Abstract
Wheat stripe rust, powdery mildew, and Fusarium head blight (FHB) are the three most important diseases in wheat worldwide. Growing resistant cultivars is the most economic and effective method to control these diseases. To assess the disease resistance of commercial wheat cultivars and regional trial wheat lines in the Huang-Huai-Hai region of China, 146 wheat entries were inoculated with the Chinese prevalent Puccinia striiformis f. sp. tritici (Pst) races CYR32, CYR33, CYR34, and Blumeria graminis f. sp. tritici (Bgt) isolate E09 under controlled greenhouse conditions, respectively; these entries were also tested with the mixed Pst races, Bgt and FHB isolates at adult-plant stage in the field, respectively. The results showed that 108 (73.97%), 83 (56.85%), 99 (67.81%), and 22 (15.07%) entries were resistant to CYR32, CYR33, CYR34, and E09 at the seedling stage, respectively; 102 (69.86%), 24 (16.44%), and 2 (1.37%) entries were resistant to stripe rust, powdery mildew, and Fusarium head blight at the adult-plant stage, respectively. Additionally, the possible resistance gene(s) in these entries were postulated by the closely linked markers of stripe rust resistance genes Yr5, Yr9, Yr10, Yr15, Yr17, Yr18, Yr26, powdery mildew resistance gene Pm21, and Fusarium head blight resistance gene Fhb1. Combined with disease resistance and molecular markers tests, 62, nine, and three wheat entries were postulated to carry the Yr9, Yr17, Yr26 gene, respectively, and no entries contained Yr5, Yr10, Yr15, Yr18, Pm21, and Fhb1 gene. This study laid a theoretical foundation for rational utilization of these entries and gene in wheat breeding programs and disease control.
1. Introduction
Wheat (Triticum aestivum L.) is one of the most important food crops in the world. China’s wheat production occupies a large proportion of the world, and its total output is third only to rice and corn (available online: http://www.fao.org/faostat/zh/#data/QC (accessed on 24 April 2021)). However, wheat yield is constantly challenged by diseases such as stripe rust, powdery mildew and Fusarium head blight (FHB).
Wheat stripe rust, also known as yellow rust, caused by Puccinia striiformis f. sp. tritici (Pst), is a major threat to most wheat growing areas in the world, especially in China. Severe national-wide stripe rust epidemics in 1950, 1964, 1990, 2002, and 2017 caused production losses of 6.0, 3.2, 1.8, 1.3, and 1.5 million metric tons, respectively [,,]. In addition, the pandemic of wheat stripe rust in 2020 was the highest in the past decade, which has once again sounded an alarm for us []. Powdery mildew, caused by the pathogen Blumeria graminis (DC.) Speer f. sp. tritici emend. É. J. Marchal (Bgt), can cause 30–40% yield losses in epidemic years [,]. In recent years, the area affected by powdery mildew in China is about 6–8 million hectares each year []. Fusarium head blight, also known as wheat scab, mainly caused by Fusarium graminearum Schwabe, occurs directly on wheat spikes and cause both grain yield losses and food toxins, which is imposing health threats to humans and livestock. Chemical controls have been proven to be an effective way for these three wheat diseases, but usually with additional cost and detrimental impact on the environment. In contrast, host resistance has been proposed as an effective and environmentally friendly measure to control the diseases. Therefore, it is necessary to explore wheat germplasm resources to identify genes resistant to the three important wheat diseases [].
Up to now, 83 Yr genes have been officially named []. Most of these genes are race-specific and confer resistance at all stages. However, due to the rapid changes in the virulence of pathogen populations, the resistance provided by these genes can be easily triumphed over by the new virulence Pst races. For example, Yr9 and Yr26 have been widely used in wheat breeding programs in China since the early 1970s and in recent years [,], respectively. However, the resistance of Yr9 and Yr26 have been overcome by the Pst race CYR29 in 1990 and V26 in 2009, respectively [,]. Except for Yr9 and Yr26, Yr17, which was also widely used in wheat breeding both in China and worldwide [], has become ineffective in China. So far, only a few Yr genes such as Yr5 and Yr15 are effectively resistant to all known Pst races in China and around the world []. Additionally, there are a few Yr genes that confer non-race-specific resistance, acting at the adult plant stage such as Yr18, which is a multi-pathogen resistance gene and confers part field resistance against stripe rust, leaf rust, stem rust, and powdery mildew have been used in breeding programs for a century and so far, no pathogen adaptability has been found [].
