Unraveling Factors Affecting Micropropagation of Four Persian Walnut Varieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Culture Conditions
2.3. In Vitro Introductions
2.4. Proliferation
2.5. Rooting
2.6. Acclimatization
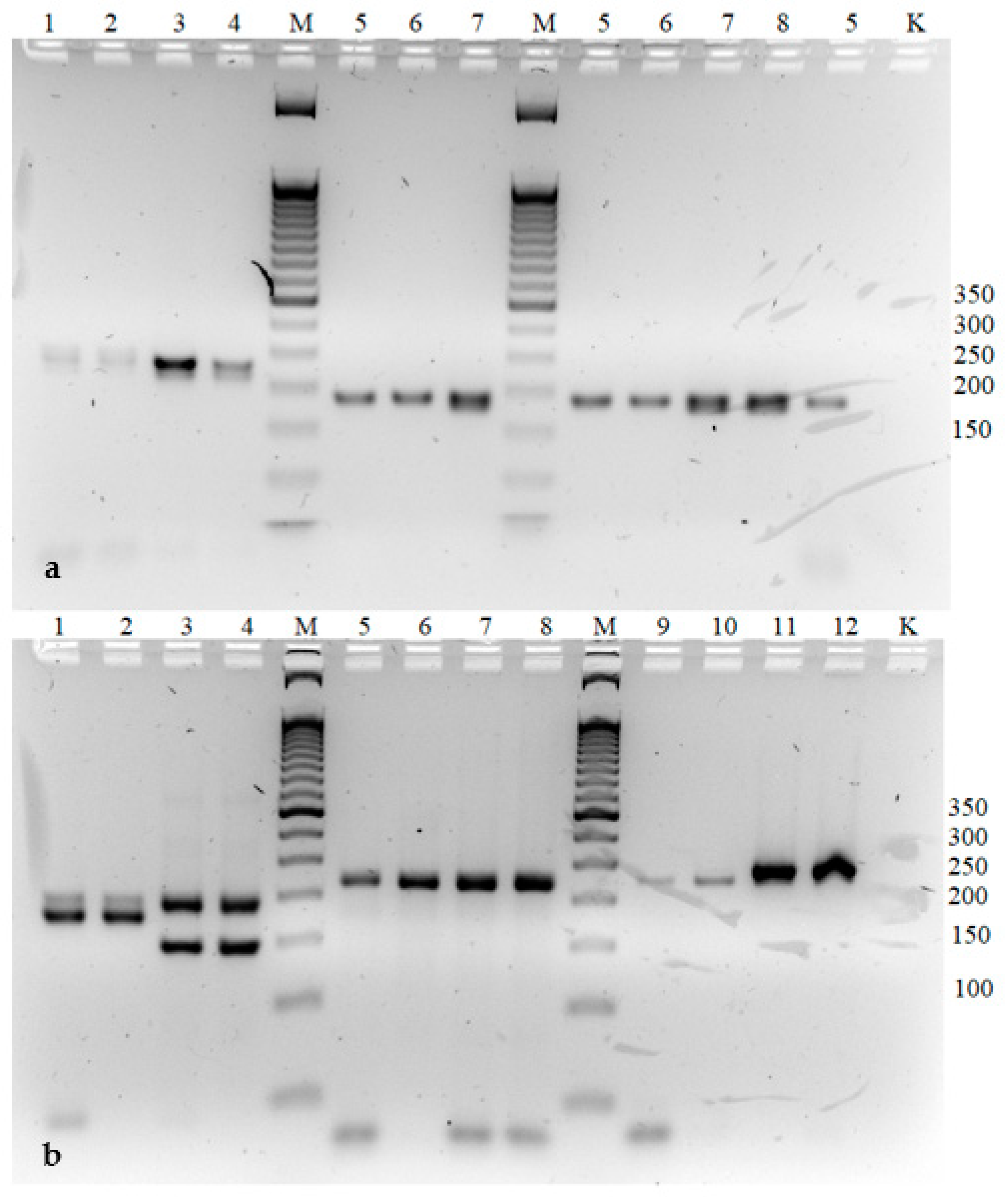
2.7. Analysis of Genetic Stability of Micropropagated Clones
2.8. Experimental Design and Statistical Data Processing
3. Results and Discussion
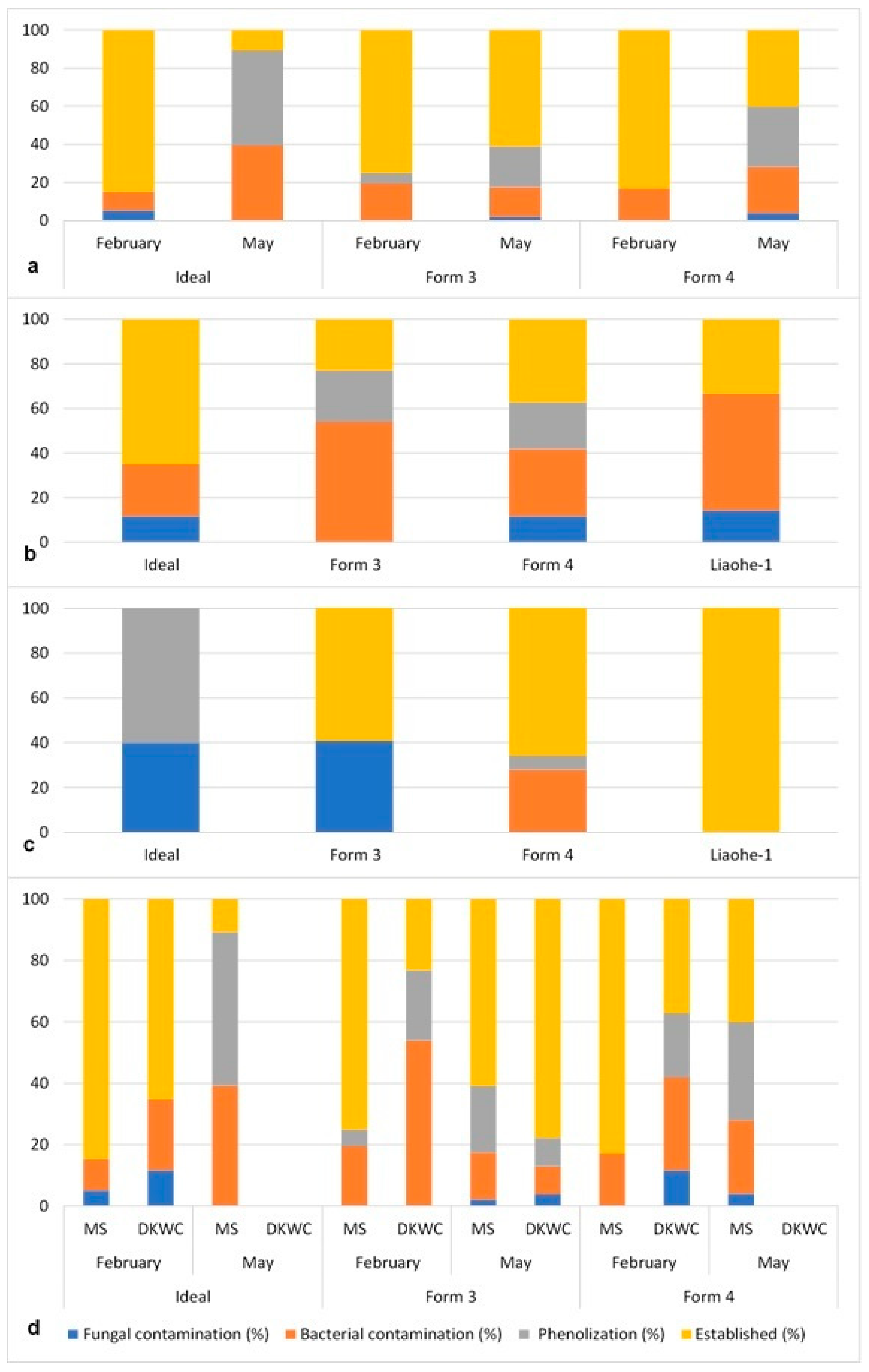
3.1. In Vitro Introduction and Establishment
3.2. Proliferation
3.3. Rooting
3.4. Acclimatization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dandekar, A.; Leslie, C.; McGranahan, G. Juglans regia Walnut. In Biotechnology of Fruit and Nut Crops; Biotechnology in Agriculture 29; Litz, R.E., Ed.; The Doyle Foundation: Glasgow, Scotland, 2005. [Google Scholar]
- Bernard, A.; Lheureux, F.; Dirlewanger, E. Walnut: Past and future of genetic improvement. Tree Genet. Genomes 2018, 141, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Pollegioni, P.; Woeste, K.; Chiocchini, F.; Del Lungo, S.; Ciolfi, M.; Olimpieri, I.; Clark, J.; Hemery, G.E.; Mapelli, S.; Malvolti, M.E.; et al. Rethinking the history of common walnut (Juglans regia L.) in Europe: Its origins and human interactions. PLoS ONE 2017, 12, e0172541. [Google Scholar] [CrossRef]
- Aradhya, M.K.; Potter, D.; Gao, F.; Simon, C.J. Molecular phylogeny of Juglans (Juglandaceae): A biogeographic perspective. Tree Genet. Genomes 2007, 3, 363–378. [Google Scholar] [CrossRef]
- McGranahan, G.; Leslie, C. Breeding walnuts (Juglans regia). In Breeding Plantation Tree Crops: Temperate Species; Mohan Jain, S., Priyadarshan, P.M., Eds.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Borchardt, P.; Schmidt, M.; Schickhoff, U. Vegetation patterns in Kyrgyzstan’s walnut-fruit forests under the impact of changing forest use in post-Soviet transformation. Die. Erde. 2010, 141, 255–275. [Google Scholar]
- Torokeldiev, N.; Ziehe, M.; Gailing, O.; Finkeldey, R. Genetic diversity and structure of natural Juglans regia L. populations in the southern Kyrgyz Republic revealed by nuclear SSR and EST-SSR markers. Tree Genet. Genomes 2019, 15, 5. [Google Scholar] [CrossRef]
- Akça, Y.; Yuldaşulu, Y.B.; Murad, E.; Vahdati, K. Exploring of Walnut Genetic Resources in Kazakhstan and Evaluation of Promising Selections. Int. J. Hort. Sci. Technol. 2020, 7, 93–102. [Google Scholar] [CrossRef]
