Empower Innovations in Routine Soil Testing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Step 1: Selection and Calibration of Rapid Broad-Spectrum Soil Testing Techniques
2.1.1. Multi-Nutrient Extractions with 0.01 M CaCl2
2.1.2. Near Infrared Spectroscopy
2.2. Step 2: Relate Results of the New Testing Techniques to Those of Conventional Soil Tests
2.3. Step 3: Improved Agronomical Calibration and Validation, and Communication
3. Results
3.1. Step1: Calibration of the Broad-Spectrum Soil Tests
3.1.1. Multi-Nutrient Extractions with 0.01 M CaCl2
3.1.2. Soil Fertility Characterization through NIRS
3.2. Step 2: Relating the New Test Data to Those of Conventional Soil Tests
3.3. Step 3: Improved Agronomical Calibration and Validation, and Communication
3.3.1. Agronomic Calibration and Validation of the Soil Tests
3.3.2. Implementation to the Market
4. Discussion
4.1. The Development of the Soil Nutrient Intensity, Buffering Capacity and Quantity Concept
4.2. Analytical Performance of the Broad-Spectrum Soil Tests
4.3. Stepwise Implementation in Practice
4.4. Outlook
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shang, Q.; Ling, N.; Feng, X.; Yang, X.; Wu, P.; Zou, J.; Shen, Q.; Guo, S. Soil fertility and its significance to crop productivity and sustainability in typical agroecosystem: A summary of long-term fertilizer experiments in China. Plant Soil 2014, 381, 13–23. [Google Scholar] [CrossRef]
- van Ittersum, M.K.; Rabbinge, R. Concepts in production ecology for analysis and quantification of agricultural input-output combinations. Field Crops Res. 1997, 52, 197–208. [Google Scholar] [CrossRef]
- FAO. Towards the Future We Want: End Hunger and Make the Transition to Sustainable Agricultural and Food Systems. Report on Rio + 20. FAO of the UN. 2012. Available online: http://www.fao.org/3/an894e/an894e00.pdf (accessed on 28 November 2021).
- Patzel, N.; Sticher, H.; Karlen, D.L. Soil fertility—phenomenon and concept. J. Plant. Nutr. Soil Sci. 2000, 163, 129–142. [Google Scholar] [CrossRef]
- Jenny, H. Great soil groups in the equatorial regions of Colombia, South America. Soil Sci. 1948, 66, 5. [Google Scholar] [CrossRef]
- Abbott, L.K.; Murphy, D.V. (Eds.) Soil Biological Fertility—A Key to Sustainable Land Use in Agriculture; Springer: Dordrecht, The Netherlands, 2007; pp. 1–15. [Google Scholar]
- Osman, K.T. Physical Properties of Soil. Soils. In Soils; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Rumpel, C.; Kögel-Knabner, I. Evaluation of an ultrasonic dispersion procedure to isolate primary organomineral complexes from soils. Eur. J. Soil Sci. 2020, 50, 87–94. [Google Scholar] [CrossRef]
- Briggs, S.; Eclair-Heath, G. Helping UK farmers to choose, use, and interpret soil test results to inform soil management decisions for soil health. Asp. Appl. Biol Crop. Prod. South. Br. 2017, 134, 161–168. [Google Scholar]
- Golicz, K.; Hallett, S.H.; Sakrabani, R.; Pan, G. The potential for using smartphones as portable soil nutrient analyzers on suburban farms in central East China. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Russell, E.J. Soil Conditions and Plant Growth, 10th ed.; Longman: London, UK, 1973. [Google Scholar]
- Domingo, W.R. The Development of Soil Fertility Research; Joh, N.V., Ed.; Ykema’s Uitgevers-Maatschappij, ‘s: Gravenhage, The Netherlands, 1955. (In Dutch) [Google Scholar]
- Daubeny, C.G.B. Memoirs on the rotation of crops and on the quantity of inorganic matters abstracted from the soil by various plants under different circumstances. Roy. Soc. Phil. Trans. Lond. 1845, 135, 79–253. [Google Scholar]
- Dyer, B. On the analytical determination of probable available mineral plant food in soils. J. Chem. Soc. Trans. 1894, 65, 115–167. [Google Scholar] [CrossRef] [Green Version]
- Kuipers, S.F. Principles of fertilization. In Nederlandse Land- en Tuinbouwbibliotheek; Wolters: Groningen, The Netherlands, 1951. (In Dutch) [Google Scholar]
- Dalal, R.C.; Hallsworth, E.G. Evaluation of the parameters of soil phosphorus availability factors in predicting yield response and phosphorus uptake. Soil Sci. Soc. Am. J. 1976, 40, 541–546. [Google Scholar] [CrossRef]
- Moody, P.W.; Aitken, R.L.; Compton, B.L.; Hunt, S. Soil phosphorus parameters affecting phosphorus availability to, and fertilizer requirements of, maize (Zea mays). Aust. J. Soil Res. 1988, 26, 611–622. [Google Scholar] [CrossRef]
- Van Rotterdam-Los, A.M.D. The Potential of Soils to Supply Phosphorus and Potassium, Processes and Predictions. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2010. [Google Scholar]
- Woods, M.S.; Ketterings, Q.M.; Rossi, F.S. Effectiveness of standard soil tests for assessing potassium availability in sand rootzones. Soil Sci. 2005, 170, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Fontana, M.; Bélanger, G.; Hirte, J.; Ziadi, N.; Elfouki, S.; Bragazza, L.; Liebisch, F.; Sinaj, S. Critical plant phosphorus for winter wheat assessed from long-term field experiments. Eur. J. Agron. 2021, 126, 126–263. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Papaioannou, B.; Gasparatos, D.; Molassiotis, A. From which soil metal fractions Fe, Mn, Zn and Cu are taken up by olive trees (Olea europaea L., cv. ‘Chondrolia Chalkidikis’) in organic groves? J. Environ. Manag. 2017, 203, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Reijneveld, J.A.; Termorshuizen, A.; Vedder, H.; Oenema, O. Strategey for innovation in soil tests illustrated for P tests. Commun. Soil Sci. Plant Anal. 2014, 45, 498–515. [Google Scholar] [CrossRef]
- Schofield, R.K.; Taylor, A.W. The measurement of soil pH. Soil Sci. Soc. Am. J. 1955, 19, 164–167. [Google Scholar] [CrossRef]
- Houba, V.J.G.; Novozamsky, I.; Lexmond, T.M.; Van der Lee, J.J. Applicability of 0.01 M CaCl2 as a single extraction solution for the assessment of the nutrient status of soils and other diagnostic purposes. Commun. Soil Sci. Plant Anal. 1990, 21, 2281–2290. [Google Scholar] [CrossRef]
- Houba, V.J.G.; Novozamsky, I.; Van der Lee, J.J. Status and future of soil and plant analysis. Commun. Soil Sci. Plant Anal. 1994, 25, 753–765. [Google Scholar] [CrossRef]
- Van Erp, P.J. The Potentials of Multi-Nutrient Soil Extraction with 0.01 M CaCl2 in Nutrient Management. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2002. [Google Scholar]
- NEN 15923-1. Water Quality—Determination of Selected Parameters by Discrete Analysis Systems—Part 1: Ammonium, Nitrate, Nitrite, Chloride, Orthophosphate, Sulfate and Silicate with Photometric Detection. 2003. Available online: https://www.nen.nl/ (accessed on 28 November 2021).
