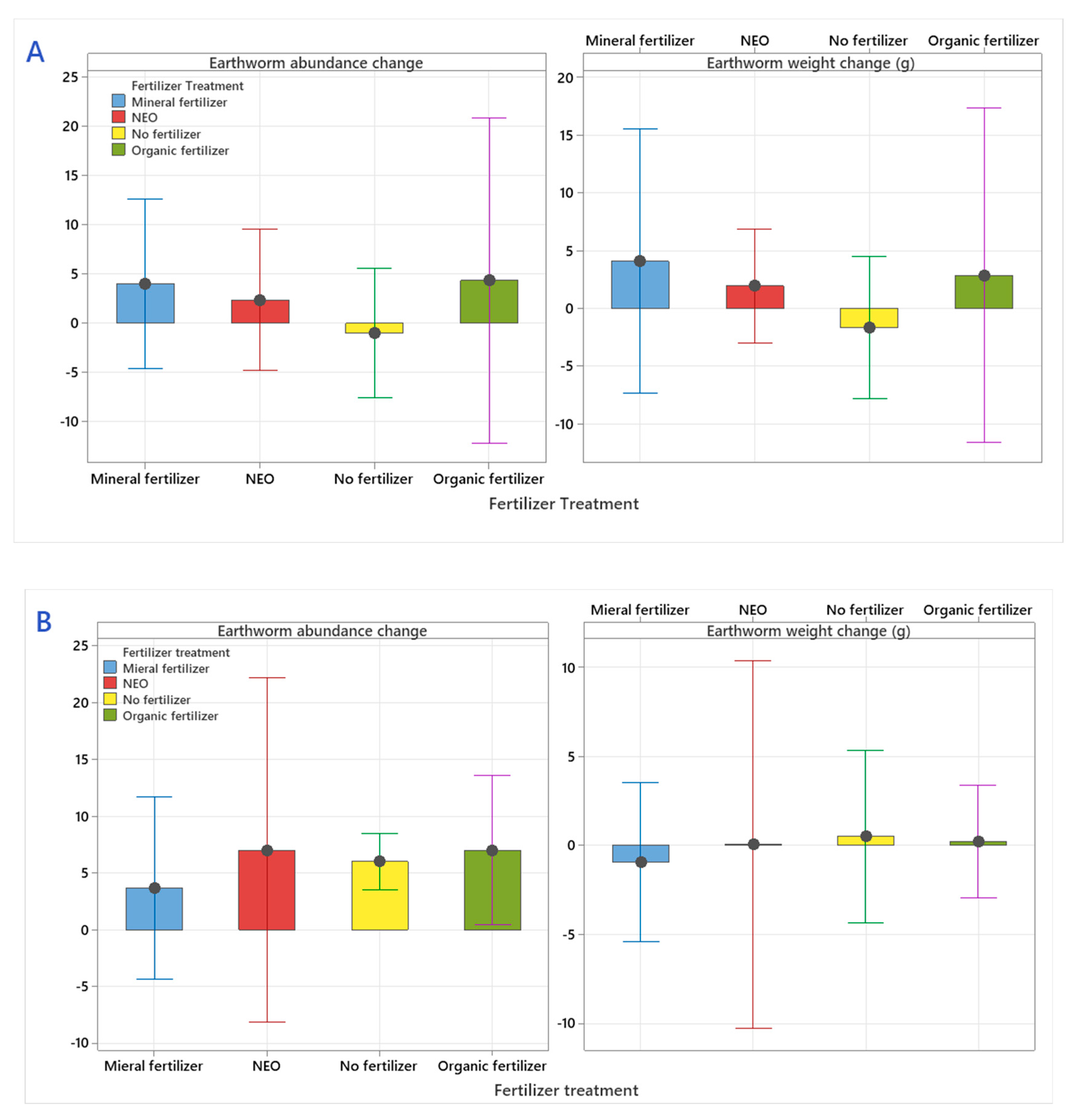
3.3. The Fate of Earthworms (Lumbricidae)
4. Discussion
4.1. Soil Fauna Feeding Activity
4.2. The Abundance of Springtails (Collembola)
4.3. The Fate of Earthworms (Lumbricidae)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mancus, P. Nitrogen Fertilizer Dependency and Its Contradictions: A Theoretical Exploration of Social-Ecological Metabolism*. Rural Sociol. 2007, 72, 269–288. [Google Scholar] [CrossRef]
- Matson, P.A.; Parton, W.J.; Power, A.G.; Swift, M.J. Agricultural intensification and ecosystem properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Tian, H. Global nitrogen and phosphorus fertilizer use for agriculture production in the past half century: Shifted hot spots and nutrient imbalance. Earth Syst. Sci. Data 2017, 9, 181–192. [Google Scholar] [CrossRef]
- FAO. Fertilizers Indicators. Available online: https://www.fao.org/faostat/en/#data/EF (accessed on 18 July 2022).