- Hassani, D.; Sarikhani, S.; Dastjerdi, R.; Mahmoudi, R.; Soleimani, A.; Vahdati, K. Situation and recent trends on cultivation and breeding of Persian walnut in Iran. Sci. Hortic. 2020, 270, 109369. [Google Scholar] [CrossRef]
- Driver, J.A.; Kuniyuki, A.H. In vitro propagation of Paradox walnut rootstock. Hortic. Sci. 1984, 19, 507–509. [Google Scholar]
- McGranahan, G.H.; Driver, J.A.; Tulecke, W. Tissue culture of Juglans. In Cell and Tissue Culture in Forestry; Bonga, J.M., Durzan, D.J., Eds.; Martinus Nijhoff: Boston, MA, USA, 1987; Volume 3, pp. 261–271. [Google Scholar]
- Cornu, D.; Jay-Allemand, C. Micropropagation of hybrid walnut trees (Juglans nigra × Juglans regia) through culture and multiplication of embryos. In Annales des Sciences Forestières 46; Dreyer, E., Ed.; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Pijut, P.M. Micropropagation of Juglans cinerea L. (butternut). In High-Tech and Micropropagation V. Biotechnology in Agriculture and Forestry, 5; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 345–357. [Google Scholar] [CrossRef]
- Vahdati, K.; Najafian Ashrafi, E.; Ebrahimzadeh, H.; Mirmasoumi, M. Improved micropropagation of walnut (Juglans regia L.) on media optimized for growth based upon mineral content of walnut seed. Acta Hortic. 2009, 839, 117–124. [Google Scholar] [CrossRef]
- Navatel, J.C.; Bourrain, L. Plant production of walnut Juglans regia L. by in vitro multiplication. Acta Hortic. 2001, 544, 465–471. [Google Scholar] [CrossRef]
- Gotea, R.; Gotea, I.; Sestras, R.E.; Vahdati, K. In vitro propagation of several walnut cultivars. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Horticulture 2012, 69, 167–171. [Google Scholar]
- Licea-Moreno, R.J.; Contreras, A.; Morales, A.V.; Urban, I.; Daquinta, M.; Gomez, L. Improved walnut mass micropropagation through the combined use of phloroglucinol and FeEDDHA. PCTOC 2015, 123, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Vahdati, K.; Leslie, C.; Zamani, Z.; McGranahan, G. Rooting and acclimatization of in vitro-grown shoots from mature trees of three Persian walnut cultivars. HortScience 2004, 39, 324–327. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plantarum. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gruselle, R.; Boxus, P. Walnut micropropagation. Acta Hortic. 1990, 284, 45–52. [Google Scholar] [CrossRef]