- NEN 6966. Environment—Analyses of Selected Elements in Water, Eluates and Destruates—Atomic Emission Spectrometry with Inductively Coupled Plasma. 2005. Available online: https://www.nen.nl/ (accessed on 28 November 2021). (In Dutch).
- NEN 17294-2. Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: DeterMination of Selected Elements Including Uranium Isotopes. 2016. Available online: https://www.nen.nl/ (accessed on 20 December 2021).
- ISO-10390. Soil Quality—Determination of pH. 2005. Available online: https://www.iso.org/standard/40879.html (accessed on 28 November 2021).
- Malley, D.F.; Williams, P. Analysis of sediments and suspended material in lake ecosystems using near-infrared spectroscopy: A review. Aquat. Ecosyst. Health Manag. 2014, 17, 447–453. [Google Scholar] [CrossRef]
- Shepherd, K.D.; Walsh, M.G. Infrared spectroscopy—enabling an evidence-based diagnostic surveillance approach to agricultural and environmental management in developing countries. J. Near Infrared Spectrosc. 2007, 15, 1–19. [Google Scholar] [CrossRef]
- Rossel, R.V.; Webster, R. Predicting soil properties from the Australian soil visible–near infrared spectroscopic database. Eur. J. Soil Sci. 2012, 63, 848–860. [Google Scholar] [CrossRef]
- Jaconi, A.; Vos, C.; Don, A. Near Infrared Spectroscopy as an easy an precise method to estimate soil texture. Geoderma 2019, 337, 906–913. [Google Scholar] [CrossRef]
- Nduwamungu, C.; Ziadi, N.; Parent, L.É.; Tremblay, G.F.; Thuriès, L. Opportunities for, and limitations of, near infrared reflectance spectroscopy applications in soil analysis: A review. Can. J. Soil Sci. 2009, 89, 531–541. [Google Scholar] [CrossRef]
- Cécillon, L.; Barthès, B.G.; Gomez, C.; Ertlen, D.; Génot, V.; Hedde, M.; Stevens, A.; Brun, J.J. Assessment and monitoring of soil quality using near-infrared reflectance spectroscopy (NIRS). Eur. J. Soil Sci. 2009, 60, 770–784. [Google Scholar] [CrossRef] [Green Version]
- FAO. Steering Committee on Soil Spectroscopy. Available online: https://www.fao.org/soils-portal/events/detail/en/c/1268465/ (accessed on 28 November 2021).
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least-Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Liu, G.; Guo, H.; Pan, T.; Wang, J.; Cao, G. Vis-NIR Spectroscopic Pattern Recognition Combined with SG Smoothing Applied to Breed Screening of Transgenic Sugarcane. Guang Pu Xue Yu Guang Pu Fen Xi 2014, 34, 2701–2706. [Google Scholar]
- Kawamura, K.; Tsujimoto, Y.; Nishigaki, T.; Andriamananjara, A.; Rabenarivo, M.; Asai, H.; Rakotoson, T.; Razafimbelo, T. Laboratory Visible and Near-Infrared Spectroscopy with Genetic Algorithm-Based Partial Least Squares Regression for Assessing the Soil Phosphorus Content of Upland and Lowland Rice Fields in Madagascar. Remote Sens. 2019, 11, 506. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Lopez, L.; Behrens, T.; Schmidt, K.; Stevens, A.; Demattê, J.A.M.; Scholten, T. The spectrum-based learner: A new local approach for modeling soil vis–NIR spectra of complex datasets. Geoderma 2013, 195, 268–279. [Google Scholar] [CrossRef]
- Liu, M.; Huang, C.; Wang, L.; Zhang, Y.; Luo, X. Short-term soil moisture forecasting via Gaussian process regression with sample selection. Water 2020, 12, 3085. [Google Scholar] [CrossRef]
- ISO-13878. Soil Quality—Determination of Total Nitrogen Content by Dry Combustion (“Elemental Analysis”). 1998. Available online: https://www.iso.org/standard/23117.html (accessed on 28 November 2021).
- NEN 15587-2. Water Quality—Digestion for the Determination of Selected Elements in Water—Part 2: Nitric Acid Digestion. 2002. Available online: https://www.nen.nl/ (accessed on 28 November 2021).
- ISO-23470. Soil Quality—Determination of Effective Cation Exchange Capacity (CEC) and Exchangeable Cations Using a Hexaminecobalt(III)chloride Solution. 2018. Available online: https://www.iso.org/standard/68765.html (accessed on 28 November 2021).
- ISO-10694. Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis). 1995. Available online: https://www.iso.org/standard/18782.html (accessed on 28 November 2021).
- NEN 5754. Soil—Calculation of Organic Matter Content on A Mass Basis as Loss-on-Ignition. 2014. Available online: https://www.nen.nl/ (accessed on 28 November 2021). (In Dutch).
- NEN-EN 15936. Sludge, Treated Biowaste, Soil and Waste—Determination of Total Organic Carbon (TOC) by Dry Combustion. 2012. Available online: https://www.nen.nl/ (accessed on 28 November 2021).
- NEN-EN 5753. Soil—Determination of Clay Content and Particle Size Distribution in Soil and Sediment by Sieve and Pipet. 2018. Available online: https://www.nen.nl/ (accessed on 28 November 2021).
- Van der Paauw, F.; Sissingh, H.A.; Ris, J. Een verbeterde fosfaatextractie van de grond met water: Het Pw-getal. Versl. Landbouwkd. Onderz. 1971, 749. Available online: https://edepot.wur.nl/218204 (accessed on 28 November 2021). (In Dutch).