Similar to Yr genes, although more than 100 Pm genes/alleles have been reported, few can provide resistance to the predominate Bgt isolates []. Generally speaking, Pm genes derived from wild relatives of wheat are often resistant to most Bgt isolates. For example, Pm21, derived from Haynaldia villosa (2n = 2x = 14, VV) [], confers high resistance to Bgt throughout all growth stages. The commercial varieties harboring Pm21 have been widely used in wheat production with more than four million hectares in China []. In addition, although more than 50 quantitative trait loci (QTLs) for FHB resistance have been documented [], only Fhb1 has a consistently significant effect on resistance to a broad spectrum of Fusarium specie []. Fhb1, derived from Chinese wheat cultivar Sumai3, is the most important quantitative trait locus (QTL) and has been reported to provide a high level of resistance against FHB. Fhb1 and those carrying Fhb1 have been considered the ideal sources of resistance all over the world for half a century [].
The Huang-Huai-Hai wheat region is the most important wheat production base in China. Identifying new resistance sources and understanding the distribution of wheat stripe rust, powdery mildew, and FHB resistance genes in wheat entries in this region may provide valuable resistance germplasms and molecular basis for predictive resistance breeding programs. A total of 146 wheat entries collected from this region were inoculated with Pst or Bgt races (isolate), both in the seedling and adult-plant stage, and the Fusarium head blight resistance were tested in the field. Additionally, the stripe rust resistance genes Yr5, Yr9, Yr10, Yr15, Yr17, Yr18, Yr26, powdery mildew resistance gene Pm21 and Fusarium head blight resistance gene Fhb1 were selected to test these wheat entries.
2. Materials and Methods
2.1. Plant Materials
A total of 146 wheat entries (Table S1) including 47 commercial wheat varieties, which were the most cultivated, and 98 regional trial wheat lines collected from the Huang-Huai-Hai region of China, were tested in this study. Wheat cultivars Mingxian169 and Avocet S were used as the susceptible controls in the resistance identification both at the seedling and adult-plant stages. Yr gene near-isogenic lines (NIL), Avocet S*6/Yr5, Avocet S*6/Yr9, Avocet S*6/Yr10, Avocet S*6/Yr15, Avocet S*6/Yr17, Avocet S*6/Yr18, and Avocet S*6/Yr26 were used as a positive controls in Yr gene detection, and Mingxian169 and Avocet S as the negative controls. Yangmai5 and Sumai3 were also used as the Pm21 and Fhb1 positive controls and Jingshuang16 and Mingxian169 as the negative controls, respectively.
2.2. Seedling Tests
Seedling tests of stripe rust resistance were conducted in controlled greenhouse conditions. For each wheat entry, about 6–8 seeds were planted in 7-cm-diameter plastic pots. When the first leaves were fully expanded (about 10 days after planting), seedlings were inoculated with the predominant Chinese Pst races, CYR32, CYR33, and CYR34, by dusting with a mixture of fresh urediniospores and talcum powder at a 1:20 ratio, respectively. After inoculation, the seedlings were incubated in a dark box at 8–10 °C and 100% relative humidity for 24 h, and then transferred to a controlled condition greenhouse for 16 h/light and 8 h/darkness, and a temperature of 15–17 °C. The infection types (ITs) were scored about 15–17 days after inoculation according to the 0–9 level described by Line and Qayoum []. Entries with IT 0–5 were considered as the all-stage resistant (ASR) group, and those with ITs 6–9 as the susceptible group.
In the meantime, seeds of the 146 cultivars (lines) and control cultivar Jingshuang16 were planted in plastic trays. When the first leaf fully expanded, inoculation was conducted with the prevalent Bgt isolate E09 by sprinkling the conidia from the spore-susceptible seedlings of Jingshuang16 onto the tested seedlings. After inoculation, the seedlings were kept in an incubator with a 12 h light/12 h dark cycle and a temperature of about 18 °C. The infection type (IT) was scored based on 0–4 levels at ten days post inoculation (dpi) []; the phenotypes of plants were considered as resistant (ITs 0–2) or susceptible (ITs 3–4) to powdery mildew.
The seedling tests of wheat stripe rust and powdery mildew were conducted three times after susceptible controls fully onset, and the highest IT was selected as the final investigation result.