- Lone, I.A.; Misger, F.A.; Banday, F.A. Effect of different growth regulator combinations on the per cent media browning in walnut in vitro studies using MS medium. Asian J. Soil Sci. 2017, 12, 135–142. [Google Scholar] [CrossRef]
- Guo-Liang, W.; Yan-Hui, C.; Peng-Fei, Z.; Jun-Qiang, Y.; Yu-Qin, S. Apomixis and new selections of walnut. Acta Hortic. 2007, 760, 541–548. [Google Scholar] [CrossRef]
- Licea-Moreno, R.J.; Morales, A.V.; Daquinta, M.; Gomez, L. Towards scaling-up the micropropagation of Juglans major (Torrey) Heller var. 209 × J. regia L., a hybrid walnut of commercial interest. In Proceedings of the IUFRO Working Party 2.09.02 Conference “Integrating Vegetative Propagation, Biotechnologies and Genetic Improvement for Tree Production and Sustainable Forest Management”; Park, Y.S., Bonga, J.M., Eds.; IUFRO: Brno, Czech Republic, 2012. [Google Scholar]
- Navatel, J.C.; Bourrain, L. Influence of the physical structure of the medium on in vitro rooting. Adv. Hort. Sci. 1994, 8, 57–59. [Google Scholar]
- Dangl, G.S.; Woeste, K.; Aradhya, M.K.; Koehmstedt, A.; Simon, C.; Potter, D.; McGranahan, G. Characterization of 14 microsatellite markers for genetic analysis and cultivar identification of walnut. J. Am. Soc. Hortic. Sci. 2005, 130, 348–354. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R. A language and environment for statistical computing. In R Foundation for Statistical Computing; R Foundation: Vienna, Austria, 2013. [Google Scholar]
- Leslie, C.; McGranahan, G. Micropropagation of Persian walnut (Juglans regia L.). In High-Tech and Micropropagation II; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 136–150. [Google Scholar]
- Scaltsoyiannes, A.; Tsoulpha, P.; Panetsos, K.P.; Moulalis, D. Effect of genotype on micropropagation of walnut trees (Juglans regia). Silvae Genet. 1998, 46, 326–331. [Google Scholar]
- Berg, G.; Grube, M.; Schloter, M.; Smalla, K. Unraveling the plant microbiome: Looking back and future perspectives. Front Microbiol. 2014, 5, 148. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.; Swarna, G.K.; Patil, P.; Rawal, R.D. Ubiquitous presence of normally non-culturable endophytic bacteria in field shoot-tips of banana and their gradual activation to quiescent cultivable form in tissue cultures. Plant Cell Tiss. Org. 2008, 93, 39–54. [Google Scholar] [CrossRef]
