Soil and Plant Responses to Phosphorus Inputs from Different Phytase-Associated Animal Diets
Abstract
:1. Introduction
2. Methods and materials
2.1. Animal Trials
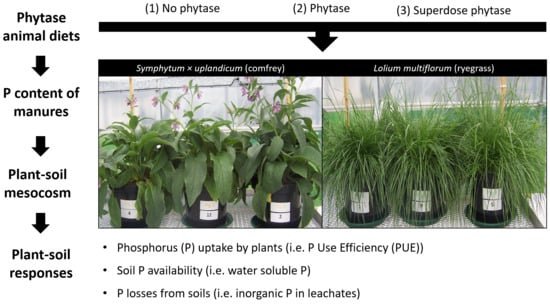
2.2. Mesocosm Experiment
2.3. Aboveground Plant Parameters
2.4. Soil and Root Parameters
2.5. Microbiota Profiling
2.6. Data Analysis
3. Results
3.1. Effects of Animal Diet on the P Content of Animal Manures
3.2. Effects of Animal Organic Amendments on Plant Biomass and P Mass
3.3. Effects of Animal Diet on Plant Phosphorus Use Efficiency (PUE)
3.4. Effects of Animal Diet on Soil P Availability
3.5. Effects of Animal Diet on P Loss in Leachates
3.6. Effects of Plant Species, Organic Amendment, and Animal Feed on Soil Microbiota Profiles
4. Discussion
4.1. Dietary Phytase Effects on P Excretion
4.2. Dietary Phytase Effects on the Plant–Soil System
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouwman, L.; Goldewijk, K.K.; van der Hoek, K.W.; Beusen, A.H.W.; van Vuuren, D.P.; Willems, J.; Rufino, M.C.; Stehfest, E. Exploring global changes in nitrogen and phosphorus cycles in agriculture induced by livestock production over the 1900–2050 period. Proc. Natl Acad. Sci. USA 2013, 110, 20882–20887. [Google Scholar] [CrossRef] [Green Version]
- Lun, F.; Liu, J.; Ciais, P.; Nesme, T.; Chang, J.; Wang, R.; Goll, D.; Sardans, J.; Peñuelas, J.; Obersteiner, M. Global and regional phosphorus budgets in agricultural systems and their implications for phosphorus-use efficiency. Earth Syst. Sci. Data 2018, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, G.K.; Bennett, E.M.; Potter, P.A.; Ramankutty, N. Agronomic phosphorus imbalances across the world’s croplands. Proc. Natl. Acad. Sci. USA 2011, 108, 3086–3091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fornara, D.A.; Flynn, D.; Caruso, T. Improving phosphorus sustainability in intensively managed grasslands: The potential role of arbuscular mycorrhizal fungi. Sci. Total Environ. 2020, 706, 135744. [Google Scholar] [CrossRef] [PubMed]
- Oster, M.; Reyer, H.; Ball, M.E.; Fornara, D.A.; McKillen, J.P.; Sørensen, K.U.; Poulsen, H.D.; Andersson, K.; Ddiba, D.; Rosemarin, A.; et al. Multi-actor approaches to bridge gaps in the agricultural phosphorus cycle from an animal husbandry perspective—The case of pigs and poultry. Sustainability 2018, 10, 1825. [Google Scholar] [CrossRef] [Green Version]
- Withers, P.J.A.; Forber, K.G.; Lyon, C.; Rothwell, S.; Doody, D.G.; Jarvie, H.P.; Martin-Ortega, J.; Jacobs, B.; Cordell, D.; Patton, M.; et al. Towards resolving the phosphorus chaos created by food systems. Ambio 2020, 49, 1076–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnett, G. Phosphorus forms in animal manure. Bioresour. Technol. 1994, 49, 139–147. [Google Scholar] [CrossRef]
- Poulsen, H.; Jongbloed, A.; Latimier, P.; Fernández, J. Phosphorus consumption, utilisation and losses in pig production in France, The Netherlands and Denmark. Livest. Prod. Sci. 1999, 58, 251–259. [Google Scholar] [CrossRef]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; Food and Agriculture Organizations of the United Nations: Rome, Italy, 2006; p. 390. [Google Scholar]
- Barquet, K.; Järnberg, L.; Rosemarin, A.; Macura, B. Identifying barriers and opportunities for a circular phosphorus economy in the Baltic Sea region. Water Res. 2020, 171, 115433. [Google Scholar] [CrossRef]