2.3. Adult-Plant Stage Tests in Field
The adult-plant resistance (APR) evaluations were performed during the 2018–2019, and 2019–2020 crop seasons in a field of the Northwest A&F University experimental station. About 20–30 seeds of each cultivar were planted in a 1-m line with 25-cm space between rows in each disease nursery. A total of three square nurseries were designed for stripe rust, powdery mildew, and head blight, separated by a protective row in the middle. Susceptible cultivars Mingxian169 (stripe rust), Jingshuang16 (powdery mildew), and the resistance cultivar Sumai3 (Fusarium head blight) were planted every 20 rows throughout each disease nursery. Stripe rust and powdery mildew inoculations with the mixture Pst races or Bgt isolates were carried out at the beginning of stem extension stage, respectively. Type II FHB resistance, defined as a disease that inhibits the spread of point infection, was employed to inoculate with F. graminearum spores with single basal florets on spikelets as previously described []. A total of 146 entries were identified by artificial inoculation, 20 spikes of each entry, and the rest of the spikes were sprayed with water as a control. Before heading and blooming of wheat, inoculating the middle spikelet of the wheat ear with single basal florets, and the inoculation amount of each spikelet was one millet mycelium. Water was sprayed on the inoculated ear and covered with a transparent plastic bag to keep it moisturized. After the inoculated spikelet turned brown, we removed the plastic bag immediately to allow it to expand under natural conditions. Disease severity (DS), scored as the percentage leaf area with disease symptoms, was evaluated three times between the early and late dough stages. According to Bariana and McIntosh [], recording IT of strip rust on a scale of 0–4 as “0”, “0;”, “1”, “2”, “3”, “4”. Accession with IT 0–2 was categorized into the resistant and those with IT 3–4 into susceptible.
ITs of wheat powdery mildew were scored according to the 0–9 scale as Saari and Prescott described []. Plants with ITs 0–6 were classified as resistant, and ITs 7–9 as susceptible. For evaluation of the type II FHB resistance of wheat, the total number of diseased spikelets and rachises were calculated as disease severity indices at 1–3 weeks after inoculation with F. graminearum, and disease index was recorded as Liu et al. []. Disease severity was calculated as the average percentage of infected spikelets. FHB severity of 0 was considered immune (I), those with severity greater than 0 and less than 2.0 were considered resistant (R), severity greater than or equal to 2.0 and less than 3.0 were considered moderately resistant (MR), those greater than or equal to 3.0 and less than 3.5 were considered moderately susceptible (MS), and 3.5 or more were considered as susceptible (S) (Table S1).
2.4. DNA Extraction
After seedling tests, the healthy leaves of 146 wheat entries and the control lines were collected for DNA extraction. Genomic DNA was isolated using the cetyltrimethylammonium bromide (CTAB) protocol with slight modifications []. The DNA was quantified using electrophoresis and spectrophotometry with NanoDrop (ND-1000, Thermo Scientific, Wilmington, DE, USA) and the concentration were adjusted to 80 ng/μL with sterilized ddH2O for use as a polymerase chain reaction (PCR) template.
2.5. PCR Amplification and Electrophoresis Analysis
Two pairs of closely linked markers for each of the resistance genes Yr5, Yr9, Yr10, Yr15, Yr17, Yr18, Yr26, Pm21, and Fhb1 were selected for molecular detection (replaced by the follow Table 1), which were synthesized by Sangon Biotech Co. Ltd. (Shanghai, China). The specific amplification procedures were in accordance with the corresponding references (Table 2 and Table 3). The test was repeated for each sample three to four times, all three repetitions were detected as present. The products of DNA amplification were separated by 2% agarose gel. A total of four pairs of KASP primers were used to detect the presence of Yr5, Yr15, Yr26, and Fhb1. Each KASP reaction was carried out using a 5.07 µL reaction mixture consisting of 2.5 µL DNA, 2.5 µL 2 × KASP master mix (LGC, Hoddesdon, Herts, UK), and 0.07 µL KBD Assay mix primer (12 mM of each allele-specific primer and 30 mM of the common primer). The KASP thermal protocol was run as outlined in Table 4. The FLUOstar Omega microplate reader (BMG Labtech, Durham, NC, USA) was used to read the different genotypes, FAM homozygotes, and HEX homozygotes using the KlusterCallerTM software (available online: http://www.lgcgroup.com/ (accessed on 25 April 2021).

Table 1.