- Wagner, M.R.; Lundberg, D.S.; Tijana, G.; Tringe, S.G.; Dangl, J.L.; Mitchell-Olds, T. Host genotype and age shape the leaf and root microbiomes of a wild perennial plant. Nat. Commun. 2016, 7, 12151. [Google Scholar] [CrossRef]
- Izumi, H. Diversity of endophytic bacteria in forest trees. In Endophytes of Forest Trees: Biology and Applications, Forestry Sciences 80; Pirttilä, A.M., Frank, A.C., Eds.; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Solar, A.; Colarič, M.; Usenik, V.; Stampar, F. Seasonal variations of selected flavonoids, phenolic acids and quinones in annual shoots of common walnut (Juglans regia L.). Plant Sci. 2006, 170, 453–461. [Google Scholar] [CrossRef]
- Licea-Moreno, R.J. Biotecnología Forestal Aplicada a la Producción de Madera de Nogal. Ph.D. Thesis, Universidad Politécnica de Mardid, Madrid, Spain, 2016. [Google Scholar]
- Licea-Moreno, R.J.; Fira, A.; Chocov, G. Micropropagation of valuable walnut genotypes for timber production: New advances and insights. Ann. Silvic. Res. 2020, 44, 5–13. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Valentão, P.; Andrade, P.B.; Ferreira, I.C.; Ferreres, F.; Bento, A.; Seabra, R.; Estevinho, L. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295. [Google Scholar] [CrossRef]
- Revilla, M.A.; Majada, J.; Rodriguez, R. Walnut (Juglans regia L.) micropropagation. In Annales des Sciences Forestières, 46; Dreyer, E., Ed.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 149s–151s. [Google Scholar]
- Heile-Sudholt, C.; Huetteman, C.A.; Preece, J.E.; Van Sambeek, J.W.; Gaffney, G.R. In vitro embryonic axis and seedling shoot tip culture of Juglans nigra L. Plant Cell Tiss. Org. 1986, 6, 189–197. [Google Scholar] [CrossRef]
- Gruselle, R.; Badia, N.; Boxus, P. Walnut micropropagation: First results. Acta Hortic. 1987, 212, 511–516. [Google Scholar] [CrossRef]
- Stevens, M.E.; Pijut, P.M. Rapid in vitro shoot multiplication of the recalcitrant species Juglans nigra L. Vitr. Cell. Dev. Biol. Plant 2018, 54, 309–317. [Google Scholar] [CrossRef]
- Pérez, L.P.; Montesinos, Y.P.; Olmedo, J.G.; Rodriguez, R.B.; Sánchez, R.R.; Montenegro, O.N.; Gómez-Kosky, R. Effect of phloroglucinol on rooting and in vitro acclimatization of papaya (Carica papaya L. var. Maradol Roja). Vitr. Cell. Dev. Biol. Plant 2016, 52, 196–203. [Google Scholar] [CrossRef]