- Houba, V.J.G.; Novozamsky, I.; Huybregts, A.W.M.; Van Der Lee, J.J. Comparison of soil extractions by 0.01 M CaCI2, by EUF and by some conventional extraction procedures. Plant Soil 1986, 96, 433–437. [Google Scholar] [CrossRef]
- De Haas, M.J.G.; Bussink, D.W.; Moolenaar, S.W. A New Route for Fertilization Research: Results of 2 Years of CaCl2 Research. NMI Rep. 936.03. 2005. Available online: https://www.nmi-agro.nl/ (accessed on 28 November 2021). (In Dutch).
- De Vries, O.; Hetterschij, C.W.G. Investigations into the methodology for potassium determinations in soil. Versl. Van Landbouwkd. Onderz. 1945, 50, 4. Available online: https://edepot.wur.nl/218017 (accessed on 28 November 2021). (In Dutch).
- Anonymous. Fertilization Recommendation for Grassland, and Forage Crops. Commissie Bemesting Grasland en Voedergewassen. 2012. Available online: www.bemestingsadvies.nl (accessed on 28 November 2021). (In Dutch).
- Anonymous. Fertilization Recommendation for Arable Crops, and Vegetable Crops. Praktijkonderzoek Voor de Akkerbouw en Vollegrondsgroenteteelt. Praktijkonderzoek voor de Akkerbouw en de Vollegrondsgroenteteelt. 1999. Available online: https://www.wur.nl/nl/Publicatie-details.htm?publicationId=publication-way-343537383933 (accessed on 28 November 2021). (In Dutch).
- Baier, J.; Baierova, V. Hundredth molar calcium chloride extraction procedure. Part IV: Calibration with conventional soil testing methods for potassium. Commun. Soil Sci. Plant Anal. 1998, 29, 11–14. [Google Scholar] [CrossRef]
- Ferrari, T.; Sluijsmans, C. Mottling and magnesium deficiency in oats and their dependence of various factors. Plant Soil 1955, 6, 262–299. [Google Scholar] [CrossRef]
- Loch, J.; Jaszberenyi, I.; Vago, I. One hundredth molar calcium chloride soil extraction procedure. Part III: Calibration with conventional soil testing methods for Magnesium. Commun. Soil Sci. Plant Anal. 1998, 29, 1633–1640. [Google Scholar] [CrossRef]
- Van Erp, P.V.; Houba, V.J.G.; Reijneveld, J.A.; Van Beusichem, M.L. Relationship between magnesium extracted by 0.01 M calcium chloride extraction procedure and conventional procedures. Commun. Soil Sci. Plant Anal. 2001, 32, 1–18. [Google Scholar] [CrossRef]
- Henkens, C.H.H. Copper measurements on arable land; the value of chemical soil test compared to the Aspergillus niger-method. Versl. Landbouwkd. Onderz. 1961, 67. ISSN 0372-6223. (In Dutch) [Google Scholar]
- Henkens, C.H.H. Cobalt on grassland. Versl. Landbouwkd. Onderz. 1959, 16, 642–651. (In Dutch) [Google Scholar]
- Berger, K.C.; Truog, E. Boron determination in soils and plants. J. Ind. Eng. Chem. 1939, 11–10, 540–545. [Google Scholar] [CrossRef]
- Novozamsky, I.; Barrera, L.L.; Houba, V.J.G.; Van der Lee, J.J.; Van Eck, R. Comparison of a hot water and cold 0.01 M CaCl2 extraction procedures for the determination of boron in soil. Commun. Soil Sci. Plant Anal. 1990, 21, 2189–2195. [Google Scholar] [CrossRef]
- Fotyma, M.; Jadczyszyn, T.; Jozefaciuk, G. Hundredth molar calcium chloride extraction procedure. Part II: Calibration with conventional soil testing methods for pH. Commun. Soil Sci. Plant Anal. 1998, 29, 1625–1632. [Google Scholar] [CrossRef]
- Minasny, B.; McBratney, A. Why you don’t need to use RPD. Pedometron 2013, 33, 14–15. [Google Scholar]
- Esbensen, K.H.; Geladi, P.; Larsen, A. The RPD myth. NIR News 2014, 25, 24–28. [Google Scholar] [CrossRef]
- Jin, X.; Li, S.; Zhang, W.; Zhu, J.; Sun, J. Prediction of soil-available potassium content with visible near-infrared ray spectroscopy of different pretreatment transformations by the boosting algorithms. Appl. Sci. 2020, 10, 1520. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-W.; Laird, D.A.; Mausbach, M.J.; Hurburgh, C.R., Jr. Near-infrared reflectance spectroscopy—Principal components regression analyses of soil properties. Soil Sci. Soc. Am. J. 2001, 3, 480–490. [Google Scholar] [CrossRef] [Green Version]
- Van Rotterdam, A.M.D.; Temminghoff, E.J.M.; Schenkeveld, W.D.L.; Hiemstra, T.; van Riemsdijk, W.H. Phosphorus removal from soil using Fe oxide-impregnated paper: Processes and applications. Geoderma 2009, 151, 282–289. [Google Scholar] [CrossRef]
- Van Rotterdam, A.M.D.; Bussink, D.W.; Temminghoff, E.J.M.; van Riemsdijk, W.H. Predicting the potential of soils to supply phosphorus by integrating soil chemical processes and standard soil tests. Geoderma 2012, 189, 617–626. [Google Scholar] [CrossRef]
- Egnér, H.; Riehm, H.; Domingo, W.R. Soil tests as basis of establishing soil nutrient status II Chemical extraction methods for phosphor and potassium (Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extraktionsmethoden zur Phospor—Und Kaliumbestimmung). Kungliga. Landbr. Ann. 1960, 26, 199–215. (in German). [Google Scholar]
- Van Rotterdam-Los, A.M.D.; Reijneveld, J.A.; van Oostrum, M.; Abbink, G.; Bussink, W. A more trustworthy P recommendation by implementing the intensity, buffering capacity, quantity concept into agricultural practice. In NUTRIHORT: Nutrient Management, Innovative Techniques and Nutrient Legislation in Intensive Horticulture for an Improved Water Quality: Proceedings; Institute for Agricultural and Fisheries Research (ILVO): Merelbeke, Belgium, 2013; pp. 136–141. Available online: https://biblio.ugent.be/publication/8520976 (accessed on 28 November 2021).