- Herrera-Estrella, L.; López-Arredondo, D. Phosphorus: The underrated element for feeding the world. Trends Plant Sci. 2016, 21, 461–463. [Google Scholar] [CrossRef]
- Oster, M.; Reyer, H.; Keiler, J.; Ball, E.; Mulvenna, C.; Muráni, E.; Ponsuksili, S.; Wimmers, K. Comfrey (Symphytum spp.) as an alternative field crop contributing to closed agricultural cycles in chicken feeding. Sci. Total Environ. 2020, 742, 140490. [Google Scholar] [CrossRef]
- Oster, M.; Reyer, H.; Keiler, J.; Ball, E.; Mulvenna, C.; Ponsuksili, S.; Wimmers, K. Comfrey (Symphytum spp.) as a feed supplement in pig nutrition contributes to regional resource cycles. Sci. Total Environ. 2021, 796, 148988. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, H.D. Reduced Dietary Phosphorus for Growing and Finishing Pigs. Effect on Performance, Retention and Excretion; Sci. Rep. 28; National Institute of Animal: Copenhagen, Denmark, 1994; Volume 29. [Google Scholar]
- Jeroch, H.; Drochner, W.; Simon, O. Ernährung landwirtschaftlicher Nutztiere; 2. Auflage; Verlag Eugen Ulmer KG: Stuttgart, Germany, 2008; ISBN 978-3-8252-8180-8189. [Google Scholar]
- Sauvant, D. Tables of Composition and Nutritional Value of Feed Materials; Sauvant, D., Perez, J.M., Tran, G., Eds.; Wageningen Academic Publisher: Gelderland, The Netherlands; INRA: Paris, France, 2004; ISBN 2-7380-1158-6. [Google Scholar]
- Dersjant-Li, Y.; Awati, A.; Schulze, H.; Partridge, G. Phytase in non-ruminant animal nutrition: A critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 2015, 95, 878–896. [Google Scholar] [CrossRef] [Green Version]
- Jongbloed, A.W.; Mroz, Z.; Kemme, P.A. The effect of supplementary Aspergillus niger phytase in diets for pigs on concentration and apparent digestibility of dry matter, total phosphorus, and phytic acid in different sections of the alimentary tract. J. Anim. Sci. 1992, 70, 1159–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selle, P.H.; Cowieson, A.J.; Cowieson, N.P.; Ravindran, V. Protein-phytate inter-actions in pig and poultry nutrition: A reappraisal. Nutr. Res. Rev. 2012, 1, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.F.; Kornegay, E.T.; Schell, T.C. Phytase supplementation of low-phosphorus growing-finishing pig diets improves performance, phosphorus digestibility, and bone mineralization and reduces phosphorus excretion. J. Anim. Sci. 1997, 75, 3174–3186. [Google Scholar] [CrossRef]
- Baxter, C.A.; Joern, B.C.; Ragland, D.; Sands, J.S.; Adeola, O. Phytase, High-Available-Phosphorus Corn, and Storage Effects on Phosphorus Levels in Pig Excreta. J. Environ. Qual. 2003, 32, 1481–1489. [Google Scholar] [CrossRef]
- Pillai, U.P.P.; Manoharan, V.; Lisle, A.; Li, X.; Bryden, W. Phytase Supplemented Poultry Diets Affect Soluble Phosphorus and Nitrogen in Manure and Manure-amended Soil. J. Environ. Qual. 2009, 38, 1700–1708. [Google Scholar] [CrossRef]
- Maguire, R.O.; Sims, J.T.; Saylor, W.W.; Turner, B.L.; Angel, R.; Applegate, T.J. Influence of Phytase Addition to Poultry Diets on Phosphorus Forms and Solubility in Litters and Amended Soils. J. Environ. Qual. 2004, 33, 2306–2316. [Google Scholar] [CrossRef]
- Yitbarek, A.; López, S.; Tenuta, M.; Asgedom, H.; France, J.; Nyachoti, C.M.; Kebreab, E. Effect of dietary phytase supplementation on greenhouse gas emissions from soil after swine manure application. J. Clean. Prod. 2017, 166, 1122–1130. [Google Scholar] [CrossRef]