Primers used to detect Yr, Pm, and Fhb resistance genes in the Huang-Huai-Hai region of China wheat cultivars (lines).

Table 2.
The PCR reactions of different selected markers.

Table 3.
The PCR protocols of different molecular markers.

Table 4.
Thermal cycle conditions for KASP genotyping reactions.
2.6. Data Analysis
Phenotype and genotype data analysis were performed using Microsoft® Excel® 2016 (MS, Redmond, WA, USA). The distribution of resistance genes in the Huang-Huai wheat region was determined by screening varieties that showed resistance in both the genotype and phenotype according to Section 2.2 and Section 2.3.
3. Results
3.1. Seedling Resistance Evaluation
Among the 146 entries, 108 were resistant to Pst race CYR32, 83 resistant to CYR33, and 99 resistant to CYR34, accounting for 73.97%, 56.85%, and 67.81% (Figure 1, Table S1), respectively; 68 entries, accounting for 46.58%, were resistant all the tested Pst races. However, only 22 entries showed resistance to Bgt isolate E09 at the seedling stage, accounting for 15.07%. In addition, 11 (7.53%) wheat varieties showed resistance to both stripe rust and powdery mildew. In total, the stripe rust resistance of these entries was better than that of powdery mildew and few entries were resistant to both stripe rust and powdery mildew.
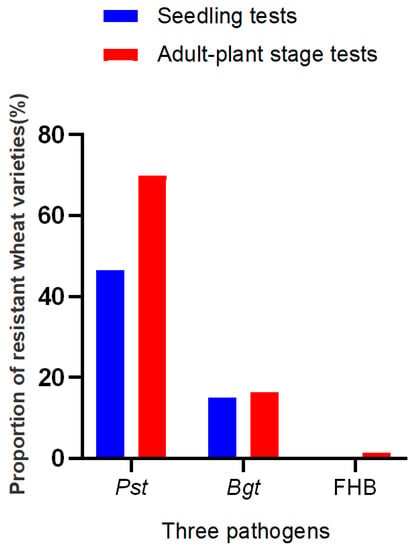
Figure 1.
The proportion of wheat entries with different types of resistance to stripe rust, powdery mildew, and FHB.
3.2. Adult-Plant Resistance Evaluation
The results of adult-plant resistance evaluation indicated that 102 entries showed resistance to stripe rust, accounting for 69.86%; 24 entries showed resistance to powdery mildew, accounting for 16.44%; and two entries showed resistance to head blight, accounting for 1.37% (Figure 1, Table S1). It was worth mentioning that among these 102 stripe rust resistance entries and 24 powdery mildew resistant entries, 48 and 10 entries were susceptible in the seedling evaluation, respectively, which indicated that these entries had adult-plant stage resistance (APR). Only two cultivars, Xinong650 and Fugao2, showed resistant to head blight, accounting for 1.37%. In addition, 17(11.64%) wheat entries showed resistance to both stripe rust and powdery mildew and only one accession, Xinong650, showed resistance to both strip rust and head blight. No variety was resistant to all three wheat diseases.
3.3. Identification of the Resistance Genes Using Molecular Markers
3.3.1. Identification of Yr Genes
The known stripe rust resistance genes Yr5 [], Yr9 [,], Yr10 [], Yr15 [,], Yr17 [], Yr18 [,], Yr26 [,], closely linked markers or functional markers, were used for molecular detection of 146 wheat varieties in the Huang-Huai-Hai wheat region, and these seven stripe rust resistance gene donors were used as positive controls (Figure 2A–G). It was concluded that among the tested wheat varieties in the Huang-Huai-Hai wheat region, only two entries (1.37%), Baojingmai186 and Baojingmai166, could produce Yr5 alleles; a total of 62 wheat entries (43.15%) produced Yr9 specific bands in both markers; nine entries (6.16%), Haomai18, Xinong328, WeifengT03, Xi’an139, Xichun919, Hangmai9, Huaikemai8, Xinong369, and Xinong528 amplified Yr17 target bands; three entries, Xinong161, Shaanza10, and Shaanmai139, may carry Yr26. Among these, Baojingmai186 may carry Yr5 and Yr9 at the same time and Shaanza10 may carry Yr9 and Yr26 at the same time; three accessions, WeifengT03, Xichun919, and Xinong528 may carry Yr9 and Yr17 at the same time. No entries that may carry Yr10, Yr15, and Yr18 were detected in the test materials (Table S1).
