Catch Crops: A Nutrient Reservoir in Post-Harvest Residues under Water Deficit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Conditions
2.2. Plant Residue and Soil Sampling
2.3. Plant Residue and Soil Analysis
2.4. Calculations and Statistical Analysis
3. Results and Discussion
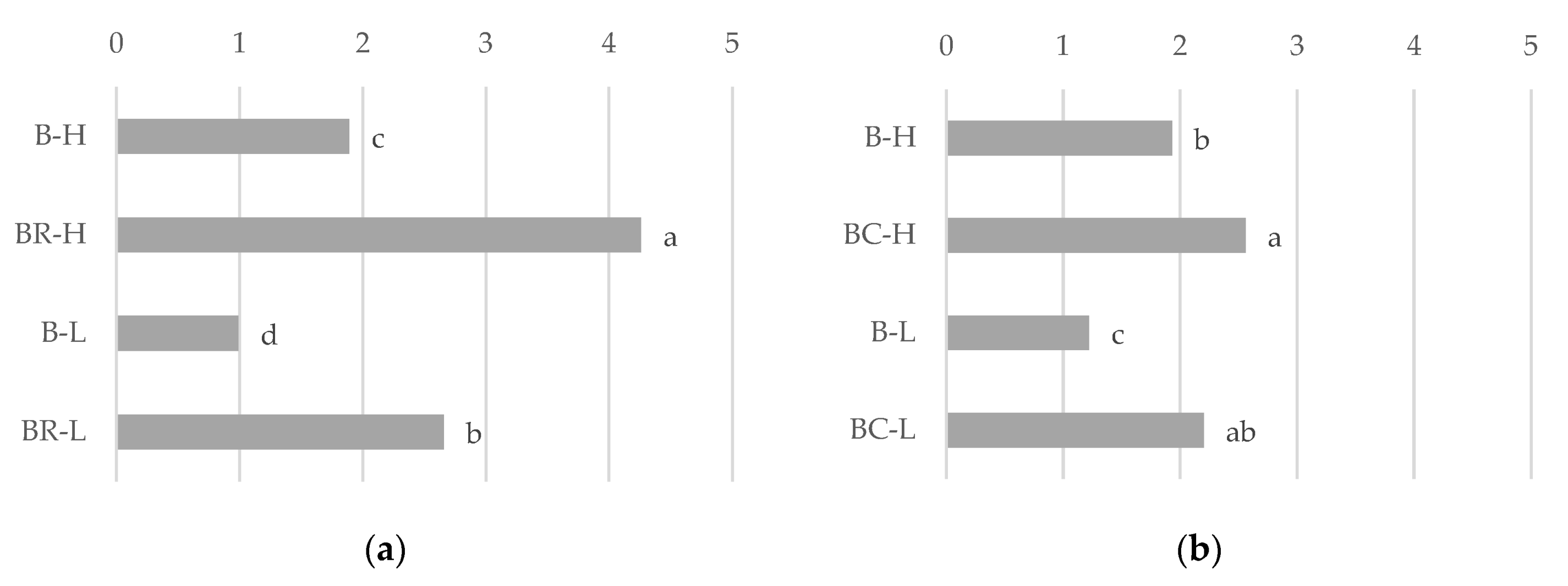
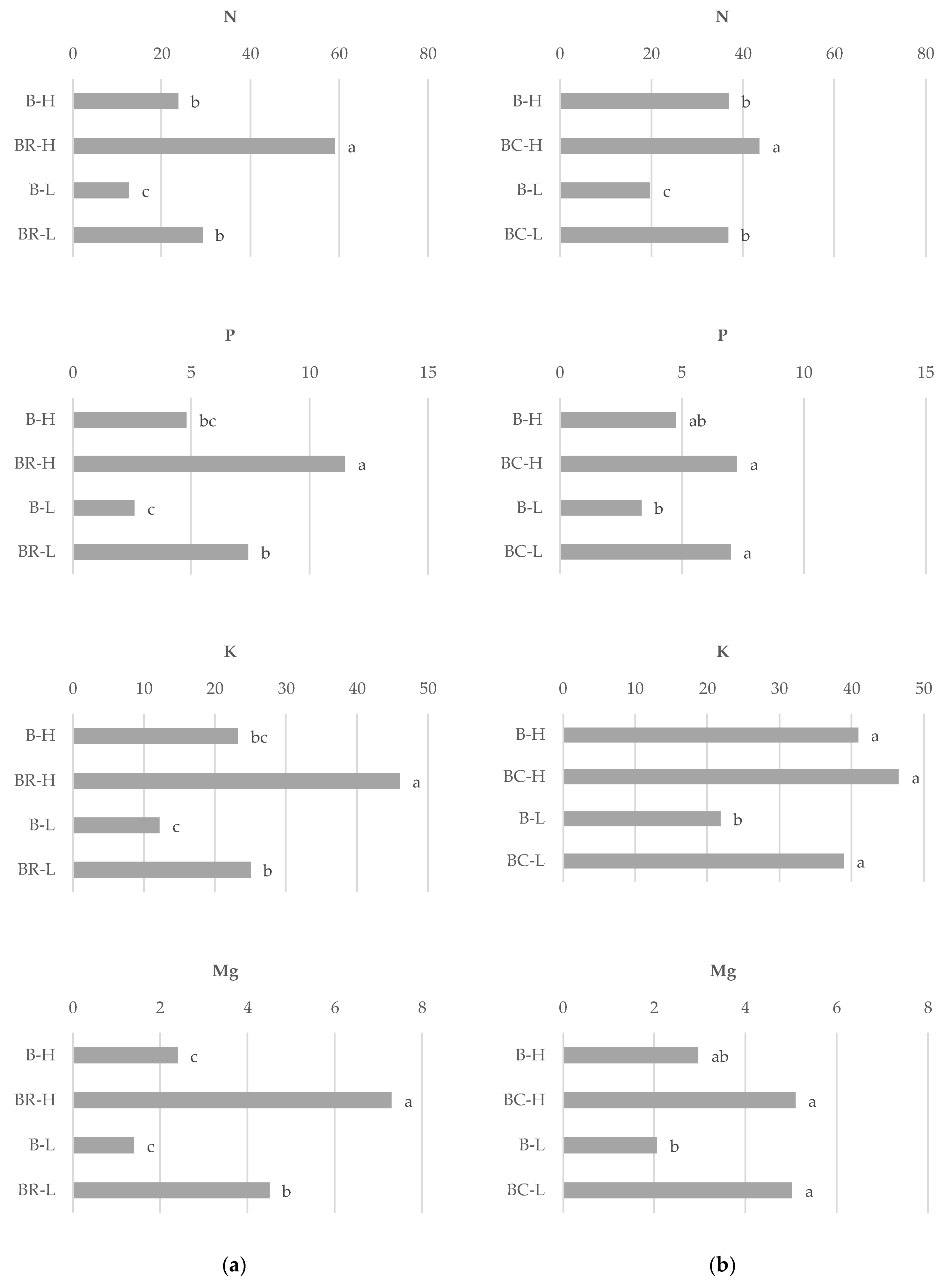
3.1. Nutrients in Post-Harvest Residues
3.2. Nutrients in the Soil
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uddin, M.N.; Bari, M.L. Governmental policies and regulations including FSMA on organic farming in the United States and around the globe. In Safety and Practice for Organic Food; Biswas, D., Micallef, S.A., Eds.; Academic Press: London, UK, 2019; pp. 33–62. [Google Scholar]
- Dar, N.A.; Lone, B.A.; Alaie, B.A.; Dar, Z.A.; Gulzafar; Bahar, F.A.; Haque, S.A.; Singh, K.N. Integrated farming systems for sustainable agriculture. In Eco-Friendly Agro-Biological Techniques for Enhancing Crop Productivity; Sengar, R.S., Singh, A., Eds.; Springer: Singapore, 2018; pp. 111–127. [Google Scholar]
- Garibaldi, L.A.; Pérez-Méndez, N.; Garratt, M.P.D.; Gemmill-Herren, B.; Miguez, F.E.; Dicks, L.V. Policies for ecological intensification of crop production. Trends Ecol. Evol. 2019, 34, 282–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abegunde, V.O.; Sibanda, M.; Obi, A. Determinants of the adoption of climate-smart agricultural practices by small-scale farming households in King Cetshwayo district municipality, South Africa. Sustainability 2020, 12, 195. [Google Scholar] [CrossRef] [Green Version]
- Kassam, A.; Friedrich, T.; Shaxson, F.; Pretty, J. The spread of conservation agriculture: Justification, sustainability and uptake. Int. J. Agric. Sustain. 2009, 7, 292–320. [Google Scholar] [CrossRef]
- Shackelford, G.E.; Kelsey, R.; Dicks, L.V. Effects of cover crops on multiple ecosystem services: Ten meta-analyses of data from arable farmland in California and the Mediterranean. Land Use Policy 2019, 88, 104204. [Google Scholar] [CrossRef]
- Valkama, E.; Lemola, R.; Känkänen, H.; Turtola, E. Meta-analysis of the effects of undersown catch crops on nitrogen leaching loss and grain yields in the Nordic countries. Agric. Ecosyst. Environ. 2015, 203, 93–101. [Google Scholar] [CrossRef]
- Lizarazo, C.I.; Tuulos, A.; Jokela, V.; Mäkelä, P.S.A. Sustainable mixed cropping systems for the boreal-nemoral region. Front. Sustain. Food Syst. 2020, 4. [Google Scholar] [CrossRef]
- Känkänen, H. Undersowing in a Northern Climate: Effects on Spring Cereal Yield and Risk of Nitrate Leaching; MTT Agrifood Research Finland: Jokioinen, Finland, 2010; pp. 1–49. [Google Scholar]