- Harris, S.L.; Thom, E.R.; Clark, D.A. Effect of high rates of nitrogen fertiliser on perennial ryegrass growth and morphology in grazed dairy pasture in northern New Zealand. New Zealand J. Agric. Res. 1996, 39, 159–169. [Google Scholar] [CrossRef]
- Cinar, S.; Özkurt, M.; Cetin, R. Effects of nitrogen fertilization rates on forage yield and quality of annual ryegrass (Lolium multiflorum L.) in central black sea climatic zone in turkey. Appl. Ecol. Environ. Res. 2020, 18, 417–432. [Google Scholar] [CrossRef]
- Abraha, A.B.; Truter, W.F.; Annandale, J.G.; Fessehazion, M.K. Forage yield and quality response of annual ryegrass (Lolium multiflorum) to different water and nitrogen levels. Afr. J. Range Forage Sci. 2015, 32, 125–131. [Google Scholar] [CrossRef]
- Savci, S. Investigation of Effect of Chemical Fertilizers on Environment. APCBEE Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef]
- Siebert, J.; Sunnemann, M.; Auge, H.; Berger, S.; Cesarz, S.; Ciobanu, M.; Guerrero-Ramirez, N.R.; Eisenhauer, N. The effects of drought and nutrient addition on soil organisms vary across taxonomic groups, but are constant across seasons. Sci. Rep. 2019, 9, 639. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Schwenke, G.D.; Van Zwieten, L. Impact of agricultural inputs on soil organisms—A review. Soil Res. 2006, 44. [Google Scholar] [CrossRef]
- European Commission. Sustainable Use of Nutrients: The Common Agricultural Policy Supports Farmers in the Safe and Efficient Use of Nutrients. Available online: https://ec.europa.eu/info/food-farming-fisheries/sustainability/environmental-sustainability/low-input-farming/nutrients_en (accessed on 18 July 2022).
- Bell, C.W.; Asao, S.; Calderon, F.; Wolk, B.; Wallenstein, M.D. Plant nitrogen uptake drives rhizosphere bacterial community assembly during plant growth. Soil Biol. Biochem. 2015, 85, 170–182. [Google Scholar] [CrossRef]
- Yuan, J.; Zhao, M.; Li, R.; Huang, Q.; Rensing, C.; Shen, Q. Lipopeptides produced by B. amyloliquefaciens NJN-6 altered the soil fungal community and non-ribosomal peptides genes harboring microbial community. Appl. Soil Ecol. 2017, 117-118, 96–105. [Google Scholar] [CrossRef]
- Körschens, M.; Albert, E.; Armbruster, M.; Barkusky, D.; Baumecker, M.; Behle-Schalk, L.; Bischoff, R. Effect of mineral and organic fertilization on crop yield, nitrogen uptake, carbon and nitrogen balances, as well as soil organic carbon content and dynamics: Results from 20 European long-term field experiments of the twenty-first century. Arch. Agron. Soil Sci. 2013, 59, 1017–1040. [Google Scholar] [CrossRef]
- N2Applied. Nitrogen Enriched Organic Fertiliser. Available online: https://n2applied.com/ (accessed on 19 July 2022).