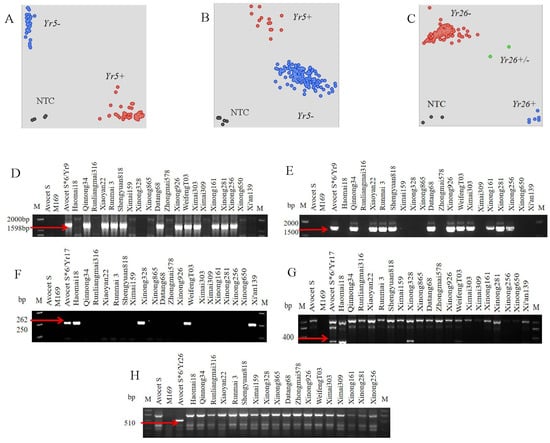
Figure 2.
Electrophoretogram and genotyping data of different markers. (A,B), identification of Yr5 resistance gene using KASP makers; (D,E), identification of Yr9; (F,G), identification of Yr17; (C,H), identification of Yr26. Yr5+/Yr26+, Yr5−/Yr26−, indicating presence/absence of Yr5/Yr26 in the tested entries for the horizontal and vertical axis, respectively. NTC, non-template control.
3.3.2. Identification of Pm Gene
Pm21, located on the short arm of chromosome 6V (6VS) of Dasypyrum villosum, confers immunity to all known Bgt isolates and has been widely used in wheat breeding []. The presence of Pm21 was identified with markers WS-1 [] and specific SCAR marker SCAR1400 [], which can produce 949 bp and 1400 bp target bands in wheat carrying Pm21, respectively. No entry can amplify the Pm21 target band of 949 bp when using the primer of WS-1. Interestingly, only Xinong136 and Gaoke1128 can amplify the target band of 1400 bp when using the primer of SCAR1400. However, both varieties showed susceptibility to powdery mildew in the identification of seedling and adult-plant stage, which means that these two entries may not carry the Pm21 gene (Table S1).
3.3.3. Identification of Fhb Gene
Fhb1, originating from Sumai 3, is one of the most important Fhb resistance genes in hexaploid wheat []. When detected with diagnostic marker His-InDel [], a 1309 bp band can be amplified in wheat entries containing the Fhb1 gene, if not, a band of 2061 bp can be amplified. Among all 146 wheat varieties, no target band of 1309 bp could be amplified. When using the Fhb1-TaHRC-KASP marker [], susceptible genotypes will cluster along the vertical axis and resistant genotypes will cluster along the horizontal axis. The test results based on the two types of markers showed that no entries may contain the resistance gene Fhb1 (Table S1).
4. Discussion
In the disease identification of 146 wheat varieties, 51 (34.93%) entries showed resistance to stripe rust both at the seedling and adult-plant stages. That is to say, these 51 entries had ASR and the remaining 51 (34.93%) had APR to stripe rust. Twenty-four (16.44%) showed resistance to Bgt mixture isolates at the adult stage and 14 wheat varieties were susceptible to powdery mildew at the seedling stage, indicating that 14 (9.59%) entries had APR to powdery mildew, and 10 (6.85%) entries showed moderate resistance to powdery mildew at all stages. Two (1.37%) entries showed resistance to head blight. It could be seen that whether it was to stripe rust or to powdery mildew, the resistance at the adult-plant stage was stronger than that at the seedling stage (Figure 1). There were still few wheat varieties that were resistant to FHB. In the molecular detection of 146 wheat varieties, the detection rates from high to low were Yr9, Yr17, Yr26, and Yr5 genes. Surprisingly, none of the entries were detected to contain Yr10, Yr15, Yr18, Pm21, and Fhb1. Two cultivars that have been detected to contain Yr5 were not resistant to stripe rust at the seedling stage, which indicated that they may not have the Yr5 gene and might be due to the false positives in molecular testing. Yr9 was susceptible to these tested Pst races, which means those that have been detected to contain Yr9 and were resistant at the seedling stage may also contain other effective ASR Yr genes. Yr17 was also susceptible to CYR32, CYR33, and CYR34, however, all nine wheat entries showed resistance to one or more Pst races in the seedling stage and were resistant to the mixture Pst race in the adult-plant stage, therefore, these entries may also carry some other effective Yr gene(s). 51 wheat entries had APR to stripe rust in this study, whereas no entries were detected to carry Yr18, which indicated that these entries may carry other effective APR gene(s). Yr26 was resistant to Pst races CYR32 and CYR33 but susceptible to CYR34 in the seedling stage. In fact, all three entries were resistant to CYR34 in the seedling tests, which suggested that these entries may also carry other Yr gene(s) besides Yr26.