- Hesami, M.; Naderi, R.; Tohidfar, M. Modeling and optimizing medium composition for shoot regeneration of Chrysanthemum via radial basis function-non-dominated sorting genetic algorithm-II (RBF-NSGAII). Sci. Rep. 2019, 9, 18237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petti, C. Phloroglucinol Mediated Plant Regeneration of Ornithogalum dubium as the Sole “Hormone-Like Supplement” in Plant Tissue Culture Long-Term Experiments. Plants 2020, 9, 929. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Shukla, M.R.; Sullivan, J.A.; Saxena, P.K. Iron supplementation promotes in vitro shoot induction and multiplication of Baptisia australis. Plant Cell Tiss. Org. 2017, 129, 145–152. [Google Scholar] [CrossRef]
- Arab, M.M.; Yadollahi, A.; Eftekhari, M.; Ahmadi, H.; Akbari, M.; Khorami, S.S. Modeling and optimizing a new culture medium for in vitro rooting of Gx N15 Prunus rootstock using artificial neural network-genetic algorithm. Sci. Rep. 2018, 8, 9977. [Google Scholar] [CrossRef]
- Eshaghi Sanayi, T.; Zare Mehrjerdi, M.; Sharifi, A. Effect of Medium, Iron-Chelating Agent and Plant Growth Regulator on Micropropagation of Pomegranate (Punica granatum L.). J. Plant Prod. 2020, 43, 309–322. [Google Scholar] [CrossRef]
- Al-Mayahi, A.M.W. In vitro plant regeneration system for date palm (Phoenix dactylifera L.): Effect of chelated iron sources. J. Genet. Eng. Biotechnol. 2021, 19, 83. [Google Scholar] [CrossRef]
- Bosela, M.J.; Michler, C.H. Media effects on black walnut (Juglans nigra L.) shoot culture growth in vitro: Evaluation of multiple nutrient formulations and cytokinin types. Vitr. Cell. Dev. Biol. Plant 2008, 44, 316–329. [Google Scholar] [CrossRef]
- Sharifian, S.; Vahdati, K.; Mirmasoumi, M.; Ghaem Maghami, S.A. Assessment of phloroglucinol effect on rooting of tissue cultured Persian walnut. Acta Hortic. 2007, 812, 189–196. [Google Scholar] [CrossRef]
- De Gryze, C.; De Riek, J.; Debergh, P.C. Water relationships in the culture vessel. Acta Hortic. 1995, 393, 39–44. [Google Scholar] [CrossRef]
- Piqueras, A.; Debergh, P.C. Morphogenesis in micropropagation. In Morphogenesis in Plant Tissue Cultures; Soh, W.Y., Bhojwani, S.S., Eds.; Springer: Dordrecht, The Netherlands, 1999. [Google Scholar]
- McGranahan, G.; Leslie, C.A.; Driver, J.A. In vitro propagation of mature Persian walnut cultivars. HortScience 1988, 23, 220. [Google Scholar]