- Kunelius, H.T.; Johnston, H.W.; MacLeod, J.A. Effect of undersowing barley with Italian ryegrass or red clover on yield, crop composition and root biomass. Agric. Ecosyst. Environ. 1992, 38, 127–137. [Google Scholar] [CrossRef]
- Doltra, J.; Olesen, J.E. The role of catch crops in the ecological intensification of spring cereals in organic farming under Nordic climate. Eur. J. Agron. 2013, 44, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Żuk-Gołaszewska, K.; Wanic, M.; Orzech, K. The role of catch crops in field plant Production—A review. J. Elem. 2019, 24, 575–587. [Google Scholar] [CrossRef]
- Salonen, J.; Ketoja, E. Undersown cover crops have limited weed suppression potential when reducing tillage intensity in organically grown cereals. Org. Agric. 2020, 10, 107–121. [Google Scholar] [CrossRef] [Green Version]
- Langdale, G.W.; Blevins, R.L.; Karlen, D.L.; McCool, D.K.; Nearing, M.A.; Skidmore, E.L.; Thomas, A.W.; Tyler, D.D.; Williams, J.R. Cover crop effects on soil erosion by wind and water. In Cover Crops for Clean Water; Hargrove, W.L., Ed.; Soil and Water Conservation Society: Ankeny, IA, USA, 1991; pp. 15–22. [Google Scholar]
- Abdin, O.A.; Coulman, B.E.; Cloutier, D.C.; Faris, M.A.; Smith, D.L. Establishment, development and yield of forage legumes and grasses as cover crops in grain corn in Eastern Canada. J. Agron. Crop. Sci. 1997, 179, 19–27. [Google Scholar] [CrossRef]
- Rayns, F.; Rosenfeld, A.; Organic, G. Green manures—Effects on soil nutrient management and soil physical and biological properties. In Factsheet 24/10, Soil Grown Crops Projects FV 299 and 299a; Horticultural Development Company: Kenilworth, UK, 2010; pp. 1–8. [Google Scholar]
- Wanic, M.; Zuk-Golaszewska, K.; Orzech, K. Catch crops and the soil Environment—A review of the literature. J. Elem. 2019, 24, 575–587. [Google Scholar] [CrossRef]
- Talgre, L.; Lauringson, E.; Roostalu, H.; Astover, A.; Makke, A. Green manure as a nutrient source for succeeding crops. Plant. Soil Environ. 2012, 58, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Hofmeijer, M.A.J.; Melander, B.; Salonen, J.; Lundkvist, A.; Zarina, L.; Gerowitt, B. Crop diversification affects weed communities and densities in organic spring cereal fields in northern Europe. Agric. Ecosyst. Environ. 2021, 308. [Google Scholar] [CrossRef]
- Rinnofner, T.; Friedel, J.K.; de Kruijff, R.; Pietsch, G.; Freyer, B. Effect of catch crops on N dynamics and following crops in organic farming. Agron. Sustain. Dev. 2008, 28, 551–558. [Google Scholar] [CrossRef]
- McKenna, P.; Cannon, N.; Conway, J.; Dooley, J. The use of red clover (Trifolium pratense) in soil fertility-building: A Review. Field Crop. Res. 2018, 221, 38–49. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Cheng, K.; Yue, Q.; Chadwick, D.; Espenberg, M.; Truu, J.; Rees, R.M.; Smith, P. A critical review of the impacts of cover crops on nitrogen leaching, net greenhouse gas balance and crop productivity. Glob. Chang. Biol. 2019, 25, 2530–2543. [Google Scholar] [CrossRef] [Green Version]