When testing 494 Chinese wheat entries for stripe rust resistance in both seedling and adult-plant stages, Zeng et al. [] found that 16 (3.24%) entries had ASR in all race tests, and 99 (20.04%) had APR. The frequencies of ASR and APR in this study were all higher than that of Zeng et al., which maybe resulted by different Pst races. Zhu et al. [] reported that 29.08% and 11.41% of wheat lines were resistant to Bgt and FHB in the field, respectively, which is similar with that in this study. Therefore, more attention should be paid to wheat resistance to stripe rust, powdery mildew, and Fusarium head blight in wheat breeding programs, especially to powdery mildew and Fusarium head blight. According to the virulence and avirulence formula of Yr genes [], Yr5 is known to have all-stage resistance to all tested Pst races, and Yr9 has become susceptible to most Chinese Pst races since 1990 [], and 43.15% of entries were detected to carry Yr9, which is consistent with previous studies [,]. Yr5 and Yr15 were still effectively resistant to almost all the known Pst races in China. However, no entries were postulated to carry Yr5 and Yr15 in this study. According to the previous studies, Yr5 has been rarely used in wheat breeding programs in China [,], and Yr15 has not been detected in Chinese cultivars [,,,]. Both Yr5 and Yr15 were successfully cloned in 2018 [,], which will facilitate their use in wheat breeding programs in the future. In fact, Yr18/Lr34/Sr57/Pm38 has been successfully used in wheat resistance breeding since the beginning of the last century, and it provides durable, adult-plant, slow-rusting resistance to stripe rust, leaf rust, stem rust, and powdery mildew. Similar to our study, Huang et al. [] also reported that no entries carried Yr18 in 66 selected commercial wheat cultivars. However, Yr18 has a high detection percent in Chinese wheat landraces [,]. Moreover, the disease resistance will be improved dramatically if Yr18 is pyramided with other all-stage resistance gene(s) or adult-plant resistance gene(s) []. Therefore, Chinese wheat landraces should be developed as the Yr18 resource for durable resistance cultivars breeding.
The Pm21 gene has been proven to be very effective against a broad-spectrum of wheat powdery mildew isolates identified in China []. Using molecular marker WS-1, Jiang et al. (2014) found that 49 (7.4%) of 662 wheat cultivars (lines) may carry Pm21 []. However, other researchers have shown that in Guizhou and Sichuan Provinces where wheat powdery mildew is severe, there is a high frequency of Pm21 in wheat varieties []. Therefore, Pm21 is not suggested for use alone in wheat breeding programs although it has low frequency occurrence in some regions. It would be great if it could be pyramided with other effective Pm resistance genes such as Pm12, Pm24 [], and Pm68 [].
Several types of FHB resistance have been identified in wheat, resistance to initial infection (Type I), and resistance to spread within the spike (Type II) following single floret injections. The greatest effect of Fhb1 imparting stable FHB type II resistance is the main source of resistance in wheat breeding. In North America, Fhb1 has been widely used in spring wheat areas where wheat scab occurred seriously, for example, the cultivars Alsen [] and Sabin []. In China, Fhb1 has been successfully used in wheat breeding programs in spring wheat areas such as Jiangsu Province, however, it has not been used widely in winter wheat areas. In this study, there were only two wheat entries resistant to head blight in the field, but Fhb1 was not detected in both of the entries, which suggested that the FHB resistance may be related to other FHB resistance gene(s). Xu et al. [] reported that none of the 22 commercial wheat cultivars in the Huang-Huai-Hai contained the Fhb1 gene and six of nine cultivars in the middle and lower reaches of the Yangtze River carried Fhb1. Zhu et al. [] found that Fhb1 contained in Chinese wheat entries were mainly derived from Sumai3 and Ningmai9, with the latter being the main one, which can be used as a valid source of resistance for breeding. Recently, another effective FHB resistance gene Fhb7, which was derived from Thinopyrum elongatum, has been isolated. The resistance to FHB of wheat cultivars can be significantly enhanced after introducing the Fhb7 gene []. Therefore, Fhb1 and Fhb7 have been suggested for use together in wheat breeding programs.