- Chalupa, V. Clonal propagation of broad-leaved forest trees in vitro. Commun. Inst. For. Czech Repub. 1981, 12, 255–271. [Google Scholar]
- Caboni, E.; Damiano, C. In vitro propagation of walnut (Juglans regia L.): Critical factors for the induction of the rooting response. In Proceeding of Vth International Walnut Symposium; Malvotti, M.E., Avanzato, D., Eds.; International Society for Horticultural Science: Leuven, Belgium, 2006; Volume 705, pp. 329–333. [Google Scholar]
- Heloir, M.C.; Kevers, C.; Hausman, J.F.; Gaspar, T. Changes in the concentrations of auxins and polyamines during rooting of in-vitro-propagated walnut shoots. Tree Physiol. 1996, 16, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Olate, M.E.; Rios, D.G.; Gea, M.A.; Rodríguez, R.; Revilla, M.A. Parameters affecting the in vitro growth and rooting of Juglans regia L. Acta Hortic. 1997, 442, 235–240. [Google Scholar] [CrossRef]
- Saadat, Y.A.; Hennerty, M.J. The effects of different in vitro and ex vitro treatments on the rooting performance of Persian walnut (Juglans regia L.) microshoots. Acta Hortic. 1999, 544, 473–480. [Google Scholar] [CrossRef]
- Dolcet-Sanjuan, R.; Claveria, E.; Gruselle, R.; Meier-Dinkel, A.; Jay-Allemand, C.; Gaspar, T. Practical factors controlling in vitro adventitious root formation from walnut shoot microcuttings. J. Am. Soc. Hort. Sci. 2004, 129, 198–203. [Google Scholar] [CrossRef]
- Voyiatzis, D.G.; McGranahan, G.H. An improved method for acclimatizing tissue-cultured walnut plantlets using an antitranspirant. HortScience 1994, 29, 42. [Google Scholar] [CrossRef] [Green Version]
- Hackett, W.P.; Leslie, C.; McGranahan, G. Acclimatization of in vitro derived plantlets of walnut rootstock clones. Acta Hortic. 2007, 812, 427–430. [Google Scholar] [CrossRef]
- Chenevard, D.; Jay-Allemand, C.; Gendraud, M.; Frossard, J.S. The effect of Sucrose on the development of hybrid walnut microcuttings (Juglans nigra × Juglans regia). Consequences on their survival during acclimatization. Ann. Sci. For. 1995, 52, 147–156. [Google Scholar] [CrossRef]



| Variation Factor | |||||
|---|---|---|---|---|---|
| Variable | Genotype (G) | Culture Medium (CM) | Laboratory (a) (Lab) | GxCM | GxLab (a) |
| Length of stem | ** | *** | nd | ns | nd |
| Proliferation rate | *** | *** | *** | ns | ns |
| Diameter of calli | ** | *** | nd | ns | nd |
| Rooting (b) | *** | *** | *** | nd | nd |