- Graves, D.B.; Bakken, L.B.; Jensen, M.B.; Ingels, R. Plasma Activated Organic Fertilizer. Plasma Chem. Plasma Processing 2019, 39, 1–19. [Google Scholar] [CrossRef]
- Ingels, R.; Graves, D.B. Improving the Efficiency of Organic Fertilizer and Nitrogen Use via Air Plasma and Distributed Renewable Energy. Plasma Med. 2015, 5, 257–270. [Google Scholar] [CrossRef]
- Edwards, C.A.; Bohlen, P.J. Biology and Ecology of Earthworms; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1996; Volume 3. [Google Scholar]
- Tiwari, S.C. Effects of organic manure and NPK fertilization on earthworm activity in an Oxisol. Biol. Fertil. Soils 1993, 16, 293–295. [Google Scholar] [CrossRef]
- Edwards, C.A.; Lofty, J.R. Nitrogenous fertilizers and earthworm populations in agricultural soils. Soil Biol. Biochem. 1982, 14, 515–521. [Google Scholar] [CrossRef]
- Edwards, C.A.; Lofty, J.R. Biology of Earthworms; Chapman and Hall; Wiley: London, UK; New York, NY, USA, 1977. [Google Scholar]
- Hendrix, P.F.; Mueller, B.R.; Bruce, R.R.; Langdale, G.W.; Parmelee, R.W. Abundance and distribution of earthworms in relation to landscape factors on the Georgia Piedmont, U.S.A. Soil Biol. Biochem. 1992, 24, 1357–1361. [Google Scholar] [CrossRef]
- Guðleifsson, B. Impact of long term use of fertilizer on surface invertebrates in experimental plots in a permanent hayfield in Northern-Iceland. Agric. Soc. Icel. 2002, 15, 37–49. [Google Scholar]
- McLaughlin, A.; Mineau, P. The impact of agricultural practices on biodiversity. Agric. Ecosyst. Environ. 1995, 55, 201–212. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms–A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Wahyuningsih, R.; Marchand, L.; Pujianto, S.; Caliman, J. Impact of inorganic fertilizer to soil biological activity in an oil palm plantation. IOP Conf. Ser. Earth Environ. Sci. 2019, 336, 012017. [Google Scholar] [CrossRef]
- Graenitz, J.; Bauer, R. The effect of fertilization and crop rotation on biological activity in a 90 year long-term experiment. Bodenkultur 2000, 51, 99–105. [Google Scholar]
- Geisseler, D.; Lazicki, P.A.; Scow, K.M. Mineral nitrogen input decreases microbial biomass in soils under grasslands but not annual crops. Appl. Soil Ecol. 2016, 106, 1–10. [Google Scholar] [CrossRef]
- Wei, M.; Hu, G.; Wang, H.; Bai, E.; Lou, Y.; Zhang, A.; Zhuge, Y. 35 years of manure and chemical fertilizer application alters soil microbial community composition in a Fluvo-aquic soil in Northern China. Eur. J. Soil Biol. 2017, 82, 27–34. [Google Scholar] [CrossRef]
- Pommeresche, R.; Løes, A.-K. Relations between Agronomic Practice and Earthworms in Norwegian Arable Soils. Dyn. Soil Dyn. Plant Glob. Sci. Books 2009, 3, 129–142. [Google Scholar]
- Pelosi, C.; Boros, G.; van Oort, F.; Schmidt, O. Soil Oligochaeta communities after 9 decades of continuous fertilization in a bare fallow experiment. Soil Org. 2020, 92, 129–141. [Google Scholar] [CrossRef]
- Pommeresche, R.; Løes, A.-K.; Torp, T. Effects of animal manure application on springtails (Collembola) in perennial ley. Appl. Soil Ecol. 2017, 110, 137–145. [Google Scholar] [CrossRef]
- Silva, D.M.d.; Jacques, R.J.S.; Silva, D.A.A.d.; Santana, N.A.; Vogelmann, E.; Eckhardt, D.P.; Antoniolli, Z.I. Effects of pig slurry application on the diversity and activity of soil biota in pasture areas. Ciênc. Rural. 2016, 46, 1756–1763. [Google Scholar] [CrossRef]
- Mousavi, H.; Cottis, T.; Hoff, G.; Solberg, S.Ø. Nitrogen Enriched Organic Fertilizer (NEO) and Its Effect on Ryegrass Yield and Soil Fauna Feeding Activity under Controlled Conditions. Sustainability 2022, 14, 2005. [Google Scholar] [CrossRef]
- Schinner, F.; Öhlinger, R.; Kandeler, E.; Margesin, R. Methods in Soil Biolology, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar] [CrossRef]
- Fründ, H.-C.; Graefe, U.; Tischer, S. Earthworms as Bioindicators of Soil Quality. In Biology of Earthworms; Karaca, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 261–278. [Google Scholar]
- Machado, J.; Oliveira Filho, L.C.; Santos, J.; Paulino, A.; Baretta, D. Morphological diversity of springtails (Hexapoda: Collembola) as soil quality bioindicators in land use systems. Biota Neotrop. 2019, 19, e20180618. [Google Scholar] [CrossRef]
- Yara. YaraMila™. Available online: https://www.yara.com/crop-nutrition/products-and-solutions/global-fertilizer-brands/yaramila/ (accessed on 20 July 2022).