- Behnke, G.D.; Villamil, M.B. Cover crop rotations affect greenhouse gas emissions and crop production in Illinois, USA. Field Crop. Res. 2019, 241, 107580. [Google Scholar] [CrossRef]
- Guardia, G.; Aguilera, E.; Vallejo, A.; Sanz-Cobena, A.; Alonso-Ayuso, M.; Quemada, M. Effective climate change mitigation through cover cropping and integrated fertilization: A global warming potential assessment from a 10-year field experiment. J. Clean. Prod. 2019, 241. [Google Scholar] [CrossRef]
- Smit, B.; Janssens, B.; Haagsma, W.; Hennen, W.; Adrados, J.; Kathage, J. Adoption of Cover Crops for Climate Change Mitigation in the EU; Publications Office of the European Union: Luxembourg, 2019; pp. 1–76. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Ruis, S.J.; Proctor, C.A.; Creech, C.F.; Drewnoski, M.E.; Redfearn, D.D. Harvesting cover crops for biofuel and livestock production: Another ecosystem service? Agron. J. 2020, 112, 2373–2400. [Google Scholar] [CrossRef]
- Krievina, A.; Leimane, I. Comparison of the support for catch crops in the Baltic Sea region countries. Res. Rural Dev. 2019, 2, 95–102. [Google Scholar] [CrossRef]
- Reimer, M.; Ringselle, B.; Bergkvist, G.; Westaway, S.; Wittwer, R.; Baresel, J.P.; Van Der Heijden, M.G.A.; Mangerud, K.; Finckh, M.R.; Brandsæter, L.O. Interactive effects of subsidiary crops and weed pressure in the transition period to non-inversion tillage, a case study of six sites across northern and central Europe. Agronomy 2019, 9, 495. [Google Scholar] [CrossRef] [Green Version]
- Chapagain, T.; Lee, E.A.; Raizada, M.N. The potential of multi-species mixtures to diversify cover crop benefits. Sustainability 2020, 12, 2058. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhong, R.; Lai, C.; Zeng, Z.; Lian, Y.; Bai, X. Climate change enhances the severity and variability of drought in the Pearl River Basin in South China in the 21st century. Agric. For. Meteorol. 2018, 249, 149–162. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and Challenges in a Changing Climate. Front. Plant. Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalak, D. Adapting to climate change and effective water management in Polish agriculture—At the level of government institutions and farms. Ecohydrol. Hydrobiol. 2020, 20, 134–141. [Google Scholar] [CrossRef]
- Hänsel, S.; Ustrnul, Z.; Łupikasza, E.; Skalak, P. Assessing seasonal drought variations and trends over Central Europe. Adv. Water Resour. 2019, 127, 53–75. [Google Scholar] [CrossRef]
- Szwed, M. Variability of precipitation in Poland under climate change. Theor. Appl. Climatol. 2019, 135, 1003–1015. [Google Scholar] [CrossRef] [Green Version]