- Gilley, J.E.; Eghball, B.; Wienhold, B.J.; Miller, P.S. Nutrients in runoff following that application of swine manure to inter-rill areas. Trans. Am. Soc. Agric. Eng. 2001, 44, 1651–1659. [Google Scholar] [CrossRef]
- Toor, G.S.; Sims, J.T. Phosphorus Leaching in Soils Amended with Animal Manures Generated from Modified Diets. J. Environ. Qual. 2016, 45, 1385–1391. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Sorn-Srivichai, P.; Syers, J.K.; Tillman, R.W.; Cornforth, I.S. An evaluation of water extraction as a soil-testing procedure for phosphorus II. Factors affecting the amounts of water-extractable phosphorus in field soils. Fertil. Res. 1988, 15, 225–236. [Google Scholar] [CrossRef]
- Oster, M.; Reyer, H.; Gerlinger, C.; Trakooljul, N.; Siengdee, P.; Keiler, J.; Ponsuksili, S.; Wolf, P.; Wimmers, K. mRNA Profiles of Porcine Parathyroid Glands Following Variable Phosphorus Supplies throughout Fetal and Postnatal Life. Biomedecines 2021, 9, 454. [Google Scholar] [CrossRef]
- Hugerth, L.W.; Wefer, H.A.; Lundin, S.; Jakobsson, H.E.; Lindberg, M.; Rodin, S.; Engstrand, L.; Andersson, A.F. DegePrime, a Program for Degenerate Primer Design for Broad-Taxonomic-Range PCR in Microbial Ecology Studies. Appl. Environ. Microbiol. 2014, 80, 5116–5123. [Google Scholar] [CrossRef] [Green Version]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.-A. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Long, W.; Chadwick, D.; Velthof, G.L.; Oenema, O.; Ma, W.; Wang, J.; Qin, W.; Hou, Y.; Zhang, F. Can dietary manipulations improve the productivity of pigs with lower environmental and economic cost? A global meta-analysis. Agric. Ecosyst. Environ. 2020, 289, 106748. [Google Scholar] [CrossRef]
- Vadas, P.A.; Meisinger, J.J.; Sikora, L.J.; McMurtry, J.P.; Sefton, A.E. Effect of Poultry Diet on Phosphorus in Runoff from Soils Amended with Poultry Manure and Compost. J. Environ. Qual. 2004, 33, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Reyer, H.; Oster, M.; Wittenburg, D.; Murani, E.; Ponsuksili, S.; Wimmers, K. Genetic Contribution to Variation in Blood Calcium, Phosphorus, and Alkaline Phosphatase Activity in Pigs. Front. Genet. 2019, 10, 590. [Google Scholar] [CrossRef] [PubMed]
- Mulvenna, C.C.; McCormick, U.; McKillen, J.; Bedford, M.R. Effect of altering broiler diet ingredients and phytase content on nitrogen and phosphorus excretion. In Proceedings of the European Federation of Animal Science, EAAP, Davos, Switzerland, 29 August–3 September 2021. [Google Scholar]
- Mulvenna, C.C.; McCormick, U.; McKillen, J.; Bedford, M.R. The use of alternative feed ingredients plus phytase on the nitrogen and phosphorus balance of pigs. In Proceedings of the European Federation of Animal Science, EAAP, Davos, Switzerland, 29 August–3 September 2021. [Google Scholar]
- Humer, E.; Schwarz, C.; Schedle, K. Phytate in pig and poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2015, 99, 605–625. [Google Scholar] [CrossRef] [PubMed]
- Pintar, J.; Homen, B.; Gazić, K.; Janječić, Z.; Sikirić, M.; Černy, T. Effects of supplemental phytase on nutrient excretion and retention in broilers fed different cereal based diets. Czech J. Anim. Sci. 2005, 50, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Ylivainio, K.; Turtola, E. Knowledge Report: Solubility and Plant-Availability of P in Manure. Available online: https://jukuri.luke.fi/bitstream/handle/10024/520267/Solubility_and_availability_of_P_in_manure_2015.pdf?sequence=1 (accessed on 6 July 2021).