| Roots/microshoot | ns | *** | *** | ns | ns |
| Survival | ** | *** | nd | ns | nd |
| Genotype | Lab | Culture Medium | Length of Stem (mm) | Proliferation Rates | Diameter of Stem (mm) | Rooting (%) | Roots/Microshoot | Survival (%) |
|---|---|---|---|---|---|---|---|---|
| Form 3 | 1 | MS-m | 15.4 ± 4.2 a | 2.0 ± 0.9 a | 3.2 ± 0.7 a | 56.7 ± 5.8 a | 2.3 ± 1.6 ab | 64.4 ± 3.8 ab |
| DKWC-m | 33.3 ± 4.4 d | 3.0 ± 0.1 bc/B | 14.2 ± 2.1 c | 76.7 ± 5.8 b/C | 3.6 ± 1.0 cd/B | 82.7 ± 6.8 cd | ||
| 2 | DKWC-m | - | 2.7 ± 0.8 B | - | 54.0 ± 6.9 B | 3.5 ± 1.2 B | - | |
| Form 4 | 1 | MS-m | 21.2 ± 5.4 b | 2.3 ± 0.6 a | 3.9 ± 1.5 ab | 76.7 ± 5.8 b | 2.2 ± 1.1 a | 52.4 ± 4.1 a |
| DKWC-m | 36.6 ± 3.7 e | 3.0 ± 0.1 bc/B | 16.5 ± 1.5 d | 73.3 ± 5.8 b/C | 3.7 ± 1.6 cd/B | 82.1 ± 15.6 cd | ||
| 2 | DKWC-m | - | 2.3 ± 0.5 A | - | 42.0 ± 6.9 AB | 2.0 ± 1.1 A | - | |
| Ideal | 1 | MS-m | 17.5 ± 2.8 a | 2.7 ± 1.3 b | 4.4 ± 1.3 b | 60.0 ± 10.0 ab | 3.1 ± 0.8 bc | 49.1 ± 8.6 a |
| DKWC-m | 39.1 ± 6.3 f | 3.6 ± 0.5 de/C | 17.3 ± 2.4 de | 70.0 ± 10.0 b/C | 3.8 ± 1.4 cd/B | 75.9 ± 16.5 bcd | ||
| 2 | DKWC-m | - | 2.2 ± 0.7 A | - | 32.0 ± 6.9 A | 2.1 ± 0.9 A | - | |
| Liaohe-1 | 1 | MS-m | 23.9 ± 3.1 c | 3.2 ± 0.9 cd | 4.2 ± 1.5 b | 56.7 ± 5.8 a | 3.1 ± 1.1 bc | 71.1 ± 7.7 bc |
| DKWC-m | 45.6 ± 7.0 g | 3.7 ± 0.5 e/C | 18.0 ± 2.3 e | 66.7 ± 15.3 ab | 4.1 ± 1.4 d | 91.1 ± 7.8 d | ||
| 2 | DKWC-m | - | 2.0 ± 0.8 A | - | 0 | 0 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yegizbayeva, T.K.; García-García, S.; Yausheva, T.V.; Kairova, M.; Apushev, A.K.; Oleichenko, S.N.; Licea-Moreno, R.J. Unraveling Factors Affecting Micropropagation of Four Persian Walnut Varieties. Agronomy 2021, 11, 1417. https://doi.org/10.3390/agronomy11071417
Yegizbayeva TK, García-García S, Yausheva TV, Kairova M, Apushev AK, Oleichenko SN, Licea-Moreno RJ. Unraveling Factors Affecting Micropropagation of Four Persian Walnut Varieties. Agronomy. 2021; 11(7):1417. https://doi.org/10.3390/agronomy11071417
Chicago/Turabian StyleYegizbayeva, Togzhan Kadylbekovna, Silvia García-García, Tatyana Viktorovna Yausheva, Markhabat Kairova, Amangeldy Kairbekovich Apushev, Sergey Nikolaevich Oleichenko, and Ricardo Julian Licea-Moreno. 2021. "Unraveling Factors Affecting Micropropagation of Four Persian Walnut Varieties" Agronomy 11, no. 7: 1417. https://doi.org/10.3390/agronomy11071417