- Valliyodan, B.; Nguyen, H.T. Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr. Opin. Plant. Biol. 2006, 9, 189–195. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.C.; Nogueira, R.; da Silva, M.A.; de Albuquerque, M.B. Drought stress and plant nutrition. Plant. Stress 2011, 5, 32–41. [Google Scholar]
- Foxx, A.J.; Fort, F. Root and shoot competition lead to contrasting competitive outcomes under water stress: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0220674. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, M.J.; Pugnaire, F.I.; Armas, C.; Rodríguez-Echeverría, S.; Schöb, C. The shift from plant-plant facilitation to competition under severe water deficit is spatially explicit. Ecol. Evol. 2017, 7, 2441–2448. [Google Scholar] [CrossRef] [Green Version]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient use efficiency in plants. Comm. Soil Sci. Plant. Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Wanic, M.; Mysliwiec, M.; Orzech, K.; Michalska, M. Nitrogen content and uptake by spring wheat and undersown Persian clover depending on plant density. J. Elem. 2016, 24, 31–45. [Google Scholar] [CrossRef]
- Wanic, M.; Myśliwiec, M.; Orzech, K.; Denert, M. Phosphorus, potassium and magnesium content and uptake by spring wheat and undersown Persian clover depending on plant density. Acta Agrophys. 2017, 24, 149–162. [Google Scholar]
- Chemical and Agricultural Research Laboratory in Olsztyn. Research Procedure of the Chemical and Agricultural Research Laboratory in Olsztyn No. PB 05 ed. 4, 14.11.2014; Chemical and Agricultural Research Laboratory in Olsztyn: Olsztyn, Poland, 2014. [Google Scholar]
- Chemical and Agricultural Research Laboratory in Olsztyn. Research Procedure of the Chemical and Agricultural Research Laboratory in Olsztyn No. PB 04 ed. 4, 14.11.2014; Chemical and Agricultural Research Laboratory in Olsztyn: Olsztyn, Poland, 2014. [Google Scholar]
- Chemical and Agricultural Research Laboratory in Olsztyn. Research Procedure of the Chemical and Agricultural Research Laboratory in Olsztyn No. PB 03 ed. 4, 14.11.2014; Chemical and Agricultural Research Laboratory in Olsztyn: Olsztyn, Poland, 2014. [Google Scholar]
- Chemical and Agricultural Research Laboratory in Olsztyn. Research Procedure of the Chemical and Agricultural Research Laboratory in Olsztyn No. PB 06 ed. 4, 14.11.2014; Chemical and Agricultural Research Laboratory in Olsztyn: Olsztyn, Poland, 2014. [Google Scholar]
- Chemical and Agricultural Research Laboratory in Olsztyn. Research Procedure of the Chemical and Agricultural Research Laboratory in Olsztyn No. PB 29 ed. 4, 27.11.2014; Chemical and Agricultural Research Laboratory in Olsztyn: Olsztyn, Poland, 2014. [Google Scholar]