- Van Rotterdam, A.M.D.; Bussink, D.W.; Reijneveld, J.A. Improved Phosphorus Fertilisation Based on Better Prediction of Availability in Soil. In Proceedings of the International Fertliser Society, London, UK, 3 July 2014; Volume 755. [Google Scholar]
- Bussink, D.W.; Reijneveld, J.A. A concept for the P-recommendation for silage maize. VDLUFA 2010, 122, 100–101. (In German) [Google Scholar]
- Bussink, D.W.; Van Schooten, H.; Van Middelkoop, J.; Holshof, G.; Doppenberg, G. Towards a new K fertilizer recommendation for silage maize in the Netherlands. VDLUFA 2018, 130, 37–38. (In German) [Google Scholar]
- Reijneveld, J.A.; van Bohemen, F.J.M.; Termorshuizen, A.J.; Oenema, O. Farmers’ perceptions of soil tests: A case study in the Netherlands. Acta Sci. Agric. 2019, 3.8, 96–103. [Google Scholar] [CrossRef]
- Schoumans, O.F.; Chardon, W.J. Risk assessment methodologies for predicting phosphorus losses. J. Plant Nutr. Soil Sci. 2003, 166, 403–408. [Google Scholar] [CrossRef]
- Amundson, R.; Berhe, A.A.; Hopmans, J.W.; Olsen, C.; Sztein, A.E.; Sparks, D.L. Soil and human security in the 21st century. Science 2015, 348, 6235. [Google Scholar] [CrossRef] [Green Version]
- Bouma, J.; Montnarella, L.; Evanylo, G. The challenge for the soil science community to contribute to the implementation of the UN sustainable development goals. Soil Use Manag. 2019, 35, 538–546. [Google Scholar] [CrossRef]
- Gil, J.D.B.; Reidsma, P.; Giller, K.; Todman, L.; Whitmore, A.; van Ittersum, M. Sustainable development goal 2: Improved targets and indicators for agriculture and food security. Ambio 2018, 48, 685–698. [Google Scholar] [CrossRef] [Green Version]
- Ehlert, P.A.I.; Morel, C.; Fotyma, M.; Destain, J.-P. Potential role of phosphate buffering capacity of soils in fertilizer management strategies fitted to environmental goals. J. Plant. Nutr. Soil Sci. 2003, 166, 409–415. [Google Scholar] [CrossRef]
- Quintero, C.E.; Boschetti, N.G.; Benavidez, R.A. Effects of soil buffer capacity on soil test phosphorus interpretation and fertilizer requirement. Commun. Soil Sci. Plant Anal. 2003, 34, 1435–1450. [Google Scholar] [CrossRef]
- Lalitha, M.; Dhakshinamoorthy, M. Quantity-intensity characteristics of Potassium (K) in relation to potassium availability under different cropping system in alluvial soils. Afr. J. Agric. Res. 2015, 10, 2097–2103. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, K.N.; Rana, D.S. The quantity-intensity relations of potassium in soils from plots having nine fixed crop rotations for six years. Plant Soil 1978, 50, 363–370. [Google Scholar] [CrossRef]
- Cervantes, C.E.; Hanson, R.G. A potential method to incorporate quantity/intensity into routine soil test interpretations. Commun. Soil Sci. Plant Anal. 1991, 22, 683–700. [Google Scholar] [CrossRef]
- Saleque, M.A.; Anisuzzaman, M.; Moslehuddin, A.Z.M. Quantity-intensity relationships and potassium buffering capacity of four Ganges river floodplain soils. Commun. Soil Sci. Plant Anal. 2009, 40, 1333–1349. [Google Scholar] [CrossRef]
- ISO-17184. Soil Quality—Determination of Carbon and Nitrogen by Near-Infrared Spectrometry (NIRS). 2014. Available online: https://www.iso.org/standard/59262.html (accessed on 28 November 2021).
- Zornoza, R.; Guerrero, C.; Mataix-Solera, K.M.; Scow, V.; Arcenequi, J. Mataix Beneyto. Soil Biol. Biochem. 2008, 40, 1923–1930. [Google Scholar] [CrossRef] [Green Version]
- Genot, V.; Colinet, G.; Bock, L.; Vanvyve, D.; Reusen, Y.; Dardenne, P. Near infrared reflectance spectroscopy for estimating soil characteristics valuable in diagnosis of soil fertility. J. Near Infrared Spectrosc. 2011, 19, 117–138. [Google Scholar] [CrossRef]
- Moron, A.; Cozzolino, D. Application of near infrared reflectance spectroscopy for the analysis of organic C, total N and pH in soils of Uruguay. J. Near Infrared Spectrosc. 2002, 10, 215–221. [Google Scholar] [CrossRef]
- Terhoeven-Urselmans, T.; Schmidt, H.; Joergensen, R.G.; Ludwig, B. Usefulness of near-infrared spectroscopy to determine biological and chemical soil properties: Importance of sample pre-treatment. Soil Biol. Biochem. 2008, 40, 1178–1188. [Google Scholar] [CrossRef]
- He, Y.; Huang, M.; García, A.; Hernandez, A.; Song, H. Prediction of soil macronutrients content using near-infrared spectroscopy. Comput. Electron. Agric. 2007, 58, 144–153. [Google Scholar] [CrossRef]
- Liu, X.-M.; Liu, J.-S. Based on the LS-SVM modeling method determination of soil available N and available K by using near-infrared spectroscopy. Spectrosc. Spectr. Anal. 2012, 32, 3019–3023. [Google Scholar]
- Jia, S.; Yang, X.; Li, G.; Zhang, J. Quantitatively Determination of Available Phosphorus and Available Potassium in Soil by Near Infrared Spectroscopy Combining with Recursive Partial Least Squares. Spectrosc. Spectr. Anal. 2015, 35, 2516–2520. [Google Scholar]
- Confalonieri, M.; Fornasier, F.; Ursino, A.; Boccardi, F.; Pintus, B.; Odoardi, M. The potential of near infrared reflectance spectroscopy as a tool for the chemical characterization of agricultural soils. J. Near Infrared Spectrosc. 2001, 9, 123–131. [Google Scholar] [CrossRef]
- Ludwig, B.; Khanna, P.K.; Bauhus, J.; Hopmans, P. Near Infrared Spectroscopy of forest soils to determine chemical and biological properties related to soil sustainability. For. Ecol. Manag. 2002, 171, 121–132. [Google Scholar] [CrossRef]
- Oliveira, J.F.; de Brossard, M.; Corazza, E.J.; Marchao, R.L.; Siqueira Vendrame, P.R.; Brito, O.R.; de Fátima Guimaraes, M. VIR-NIR spectrometry, soil phosphate extraction methods and interactions of soil attributes. Quim Nova 2015, 38, 342–350. [Google Scholar]
- Zhang, L.; Zhang, R. Fast detection of inorganic phosphorus fractions and their phosphorus content in soil based on near-infrared spectroscopy. Chem. Eng. Trans. 2015, 46, 1405–1410. [Google Scholar]
- Rakotonindrina, H.; Kawamura, K.; Tsujimoto, Y.; Nishigaki, T.; Razakamanarivo, H.; Andrianary, B.H.; Andriamananjara, A. Prediction of soil oxalate phosphorus using visible and near-infrared spectroscopy in natural and cultivated system soils of madagascar. Agriculture 2020, 10, 177. [Google Scholar] [CrossRef]
- Niederberger, J.; Todt, B.; Boča, A.; Nitschke, R.; Kohler, M.; Kühn, P.; Bauhus, J. Use of near-infrared spectroscopy to assess phosphorus fractions of different plant availabilty in forest soils. Biogeosciences 2015, 12, 3415–3428. [Google Scholar] [CrossRef] [Green Version]
- Brolsma, K.M.; van Oostrum, M.J.; Elbers, H.L.; van Vliet, P.C.J. Soil phosphorus with NIRS. Eurofins Agro Rep. 2018, 30. (In Dutch) [Google Scholar]
- Van der Paauw, F.; Ris, J. A new K-number for arable crops on sand and reclaimed peat soils. Landbouwvoorlichting 1960, 19, 719–725. (In Dutch) [Google Scholar]
- Prummel, J. Autumn and spring fertilization with phosphorus and potassium on arable land. Landbouwkd. Tijdschr. 1962, 74, 252–260. (In Dutch) [Google Scholar]
- Boskma, K.; van der Heij, D. Potassium fertilization of potatoes on peaty soil. Versl. Landbouwkd. Onderz. 1964, 644. Available online: https://www.wur.nl/de/Publicatie-details.htm?publicationId=publication-way-353337323938 (accessed on 28 November 2021). (In Dutch).