- Yara. YaraLiva™. Available online: https://www.yara.com/crop-nutrition/products-and-solutions/global-fertilizer-brands/yaraliva/ (accessed on 20 July 2022).
- Lumatek. ATS300W Specs Sheet. Available online: https://lumatek-lighting.com/lumatek-ats-300w/ (accessed on 21 July 2022).
- Yiotis, C.; McElwain, J.C.; Osborne, B.A. Enhancing the productivity of ryegrass at elevated CO2 is dependent on tillering and leaf area development rather than leaf-level photosynthesis. J. Exp. Bot. 2020, 72, 1962–1977. [Google Scholar] [CrossRef] [PubMed]
- TerraProtecta. The Bait-Lamina Test. Available online: http://www.terra-protecta.de/en/bait_strips.html (accessed on 24 July 2022).
- Törne, E.v. Assessing feeding activities of soil-living animals. Bait-lamina-tests. Pedobiologia 1990, 34, 89–101. [Google Scholar]
- Hamel, C.; Schellenberg, M.P.; Hanson, K.; Wang, H. Evaluation of the “bait-lamina test” to assess soil microfauna feeding activity in mixed grassland. Appl. Soil Ecol. 2007, 36, 199–204. [Google Scholar] [CrossRef]
- Kratz, W. Bait-lamina test, general aspect, applications and perspectives. Environ. Sci. Pollut. Res. 1998, 5, 3. [Google Scholar]
- Jänsch, S.; Scheffczyk, A.; Römbke, J. The bait-lamina earthworm test: A possible addition to the chronic earthworm toxicity test? Euro-Mediterr. J. Environ. Integr. 2017, 2, 5. [Google Scholar] [CrossRef]
- ISO18311(E). Soil Quality–Method for Testing Effects of Soil Contaminants on The Feeding Activity Of Soil Dwelling Organisms in The Field–Bait-Lamina Test" for Approval for The Next Stage (FDIS) until 2015-04-17 (see Recommendation Paris-2). 2016. Available online: https://www.iso.org/standard/62102.html (accessed on 21 July 2022).
- Gardi, C.; Montanarella, L.; Arrouays, D.; Bispo, A.; Lemanceau, P.; Jolivet, C.; Mulder, C.; Ranjard, L.; Römbke, J.; Rutgers, M.; et al. Soil biodiversity monitoring in Europe: Ongoing activities and challenges. Eur. J. Soil Sci. 2009, 60, 807–819. [Google Scholar] [CrossRef]
- Simpson, J.E.; Slade, E.; Riutta, T.; Taylor, M.E. Factors Affecting Soil Fauna Feeding Activity in a Fragmented Lowland Temperate Deciduous Woodland. PLoS ONE 2012, 7, e29616. [Google Scholar] [CrossRef]
- Rożen, A.; Sobczyk, Ł.; Liszka, K.; Weiner, J. Soil faunal activity as measured by the bait-lamina test in monocultures of 14 tree species in the Siemianice common-garden experiment, Poland. Appl. Soil Ecol. 2010, 45, 160–167. [Google Scholar] [CrossRef]
- Verhoef, H.A.; Brussaard, L. Decomposition and nitrogen mineralization in natural and agroecosystems: The contribution of soil animals. Biogeochemistry 1990, 11, 175. [Google Scholar] [CrossRef]
- Hopkin, S.P. Biology of the Springtails (Insecta-Collembola); Oxford University Press: Oxford, UK, 1997; p. 330. [Google Scholar]
- Tullgren. Ein sehr einfacher Ausleseapparat für terricole Tierformen. Z. Angew. Entomol. 1918, 4, 149–150. [Google Scholar] [CrossRef]
- Berlese, A. Apparecchio per Raccogliere Presto ed in Gran Numero Piccoli Arthropodi; Kessinger Publishing, LLC: Whitefish, MT, USA, 1905; Volume 2, pp. 85–89. [Google Scholar]
- O’Connor, F.B. The extraction of Enchytraeidae from soil. Prog. Soil Zool. 1962, 279–285. [Google Scholar]
- Bouché, M.B. Strategies lombriciennes. Ecol. Bull. 1977, 25, 122–132. [Google Scholar]
- Graff, O. Unsere Regenwürmer (Lexikon für Freunde der Bodenbiologie). Z. Pflanz. Bodenkd. 1984, 147, 530. [Google Scholar] [CrossRef]
- Sims, R.W.; Gerard, B.M.; London, L.S.; Association, E.B.W.S. Earthworms: Keys and Notes for the Identification and Study of the Species; Linnean Society of London and the Estuarine and Brackish-Water Sciences Association; Linnean Society of London and the Estuarine and Brackish-Water Sciences Association, Brill Archive: London, UK.