- Polish Standard. Determination of Available Phosphorus in Mineral Soils, PN-R-04023:1996; Polish Committee for Standardization: Warsaw, Poland, 1996. [Google Scholar]
- Polish Standard. Determination of Available Potassium in Mineral Soils, PN-R-04022:1996/Az1:2002; Polish Committee for Standardization: Warsaw, Poland, 1996. [Google Scholar]
- Polish Standard. Determination of Available Magnesium in Mineral Soils, PN-R-04020:1994/Az1:2004; Polish Committee for Standardization: Warsaw, Poland, 1994. [Google Scholar]
- Torma, S.; Vilček, J.; Lošák, T.; Kužel, S.; Martensson, A. Residual plant nutrients in crop Residues—An important resource. Acta Agric. Scand. B Soil Plant. Sci. 2018, 68, 358–366. [Google Scholar] [CrossRef]
- Holmstrom, D.A.; Kunelius, H.T.; Ivany, J.A. Forages underseeded in barley for residue management for potatoes. Can. J. Plant. Sci. 2001, 81, 205–210. [Google Scholar] [CrossRef]
- Hinsinger, P.; Betencourt, E.; Bernard, L.; Brauman, A.; Plassard, C.; Shen, J.; Tang, X.; Zhang, F. P for two, sharing a scarce resource: Soil phosphorus acquisition in the rhizosphere of intercropped species. Plant. Physiol. 2011, 156, 1078–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, E.S.; Bedoussac, L.; Carlsson, G.; Journet, E.-P.; Justes, E.; Hauggaard-Nielsen, H. Enhancing yields in organic crop production by eco-functional intensification. Sustain. Agric. Res. 2015, 4, 42–50. [Google Scholar] [CrossRef]
- Gürel, F.; Öztürk, Z.N.; Uçarlı, C.; Rosellini, D. Barley genes as tools to confer abiotic stress tolerance in crops. Front. Plant. Sci. 2016, 7. [Google Scholar] [CrossRef]
- Bista, D.R.; Heckathorn, S.A.; Jayawardena, D.M.; Mishra, S.; Boldt, J.K. Effects of drought on nutrient uptake and the levels of nutrient-uptake proteins in roots of drought-sensitive and -tolerant grasses. Plants 2018, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Norris, I.B.; Thomas, H. Recovery of ryegrass species from drought. J. Agric. Sci. Camb. 1982, 98, 623–628. [Google Scholar] [CrossRef]
- Lang, J.; Vejražka, K. Yields and quality of forage legumes under imbalanced year precipitation conditions on south Moravia. Acta Uni. Agric. Silvic. Mendel. Brun. 2012, 60, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Wanic, M.; Jastrzebska, M.; Kostrzewska, M.K.; Treder, K. Competition between spring barley (Hordeum vulgare L.) and Italian ryegrass (Lolium multiflorum Lam.) under different water supply conditions. Acta Agrobot. 2013, 66, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Treder, K.; Jastrzebska, M.; Kostrzewska, M.K.; Makowski, P.; Wanic, M. Effect of competitive interactions and water stress on the morphological characteristics of red clover (Trifolium pretense L.) cultivated with spring barley (Hordeum vulgare L.). Acta Sci. Pol. Agric. 2016, 15, 83–94. [Google Scholar]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH-Monograph; Blackwell Wissenschafts-Verlag: Berlin, Germany; Boston, MA, USA, 1997; p. 622. [Google Scholar]