Compared to other studies that have only focused on one type of wheat disease at a time, this research is more in line with agricultural practices. It not only lays a theoretical foundation for the balanced application of these genotypes and genes in wheat breeding programs and disease control, but also improves the host resistance to these three dangerous pathogens widespread in nature at the same time.
5. Conclusions
The results revealed that the number of resistant cultivars to Bgt and FHB in Huang-Huai-Hai region is small, and the diversity of resistance genes is also low. Due to Yr9, Yr10, Yr17, and Yr26 have become ineffective to Pst prevalent races, these genes are not recommended for use in wheat breeding; Yr5, Yr15, Yr18, Pm21, and Fhb1 should not be used alone, but can be used with other effective gene(s) to get broad-spectrum and durable resistance cultivars. The use of resistant cultivars with multiple or different resistance genes can reduce the accumulation of inoculum. If the cultivar is susceptible, it should be withdrawn from production as soon as possible.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agronomy11061025/s1, Table S1: Infection types to Pst races, Bgt and FHB isolates and molecular detection results with the specific markers. Notes: NT, not tested; “+”, had the same genotyping to the reference primers, may carry the tested gene(s); “−”, indicating that the entry may not carry the resistance gene(s) as the target band could not be amplified.
Author Contributions
K.M., X.L., Y.L., and B.Z. investigated the data. K.M. interpreted the data and wrote the manuscript. X.L. conceived the idea to plan this study. Z.W., data curation, formal analysis. Q.L. and B.W. designed the project and critically reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key Research and Development Program of China (2018YFD0200404 and 2016YFD0300705), the Natural Science Foundation of Shaanxi Province (2019JZ-17), the Technical Guidance Project of Shaanxi Province (2017CGZH-HJ-01), and the National Natural Science Foundation of China (31701745).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| APR | Adult-plant resistance |
| ASR | All-stage resistance |
| Bgt | Blumeria graminis f.sp. tritici Em. Marchal |
| CYR | Chinese yellow rust |
| DNA | Deoxyribonucleic acid |
| DS | Disease severity |
| EST | Expressed sequence tag |
| FHB | Fusarium head blight |
| Fhb | Fusarium head blight resistance genes |
| IT | Infection type |
| KASP | Kompetitive allele-specific PCR |
| NIL | near isogenic line |
| PCR | Polymerase chain reaction |
| Pm | Powdery mildew resistance genes |
| Pst | Puccinia striiformis Westend. f. sp. tritici Erikss |
| SSR | Simple sequence repeat |
| STS | Sequence-tagged site |
| Yr | Yellow rust (stripe rust) resistance genes |
References
- Chen, X.M. Epidemiology and control of stripe rust (Puccinia striiformis f. sp. tritici) on wheat. Can. J. Plant Pathol. 2005, 27, 314–337. [Google Scholar] [CrossRef]
- Wan, A.M.; Chen, X.M.; He, Z.H. Wheat stripe rust in China. Aust. J. Agric. Res. 2007, 58, 605–619. [Google Scholar] [CrossRef]
- Ma, Z.H. Researches and control of wheat stripe rust in China. J. Plant Prot. 2018, 45, 1–6. [Google Scholar] [CrossRef]
- Wu, J.; Yu, R.; Wang, H.; Zhou, C.; Huang, S.; Jiao, H.; Yu, S.; Nie, X.; Wang, Q.; Liu, S.; et al. A large-scale genomic association analysis identifies the candidate causal genes conferring stripe rust resistance under multiple field environments. Plant Biotechnol. J. 2021, 19, 177–191. [Google Scholar] [CrossRef]
- Samobor, V.; Vukobratović, M.; Jošt, M. Effect of powdery mildew attack on quality parameters and experimental bread baking of wheat. Acta Agric. Slov. 2006, 87, 381–391. Available online: http://aas.bf.uni-lj.si/september2006/18jost.pdf (accessed on 24 April 2021).