- Harmsen, K. The Institute for Soil Fertility Research 1890–1990. Neth. J. Agric. Sci. 1991, 39, 205–220. [Google Scholar] [CrossRef]
- Bouma, J.; Hartemink, A.E. Soil science and society in the Dutch context. NJAS Wagen. J. Life Sci. 2003, 50, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Sims, J.T. Comparison of Mehlich 1 and Mehlich 3 extractants for P, K, Ca, Mg, Mn, Cu and Zn in Atlantic coastal plain soils. Commun. Soil Sci. Plant Anal. 1989, 20, 1707–1726. [Google Scholar] [CrossRef]
- Frostegård, Ǻ.A.; Tunlid, E.; Bååth, E. Use and misuse of PLFA measurements in soil. Soil Biol. Biochem. 2011, 43, 723–730. [Google Scholar] [CrossRef]
- Barthès, B.G.; Brunet, D.; Rabary, B.; Ba, O.; Villenave, C. Near infrared reflectance spectroscopy (NIRS) could be used for characterization of soil nematode community. Soil Biol. Biochem. 2011, 43, 1649–1659. [Google Scholar] [CrossRef]
- Comino, F.; Arand, V.; García-Ruizc, R.; Ayora-Cañada, M.J.; Domínguez-Vidala, A. Infrared spectroscopy as a tool for the assessment of soil biological quality in agricultural soils under contrasting management practices. Ecol. Indic. 2018, 87, 117–126. [Google Scholar] [CrossRef]
- Zimmermann, M.; Leifeld, J.; Fuhrer, J. Quantifying soil organic carbon fractions by infrared-spectroscopy. Soil Biol. Biochem. 2007, 39, 224–231. [Google Scholar] [CrossRef]
- Kusumo, B.H.; Arbestain, M.C.; Mahmud, A.F.; Hedley, M.J.; Hedley, C.B.; Pereira, R.C.; Wang, T.; Singh, B.P. Assessing biochar stability indices using Near Infrared Spectroscopy. J. Near Infrared Spectrosc. 2014, 22, 313–328. [Google Scholar] [CrossRef]
- Girona-García, A.; Badía-Villas, D.; Jiménez-Morillo, N.T.; González-Pérez, J.A. Changes in soil organic matter composition after Scots pine afforestation in a native European beech forest revealed by analytical pyrolysis (Py-GC/MS). Sci. Total Environ. 2019, 691, 1155–1161. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Vitousek, P. An experiment f or the world. Nature 2013, 497, 33–35. [Google Scholar] [CrossRef]
- Sutton, M.A.; Bleeker, A.; Howard, C.M.; Erisman, J.W.; Abrol, Y.P.; Bekunda, M.; Datta, A.; Davidson, E.; De Vries, W.; Oenema, O.; et al. Our Nutrient World. The Challenge to Produce More Food & Energy with Less Pollution; Centre for Ecology & Hydrology: Bailrigg, UK, 2013. [Google Scholar]
- Mohamed, E.S.; Saleh, A.M.; Belal, A.B.; Gad, A. Application of near-infrared reflectance for quantitative assessment of soil properties. Egypt. J. Remote Sens. Space Sci. 2018, 21, 1–14. [Google Scholar] [CrossRef]
- Chen, L.; Xing, L.; Han, L. Review of the application of near-infrared spectroscopy technology to determine the chemical composition of animal manure. J. Environ. Qual. 2013, 42, 1015–1028. [Google Scholar] [CrossRef]