- Zhou, G.; Zhou, X.; Nie, Y.; Bai, S.H.; Zhou, L.; Shao, J.; Cheng, W.; Wang, J.; Hu, F.; Fu, Y. Drought-induced changes in root biomass largely result from altered root morphological traits: Evidence from a synthesis of global field trials. Plant. Cell Environ. 2018, 41, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Steynberg, R.E.; Nel, P.C.; Rethman, N.F.G. Soil water use and rooting depth of Italian ryegrass (Lolium multiflorum Lam.) in a small plot experiment. S. Afr. J. Plant. Soil 1994, 11, 80–83. [Google Scholar] [CrossRef] [Green Version]
- Juknevičius, S.; Sabienė, N. The content of mineral elements in some grasses and legumes. Ekologija 2007, 53, 44–52. [Google Scholar]
- Dakora, F.D. Defining new roles for plant and rhizobial molecules in sole and mixed plant cultures involving symbiotic legumes. New Phytol. 2003, 158, 39–49. [Google Scholar] [CrossRef]
- Yan, F.; Schubert, S.; Mengel, K. Soil pH changes during legume growth and application of plant material. Biol. Fertil. Soils 1996, 23, 236–242. [Google Scholar] [CrossRef]
- Yao, Q.; Li, X.; Ai, W.; Christie, P. Bi-directional transfer of phosphorus between red clover and perennial ryegrass via arbuscular mycorrhizal hyphal links. Eur. J. Soil Biol. 2003, 39, 47–54. [Google Scholar] [CrossRef]
- He, M.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant. Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahieu, S.; Germon, F.; Aveline, A.; Hauggaard-Nielsen, H.; Ambus, P.; Jensen, E.S. The influence of water stress on biomass and N accumulation, N partitioning between above and below ground parts and on N rhizodeposition during reproductive growth of pea (Pisum sativum L.). Soil Biol. Biochem. 2009, 41, 380–387. [Google Scholar] [CrossRef]
- Soares, M.M.; Freitas, C.D.M.; Oliveira, F.S.D.; Mesquita, H.C.D.E.; Silva, T.S.; Silva, D.V. Effects of competition and water deficiency on sunflower and weed growth. Rev. Caatinga 2019, 32, 318–328. [Google Scholar] [CrossRef] [Green Version]
- Kostrzewska, M.K.; Jastrzębska, M.; Treder, K.; Wanic, M. Phosphorus in spring barley and Italian ryegrass biomass as an effect of inter-species interactions under water deficit. Agriculture 2020, 10, 329. [Google Scholar] [CrossRef]
- Jarrell, W.M.; Beverly, R.B. The Dilution Effect in Plant Nutrition Studies. In Advances in Agronomy; Brady, N.C., Ed.; Academic Press: London, UK, 1981; Volume 34, pp. 197–224. [Google Scholar]