- Birkhofer, K.; Baulechner, D.; Diekötter, T.; Zaitsev, A.; Wolters, V. Fertilization Rapidly Alters the Feeding Activity of Grassland Soil Mesofauna Independent of Management History. Front. Ecol. Evol. 2022, 10, 864470. [Google Scholar] [CrossRef]
- Butterfield, J. Changes in decomposition rates and Collembola densities during the forestry cycle in conifer plantations. J. Appl. Ecol. 1999, 36, 92–100. [Google Scholar] [CrossRef]
- Christiansen, K.A.; Bellinger, P.; Janssens, F. Chapter 55 - Collembola: (Springtails, Snow Fleas). In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 206–210. [Google Scholar]
- Gergócs, V.; Flórián, N.; Tóth, Z.; Szili-Kovács, T.; Mucsi, M.; Dombos, M. Crop species and year affect soil-dwelling Collembola and Acari more strongly than fertilisation regime in an arable field. Appl. Soil Ecol. 2022, 173, 104390. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.Y.H.; Tan, Y.; Fan, H.; Ruan, H. Fertilizer regime impacts on abundance and diversity of soil fauna across a poplar plantation chronosequence in coastal Eastern China. Sci. Rep. 2016, 6, 20816. [Google Scholar] [CrossRef] [Green Version]
- Lalthanzara, H.; Ramanujam, S.N. Effect of fertilizer (NPK) on earthworm population in the Agroforestry system of Mizoram, India. Sci. Vis. 2010, 10, 159–167. [Google Scholar]
- Ernst, D. The Farmer’s Earthworm Handbook: Managing Your Underground Money-Makers; Lessiter Publications, Inc.: Brookfield, WI, USA, 1995. [Google Scholar]



| Fertilizing Treatment | Organic Fertilizer (Tons ha−1) | Kg N in Yara Mila18-3-15 (kg ha−1) | Kg N in Organic Fertilizer (kg ha−1) | Kg N in Yara Liva 16-0-0 (kg ha−1) | Total kg N (kg ha−1) | |
|---|---|---|---|---|---|---|
| 1 | No fertilizer | - | - | - | - | 0 |
| 2 | Mineral fertilizer 73 kg N ha−1 | 73 | 73 | |||
| 3 | Mineral fertilizer 175 kg N ha−1 | 175 | 175 | |||
| 4 | NEO type D 73 kg N ha−1 | 22 | 73 | 73 | ||
| 5 | NEO type D 175 kg N ha−1 | 50 | 175 | 175 | ||
| 6 | Organic fertilizer 73 kg N ha−1 | 55 | 73 | 73 | ||
| 7 | Organic fertilizer + mineral fertilizer 175 kg N ha−1 | 55 | 73 | 102 | 175 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi, H.; Cottis, T.; Pommeresche, R.; Dörsch, P.; Solberg, S.Ø. Plasma-Treated Nitrogen-Enriched Manure Does Not Impose Adverse Effects on Soil Fauna Feeding Activity or Springtails and Earthworms Abundance. Agronomy 2022, 12, 2314. https://doi.org/10.3390/agronomy12102314
Mousavi H, Cottis T, Pommeresche R, Dörsch P, Solberg SØ. Plasma-Treated Nitrogen-Enriched Manure Does Not Impose Adverse Effects on Soil Fauna Feeding Activity or Springtails and Earthworms Abundance. Agronomy. 2022; 12(10):2314. https://doi.org/10.3390/agronomy12102314
Chicago/Turabian StyleMousavi, Hesam, Thomas Cottis, Reidun Pommeresche, Peter Dörsch, and Svein Øivind Solberg. 2022. "Plasma-Treated Nitrogen-Enriched Manure Does Not Impose Adverse Effects on Soil Fauna Feeding Activity or Springtails and Earthworms Abundance" Agronomy 12, no. 10: 2314. https://doi.org/10.3390/agronomy12102314