- McGrath, J.M.; Sims, J.T.; Maguire, R.O.; Saylor, W.W.; Angel, C.R.; Turner, B.L. Broiler Diet Modification and Litter Storage. J. Environ. Qual. 2005, 34, 1896–1909. [Google Scholar] [CrossRef]
- Applegate, T.J.; Joern, B.C.; Nussbaum-Wagler, D.L.; Angel, R. Water-soluble phosphorus in fresh broiler litter is dependent upon phosphorus concentration fed but not on fungal phytase supplementation. Poult. Sci. 2003, 82, 1024–1029. [Google Scholar] [CrossRef]
- Manangi, M.K.; Sands, J.S.; Coon, C.N. Effect of Adding Phytase to Broiler Diets Containing Low and High Phytate Phosphorus: 1. Performance, Phytate P Hydrolysis, Tibia Ash, Litter Phosphorus and Ca and P Digestion and Retention. Int. J. Poult. Sci. 2009, 8, 919–928. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.R.; Moore, P.A., Jr.; Miles, D.M. Soil extractable phosphorus changes with time after application of fertilizer: I. Litter from poultry-fed modified diets. Soil Sci. 2005, 170, 530–542. [Google Scholar] [CrossRef]
- Angel, C.R.; Powers, W.J.; Applegate, T.J.; Tamim, N.M.; Christman, M.C. Influence of Phytase on Water-Soluble Phosphorus in Poultry and Swine Manure. J. Environ. Qual. 2005, 34, 563–571. [Google Scholar] [CrossRef]
- Ikoyi, I.; Egeter, B.; Chaves, C.; Ahmed, M.; Fowler, A.; Schmalenberger, A. Responses of soil microbiota and nematodes to application of organic and inorganic fertilizers in grassland columns. Biol. Fertil. Soils 2020, 56, 647–662. [Google Scholar] [CrossRef]
- Kumaragamage, D.; Akinremi, O.O. Manure Phosphorus: Mobility in Soils and Management Strategies to Minimize Losses. Curr. Pollut. Rep. 2018, 4, 162–174. [Google Scholar] [CrossRef]
- Hacquard, S.; Garrido-Oter, R.; González, A.; Spaepen, S.; Ackermann, G.; Lebeis, S.; McHardy, A.C.; Dangl, J.L.; Knight, R.; Ley, R.; et al. Microbiota and Host Nutrition across Plant and Animal Kingdoms. Cell Host Microbe 2015, 17, 603–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jendza, J.A.; Adeola, O. Water-soluble phosphorus excretion in pigs fed diets supplemented with microbial phytase. Anim. Sci. J. 2009, 80, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Ball, M.E.E.; Magowan, E.; McCracken, K.J.; Beattie, V.E.; Bradford, R.; Gordon, F.J.; Robinson, M.J.; Smyth, S.; Henry, W. The Effect of Level of Crude Protein and Available Lysine on Finishing Pig Performance, Nitrogen Balance and Nutrient Digestibility. Asian-Australas. J. Anim. Sci. 2013, 26, 564–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Phosphorus Content | Nitrogen Content | |||
|---|---|---|---|---|
| Pig Slurry (% DM) | Broiler Litter (% DM) | Pig Slurry (% DM) | Broiler Litter (% DM) | |