- Marles, R.J. Mineral nutrient composition of vegetables, fruits and grains: The context of reports of apparent historical declines. J. Food Comp. Anal. 2017, 56, 93–103. [Google Scholar] [CrossRef]
- Ciećko, Z.; Kalembasa, S.; Wyszkowski, M.; Rolka, E. The magnesium content in plants in soil contaminated with cadmium. Pol. J. Environ. Stud. 2005, 14, 365–370. [Google Scholar]
- Styrczula, P.; Możdżer, E. Wpływ wieloskładnikowych nawozów mineralnych oraz nawożenia organicznego na zawartość i pobranie NPK przez biomasę życicy trwałej. Chemik 2013, 67, 604–615. [Google Scholar]
- Thilakarathna, R.M.M.S.; Papadopoulos, Y.A.; Rodd, A.V.; Gunawardena, A.N.; Fillmore, S.A.E.; Prithiviraj, B. Characterizing nitrogen transfer from red clover populations to companion bluegrass under field conditions. Can. J. Plant. Sci. 2012, 92, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, K.; Makaju, S.; Ibrahim, R.; Missaoui, A. Current progress in nitrogen fixing plants and microbiome research. Plants 2020, 9, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordeleau, L.M.; Prévost, D. Nodulation and nitrogen fixation in extreme environments. Plant. Soil 1994, 161, 115–125. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, L.; Baddeley, J.A.; Watson, C.A. Models of biological nitrogen fixation of legumes. A review. Agron. Sustain. Dev. 2011, 31, 155–172. [Google Scholar] [CrossRef]
- Balemi, T.; Negisho, K. Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: A review. J. Soil Sci. Plant. Nutr. 2012, 12, 547–561. [Google Scholar] [CrossRef] [Green Version]
- Hunt, I.V.; Frame, J.; Harkess, R.D. Removal of mineral nutrients by red clover varieties. Grass Forage Sci. 1976, 31, 171–179. [Google Scholar] [CrossRef]
- Mengel, K.; Steffens, D. Potassium uptake of rye-grass (Lolium perenne) and red clover (Trifolium pratense) as related to root parameters. Biol. Fertil. Soils 1985, 1, 53–58. [Google Scholar] [CrossRef]
- Mäkelä, P.S.A.; Manninen-Egilmez, P.; Santanen, A.; Kleemola, J. Role of potassium in barley plant stand architecture and yield formation. Comm. Soil Sci. Plant. Anal. 2012, 43, 2603–2614. [Google Scholar] [CrossRef]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ nutrition in plants. Front. Plant. Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Item | Experiment I | Experiment II |
|---|---|---|
| Barley cultivar | Rastik 1 | Rastik |
| Undersown catch crop (CC), cultivar | Italian ryegrass, Gaza 2 | Red clover, Bona 2 |
| Plant number in pot | ||
| barley | 19 | 19 |
| barley + CC | 19 + 19 | 19 + 8 |
| Seed planting depth (barley / CC), cm | 3/3 | 3/1.5 |
| Soil type | Cambisols | Cambisols |
| Soil texture | slightly loamy sand | clay loam |
| Soil pH, in 1M KCl | 6.06 | 5.80 |
| Average soil content of | ||
| Corganic, g kg–1 | 9.1 | 12.7 |
| Ntotal, mg kg–1 | 699 | 823 |