- Rietra, R.P.J.J.; Oenema, O. Using NIRS to determine composition of solid manure. In Wageningen Environmental Research; Report 2837; Springer: Berlin/Heidelberg, Germany, 2017; Available online: https://edepot.wur.nl/423875 (accessed on 28 November 2021). (In Dutch)
- Galvez-Sola, L.; Moral, R.; Perez-Murcia, M.D.; Perez-Espinosa, A.; Bustamante, M.A.; Martinez-Sabater, E.; Paredes, C. The potential of near infrared reflectance spectroscopy (NIRS) for the estimation of agro-industrial compost quality. Sci. Total Environ. 2010, 408, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Van Vuuren, J.A.; Groenewald, C.A. Use of Scanning Near-Infrared Spectroscopy as a Quality Control Indicator for Bulk Blended Inorganic Fertilizers. Commun. Soil Sci. Plant Anal. 2013, 44, 120–135. [Google Scholar] [CrossRef]
- Prananto, J.A.; Minasny, B.; Weaver, T. Near infrared (NIR) spectroscopy as a rapid and cost-effective method for nutrient analysis of plant leaf tissues. Adv. Agron 2020, 164, 1–49. [Google Scholar]
- Park, R.S.; Agnew, R.E.; Gordon, F.J.; Steen, R.W.J. The use of near infrared reflectance spectroscopy (NIRS) on undried samples of grass silage to predict chemical composition and digestibility parameters. Anim. Feed Sci. Technol. 1998, 72, 155–167. [Google Scholar] [CrossRef]
- Aernouts, B.; Polshin, E.; Lammertyn, J.; Saeys, W. Visible and near-infrared spectroscopic analysis of raw milk for cow health monitoring: Reflectance or transmittance. Int. J. Dairy Sci. 2011, 94, 5315–5329. [Google Scholar] [CrossRef] [Green Version]
- López, A.; Arazuri, S.; García, I.; Mangado, J.; Jarén, C. A review of the application of near-infrared spectroscopy for the analysis of potatoes. J. Agric. Food Chem. 2013, 61, 5413–5424. [Google Scholar] [CrossRef] [Green Version]

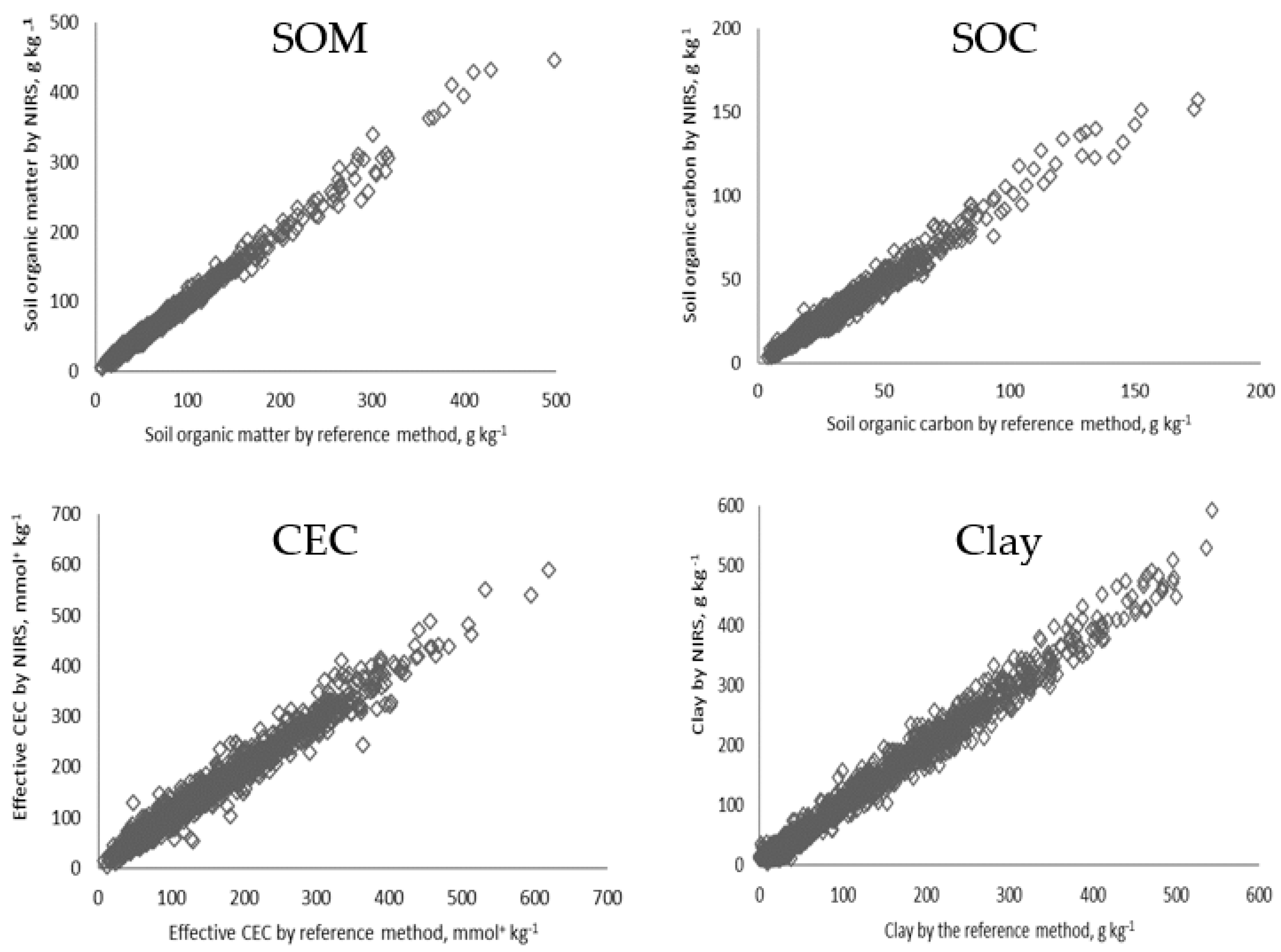
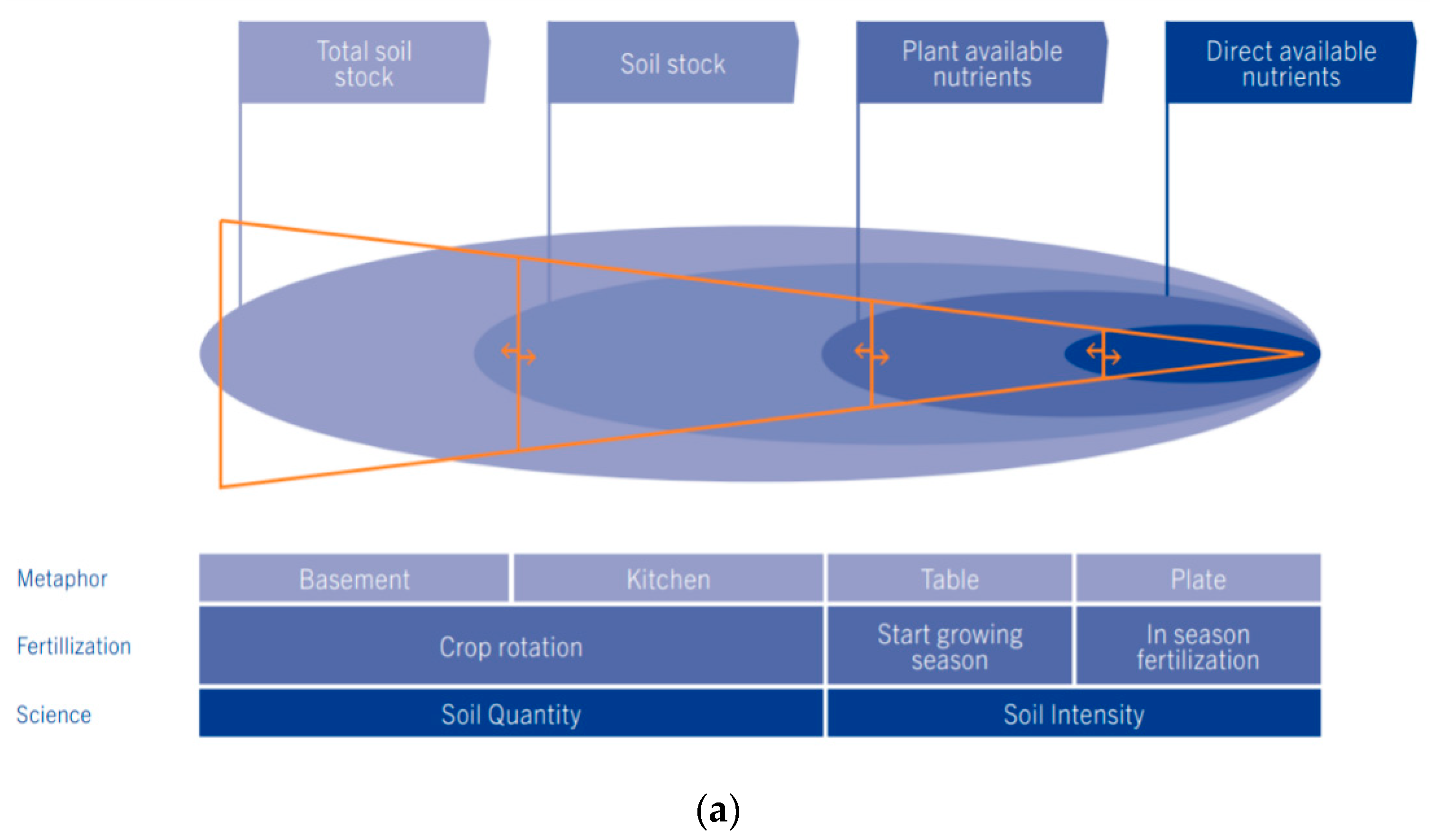
| Soil Characteristic | Year | Reporting | First | Median | Third | Average | St. Dev. | n | Unit | Analyses/ |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 M CaCl2 | Limit | Quartile | Quartile | Reference | ||||||
| DON | 2007 | 3.0 | 19 | 26 | 40 | 31.9 | 18.6 | 7175 | mg kg−1 | DA; NEN-ISO 15923-1 [27] |
| NH4-N | 2004 | 0.5 | 4.4 | 6.7 | 9.9 | 7.7 | 4.7 | 7175 | mg kg−1 | DA; NEN-ISO 15923-1 [27] |
| NO3-N | 2004 | 1.2 | 3.4 | 6.3 | 14 | 11.2 | 12.2 | 7175 | mg kg−1 | DA; NEN-ISO 15923-1 [27] |
| S | 2017 | 2.0 | 3.7 | 5.5 | 10.1 | 18.4 | 78 | 16,085 | mg kg−1 | ICP-AES; NEN 6966 [28] |
| P | 2004 | 0.2 | 1.1 | 2.0 | 3.8 | 3.0 | 3.2 | 326,957 | mg kg−1 | DA; NEN-ISO 15923-1 [27] |
| K | 2004 | 8.0 | 58 | 83 | 121 | 100 | 67 | 310,417 | mg kg−1 | ICP-AES; NEN 6966 [28] |
| Mg | 2004 | 4.5 | 76 | 127 | 216 | 170 | 132 | 310,048 | mg kg−1 | ICP-AES; NEN 6966 [28] |
| Na | 2004 | 5.0 | 9.0 | 15 | 27 | 25 | 46 | 239,181 | mg kg−1 | ICP-AES; NEN 6966 [28] |
| Si | 2012 | 3.0 | 4.1 | 6.3 | 12 | 13 | 16 | 45,750 | mg kg−1 | ICP-MS; NEN 17294-2 [29] |