| By-product diet + superdose phytase | 1.34 ± 0.21 | 0.85 ± 0.07 | 8.89 ± 0.31 | 5.03 ± 0.48 |
| By-product diet + phytase | 1.76 ± 0.31 | 0.96 ± 0.06 * | 8.50 ± 0.31 | 4.89 ± 0.34 |
| By-product diet no-phytase | 1.42 ± 0.22 | 0.84 ± 0.10 | 8.95 ± 0.22 | 4.60 ± 0.35 |
| Pig Slurry | Broiler Litter | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comfrey | Ryegrass | Comfrey | Ryegrass | |||||||||||||
| Control | No Phytase | Phytase | Super-phytase | Control | No Phytase | Phytase | Super-phytase | Control | No Phytase | Phytase | Super- phytase | Control | No Phytase | Phytase | Super-Phytase | |
| Shoot biomass (g) | 10.2 ± 5.63 c | 74.2 ± 4.35 a | 77.4 ± 5.76 a | 64.5 ± 5.16 b | 1.93 ± 1.25 b | 55 ± 4.32 a | 54.8 ± 4.66 a | 54.5 ± 5.13 a | 10.2 ± 5.6 b | 21 ± 2.3 a | 22.3 ± 2.7 a | 21.5 ± 5.1 a | 1.93 ± 1.2 b | 4.5 ± 0.43 a | 4.7 ± 0.46 a | 4.6 ± 0.51 a |
| Shoot P mass (g) | 0.02 ± 0.01 c | 0.8 ± 0.01 b | 0.14 ± 0.01 a | 0.8 ± 0.01 b | 0.02 ± 0.01 | 0.1 ± 0.01 | 0.11 ± 0.01 | 0.2 ± 0.01 | 0.02 ± 0.01 | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.05 ± 0.01 | 0.02 ± 0.01 | 0.007 ± 0.001 | 0.008 ± 0.001 | 0.008 ± 0.001 |
| Root biomass (g) | 2.2 ± 1.62 b | 9.73 ± 2.3 a | 6.1 ± 1.7 a | 5.75 ± 5.1 ab | 0.7 ± 0.3 c | 2.85 ± 0.8 a | 1.32 ± 0.6 b | 1.35 ± 0.7 b | 2.2 ± 1.6 b | 6.93 ± 3.3 a | 7.46 ± 2.7 a | 10 ± 3.1 a | 0.7 ± 0.3 b | 1.29 ± 0.23 a | 1.33 ± 0.3 a | 1.08 ± 0.25 a |
| Root P mass (g) | 0.3 ± 0.49 | 2.7 ± 0.5 | 1.33 ± 0.7 | 1.27 ± 0.4 | 0.08 ± 0.01 b | 0.47 ± 0.5 a | 0.23 ± 0.7 a | 0.34 ± 0.4 a | 0.3 ± 0.49 b | 1.57 ± 0.56 a | 1.18 ± 0.73 a | 2.1 ± 0.68 a | 0.08 ± 0.01 b | 0.13 ± 0.02 a | 0.14 ± 0.03 a | 0.12 ± 0.03 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fornara, D.; Ball, E.M.E.; Mulvenna, C.; Reyer, H.; Oster, M.; Wimmers, K.; Damgaard Poulsen, H.; Rosemarin, A. Soil and Plant Responses to Phosphorus Inputs from Different Phytase-Associated Animal Diets. Agronomy 2022, 12, 130. https://doi.org/10.3390/agronomy12010130
Fornara D, Ball EME, Mulvenna C, Reyer H, Oster M, Wimmers K, Damgaard Poulsen H, Rosemarin A. Soil and Plant Responses to Phosphorus Inputs from Different Phytase-Associated Animal Diets. Agronomy. 2022; 12(1):130. https://doi.org/10.3390/agronomy12010130
Chicago/Turabian StyleFornara, Dario, Elizabeth M. E. Ball, Christina Mulvenna, Henry Reyer, Michael Oster, Klaus Wimmers, Hanne Damgaard Poulsen, and Arno Rosemarin. 2022. "Soil and Plant Responses to Phosphorus Inputs from Different Phytase-Associated Animal Diets" Agronomy 12, no. 1: 130. https://doi.org/10.3390/agronomy12010130