| Pavailable, mg kg–1 | 91 | 116 |
| Kavailable, mg kg–1 | 196 | 168 |
| Mgavailable, mg kg–1 | 30.8 | 65.7 |
| Experiment | Water Supply | Crops | N | P | K | Mg |
|---|---|---|---|---|---|---|
| I | H | B | 12.1 ± 0.14 a 1 | 2.37 ± 0.03 a | 10.7 ± 0.15 a | 1.37 ± 0.12 a |
| BR | 13.0 ± 0.18 a | 2.60 ± 0.04 a | 11.5 ± 0.15 a | 1.47 ± 0.12 a | ||
| L | B | 13.9 ± 0.14 a | 2.70 ± 0.06 a | 11.0 ± 0.22 a | 1.70 ± 0.02 a | |
| BR | 10.8 ± 0.22 a | 2.80 ± 0.07 a | 9.6 ± 0.11 a | 1.67 ± 0.02 a | ||
| II | H | B | 18.5 ± 0.15 a | 2.41± 0.01 c | 21.6 ± 0.46 a | 1.54 ± 0.02 b |
| BC | 16.5 ± 0.43 a | 2.80 ± 0.12 b | 18.1 ± 0.47 b | 1.97 ± 0.11 a | ||
| L | B | 16.5 ± 0.11 a | 2.74 ± 0.04 b | 17.8 ± 0.59 b | 1.66 ± 0.01 b | |
| BC | 17.7 ± 0.13 a | 3.15 ± 0.08 a | 18.4 ± 0.38 b | 2.09 ± 0.03 a |
| Experiment | Post-Harvest Residue | N | P | K | Mg |
|---|---|---|---|---|---|
| I | biomass | 0.985 1 | 0.998 | 0.980 | 0.991 |
| nutrient content | ns | ns | ns | 0.847 | |
| II | biomass | 0.862 | 0.964 | 0.962 | 0.958 |
| nutrient content | 0.464 | 0.400 | ns | 0.686 |
| Experiment | Water Supply | Crops | N | P | K | Mg |
|---|---|---|---|---|---|---|
| I | H | B | 667 ± 48 ab↓ 1 | 87 ± 4.4 a | 132 ± 5.8 ab↓ | 29.7 ± 1.45 a |
| BR | 643 ± 44 b↓ | 86 ± 4.3 a | 116 ± 7.8 b↓ | 28.7 ± 1.06 a↓ | ||
| L | B | 683 ± 67 a | 88 ± 6.4 a | 150 ± 6.1 a↓ | 30.0 ± 0.91 a | |
| BR | 643 ± 33 b↓ | 85 ± 5.3 a | 126 ± 6.9 ab↓ | 28.7 ± 0.66 a↓ | ||
| II | H | B | 587 ± 44 b↓ | 89 ± 5.7 b ↓ | 109 ± 5.9 b↓ | 55.7 ± 1.76 b↓ |
| BC | 690 ± 40a↓ | 98 ± 5.6 ab↓ | 106 ± 7.4 b↓ | 57.7 ± 1.20 ab↓ | ||
| L | B | 705 ± 50 a↓ | 103 ± 7.0 a↓ | 132 ± 2.9 a↓ | 61.3 ± 0.67 a | |
| BC | 680 ± 25 ab↓ | 101 ± 7.8 ab↓ | 130 ± 6.6 a↓ | 60.7 ± 0.88 a↓ |
| Experiment | Biomass (of) | N | P | K | Mg |
|---|---|---|---|---|---|
| I | removed | −0.657 1 | ns | −0.855 | −0.632 |
| post-harvest residues | −0.880 | −0.593 | −0.947 | −0.874 | |
| II | removed | −0.586 | −0.915 | −0.906 | −0.972 |
| post-harvest residues | ns | ns | −0.540 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jastrzębska, M.; Kostrzewska, M.K.; Wanic, M.; Marks, M.; Treder, K. Catch Crops: A Nutrient Reservoir in Post-Harvest Residues under Water Deficit. Agronomy 2021, 11, 1501. https://doi.org/10.3390/agronomy11081501
Jastrzębska M, Kostrzewska MK, Wanic M, Marks M, Treder K. Catch Crops: A Nutrient Reservoir in Post-Harvest Residues under Water Deficit. Agronomy. 2021; 11(8):1501. https://doi.org/10.3390/agronomy11081501
Chicago/Turabian StyleJastrzębska, Magdalena, Marta K. Kostrzewska, Maria Wanic, Marek Marks, and Kinga Treder. 2021. "Catch Crops: A Nutrient Reservoir in Post-Harvest Residues under Water Deficit" Agronomy 11, no. 8: 1501. https://doi.org/10.3390/agronomy11081501