| Fe | 2012 | 2.0 | 2.1 | 3.0 | 3.1 | 3.4 | 2.4 | 45,724 | mg kg−1 | ICP-AES; NEN 6966 [28] |
| Zn | 2004 | 0.1 | 0.4 | 1.3 | 2.2 | 1.5 | 1.5 | 66,541 | mg kg−1 | ICP-MS; NEN 17294-2 [29] |
| Mn | 2004 | 0.25 | 1.5 | 4.1 | 8.1 | 6.2 | 7.4 | 80,001 | mg kg−1 | ICP-AES; NEN 6966 [28] |
| Cu | 2004 | 20 | 21 | 30 | 43 | 37 | 34 | 73,128 | ug kg−1 | ICP-MS; NEN 17294-2 [29] |
| Co | 2004 | 2.5 | 4.6 | 10 | 21 | 17 | 24 | 58,556 | ug kg−1 | ICP-MS; NEN 17294-2 [29] |
| B | 2004 | 75 | 103 | 145 | 219 | 203 | 197 | 72,897 | ug kg−1 | ICP-AES; NEN 6966 [28] |
| Mo | 2012 | 3.0 | 3.8 | 4.0 | 4.2 | 4.9 | 5.2 | 45,725 | ug kg−1 | ICP-MS; NEN 17294-2 [29] |
| Se | 2008 | 2.0 | 2.1 | 2.8 | 3.9 | 3.5 | 2.5 | 58,528 | ug kg−1 | ICP-MS; NEN 17294-2 [29] |
| pH | 2004 | - | 5.0 | 5.3 | 6.2 | 5.6 | 0.90 | 354,187 | - | Potentiometric ISO 10390 [30] |
| Soil Characteristic | Calibration | |||||||
|---|---|---|---|---|---|---|---|---|
| n | year | R2 | RPD | RMSE | Bias | Sres | Reference | |
| N-total | 55,947 | 2004 | 0.99 | 8.6 | 0.53 | 0.002 | 0.53 | ISO 13878 [43]; NEN 6966 [28] |
| S-total | 37,783 | 2004 | 0.97 | 5.5 | 0.21 | −0.000 | 0.21 | NEN 15587-2 [44]; NEN 6966 [28] |
| K-CEC | 16,144 | 2006 | 0.79 | 2.0 | 2.19 | −0.040 | 2.19 | ISO 23470 [45]; NEN 6966 [28] |
| Ca-CEC | 15,742 | 2006 | 0.97 | 5.5 | 17.53 | 0.483 | 17.52 | ISO 23470 [45]; NEN 6966 [28] |
| Mg-CEC | 15,732 | 2006 | 0.88 | 2.7 | 6.32 | −0.015 | 6.32 | ISO 23470 [45]; NEN 6966 [28] |
| pH-CaCl2 | 89,075 | 2013 | 0.97 | 5.3 | 0.18 | −0.004 | 0.18 | Potentiometric ISO 10390 [30] |
| Soil organic carbon (SOC) | 21,976 | 2004 | 0.99 | 12.9 | 4.93 | 0.066 | 4.93 | ISO 10694 [46] |
| Soil organic matter (SOM) | 24,825 | 2004 | 1.00 | 17.5 | 6.46 | 0.007 | 6.46 | NEN 5754 [47] |
| Soil inorganic carbon (SIC) | 15,864 | 2004 | 0.97 | 5.6 | 1.45 | 0.001 | 1.45 | NEN-EN 15936 [48] |
| Clay (<2 µm) | 49,121 | 2004 | 0.98 | 7.0 | 17.99 | 0.664 | 17.97 | NEN 5753 [49] |
| Sand (>50 µm <2000 µm) | 8,419 | 2015 | 0.96 | 4.7 | 58.39 | 1.390 | 58.37 | NEN 5753 [49] |
| Effective CEC (ECEC) | 16,122 | 2005 | 0.97 | 5.8 | 20.44 | 0.125 | 20.44 | ISO 23470 [45], 2018; NEN 6966 [28] |
| Element | Conventional Method (CM) | Reference of CM | R2 | Reference Step 2 |
|---|---|---|---|---|
| P | Pw; 1: 60 (v/v) water | Van der Paauw, 1971 [50] | 0.79–0.93 | Houba et al., 1986 [51]; De Haas et al., 2005 [52]; Reijneveld et al., 2014 [22] |
| K | HCl; 1: 10 (w/v) 0.1 M hydrochloric acid–0.02M oxalic acid | De Vries & Hetterschij, 1945 [53]; Anonymous, 2012a; 2012b [54,55] | 0.77–0.90 | Houba et al., 1986 [51]; Baier & Baierova, 1998 [56]; De Haas et al. [52] |
| Mg | NaCl; 1: 5 (w/v) 0.5 M sodiumchloride | Ferrari & Sluijsmans, 1995 [57]; Anonymous, 2012a; 2012b [54,55] | 0.88–0.97 | Loch et al., 1998 [58]; Van Erp et al., 2001 [59] |
| Na | HCl; 1: 10 (w/v) hydrochloric acid–oxalic acid | Anonymous, 2012b [54] | 0.82–0.93 | De Haas et al., 2005 [52] |
| Mn | Mn-reducible; 1: 20 (w/v) ammonium acetate–1N hydroquinone | Anonymous, 2012a [55] | 0.95 | De Haas et al., 2005 [52] |
| Cu | HNO3; 1: 10 (w/v) 0.43 M Nitric acid | Henkens, 1961 [60]; Anonymous, 2012a; 2012b [14,54] | 0.87 | De Haas et al., 2005 [52] |
| Co | 1: 40 (w/v) 0.4 M Acetic acid | Henkens, 1959 [61]; Anonymous, 2012b [54] | 088 | De Haas et al., 2005 [52] |
| B | Hot water; 1: 10 (w/v) hot water | Berger & Truog 1939 [62] | 0.74 | Novozamsky et al., 1990 [63] |
| pH | KCl; 1: 5 (v/v) 1 M potassiumchloride | Anonymous, 2012a; 2012b [54,55] | 0.98 | Houba et al., 1990 [24]; Fotyma et al., 1998 [64] |
| Soil Characteristic | Reference versus NIRS | Reference vs. Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | β | R2 | RPD | RMSE | Bias | SE | F | p (0.05) | R2 | RMSE | |
| N-total | 1502 | 0.94 | 0.97 | 5.4 | 0.26 | −0.05 | 0.01 | 0.98 | 0.32 | 0.98 | 0.21 |
| S-total | 1497 | 0.93 | 0.90 | 3.0 | 0.11 | −0.01 | 0.00 | 0.79 | 0.38 | 0.95 | 0.08 |
| K-CEC | 1934 | 1.05 | 0.69 | 1.4 | 1.68 | 0.09 | 0.04 | 1.17 | 0.28 | 0.99 | 0.28 |
| Ca-CEC | 1930 | 0.94 | 0.97 | 5.7 | 14.21 | −0.89 | 0.32 | 0.11 | 0.74 | 0.99 | 5.62 |
| Mg-CEC | 1922 | 1.01 | 0.91 | 3.1 | 4.61 | −0.05 | 0.11 | 0.01 | 0.92 | 0.99 | 0.96 |
| pH | 1843 | 0.94 | 0.95 | 4.4 | 0.21 | −0.04 | 0.00 | 1.72 | 0.19 | 0.99 | 0.07 |
| SOC | 1840 | 0.98 | 0.98 | 6.4 | 2.98 | −0.12 | 0.07 | 0.04 | 0.85 | 0.98 | 3.11 |
| SOM | 2259 | 0.99 | 0.99 | 10.6 | 4.62 | −0.02 | 0.10 | 0.00 | 0.99 | 0.99 | 4.14 |
| SIC | 1863 | 0.98 | 0.96 | 5.1 | 0.61 | −0.11 | 0.01 | 1.22 | 0.27 | 0.99 | 0.33 |
| Clay (<2 µm) | 1852 | 0.98 | 0.99 | 8.5 | 13.24 | 0.45 | 0.31 | 0.02 | 0.90 | 0.99 | 8.20 |
| Sand (>50 µm) | 1796 | 0.95 | 0.97 | 5.3 | 46.73 | 4.91 | 1.10 | 0.34 | 0.56 | 0.99 | 15.71 |
| ECEC | 1981 | 0.95 | 0.97 | 6.0 | 15.26 | 0.35 | 0.34 | 0.01 | 0.90 | 0.99 | 7.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reijneveld, J.A.; van Oostrum, M.J.; Brolsma, K.M.; Fletcher, D.; Oenema, O. Empower Innovations in Routine Soil Testing. Agronomy 2022, 12, 191. https://doi.org/10.3390/agronomy12010191
Reijneveld JA, van Oostrum MJ, Brolsma KM, Fletcher D, Oenema O. Empower Innovations in Routine Soil Testing. Agronomy. 2022; 12(1):191. https://doi.org/10.3390/agronomy12010191
Chicago/Turabian StyleReijneveld, Jan Adriaan, Martijn Jasper van Oostrum, Karst Michiel Brolsma, Dale Fletcher, and Oene Oenema. 2022. "Empower Innovations in Routine Soil Testing" Agronomy 12, no. 1: 191. https://doi.org/10.3390/agronomy12010191
APA StyleReijneveld, J. A., van Oostrum, M. J., Brolsma, K. M., Fletcher, D., & Oenema, O. (2022). Empower Innovations in Routine Soil Testing. Agronomy, 12(1), 191. https://doi.org/10.3390/agronomy12010191