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jorgensen, L.N.; Hovmoller, M.S.; Huerta-Espino, J. Disease Impact on Wheat Yield Potential and Prospects of Genetic Control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef]
- Sun, H.G.; Hu, J.H.; Song, W.; Qiu, D.; Cui, L.; Wu, P.P.; Zhang, H.J.; Liu, H.W.; Yang, L.; Qu, Y.F.; et al. Pm61: A recessive gene for resistance to powdery mildew in wheat landrace Xuxusanyuehuang identified by comparative genomics analysis. Theor. Appl. Genet. 2018, 131, 2085–2097. [Google Scholar] [CrossRef]
- Wang, H.W.; Sun, S.L.; Ge, W.Y.; Zhao, L.F.; Hou, B.Q.; Wang, K.; Lyu, Z.F.; Chen, L.Y.; Xu, S.S.; Guo, J.; et al. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 2020, 368, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Li, J.B.; Dundas, I.; Dong, C.M.; Li, G.R.; Trethowan, R.; Yang, Z.J.; Hoxha, S.; Zhang, P. Identification and characterization of a new stripe rust resistance gene Yr83 on rye chromosome 6R in wheat. Theor. Appl. Genet. 2020, 133, 1095–1107. [Google Scholar] [CrossRef]
- Zhou, Y.; He, Z.; Zhang, G.S.; Xia, L.Q. Utilization of 1BL/1RS translocation in wheat breeding in China. Zuo Wu Xue Bao 2004, 30, 531–535. [Google Scholar]
- Han, D.J.; Wang, Q.L.; Chen, X.M.; Zeng, Q.D.; Wu, J.H.; Xue, W.B.; Zhan, G.M.; Huang, L.L.; Kang, Z.S. Emerging Yr26-Virulent Races of Puccinia striiformis f. sp. tritici Are Threatening Wheat Production in the Sichuan Basin, China. Plant Dis. 2015, 99, 754–760. [Google Scholar] [CrossRef]
- McIntosh, R.; Mu, J.M.; Han, D.J.; Kang, Z.S. Wheat stripe rust resistance gene Yr24/Yr26: A retrospective review. Crop. J. 2018, 6, 321–329. [Google Scholar] [CrossRef]
- Liu, J.; Chang, Z.J.; Zhang, X.J.; Yang, Z.J.; Li, X.; Jia, J.Q.; Zhan, H.X.; Guo, H.J.; Wang, J.M. Putative Thinopyrum intermedium-derived stripe rust resistance gene Yr50 maps on wheat chromosome arm 4BL. Theor. Appl. Genet. 2013, 126, 265–274. [Google Scholar] [CrossRef]
- Ash, G. Wheat Rusts: An Atlas of Resistance Genes. Australas. Plant Pathol. 1996, 25, 70. [Google Scholar] [CrossRef]
- Sharma-Poudyal, D.; Chen, X.M.; Wan, A.M.; Zhan, G.M.; Kang, Z.S.; Cao, S.Q.; Jin, S.L.; Morgounov, A.; Akin, B.; Mert, Z.; et al. Virulence Characterization of International Collections of the Wheat Stripe Rust Pathogen, Puccinia striiformis f. sp. tritici. Plant Dis. 2013, 97, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Krattinger, S.G.; Sucher, J.; Selter, L.L.; Chauhan, H.; Zhou, B.; Tang, M.Z.; Upadhyaya, N.M.; Mieulet, D.; Guiderdoni, E.; Weidenbach, D.; et al. The wheat durable, multipathogen resistance gene Lr34 confers partial blast resistance in rice. Plant Biotechnol. J. 2016, 14, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Jia, H.Y.; Zhang, X.J.; Qiao, L.Y.; Li, X.; Zheng, J.; Guo, H.J.; Powers, C.; Yan, L.L.; Chang, Z.J. Positional cloning of PmCH1357 reveals the origin and allelic variation of the Pm2 gene for powdery mildew resistance in wheat. Crop. J. 2019, 7, 771–783. [Google Scholar] [CrossRef]
- He, H.G.; Zhu, S.Y.; Zhao, R.H.; Jiang, Z.N.; Ji, Y.Y.; Ji, J.; Qiu, D.; Li, H.J.; Bie, T.D. Pm21, Encoding a Typical CC-NBS-LRR Protein, Confers Broad-Spectrum Resistance to Wheat Powdery Mildew Disease. Mol. Plant 2018, 11, 879–882. [Google Scholar] [CrossRef]
- Xing, L.; Hu, P.; Liu, J.; Witek, K.; Zhou, S.; Xu, J.; Zhou, W.; Gao, L.; Huang, Z.; Zhang, R.; et al. Pm21 from Haynaldia villosa Encodes a CC-NBS-LRR Protein Conferring Powdery Mildew Resistance in Wheat. Mol. Plant 2018, 11, 874–878. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Ban, T.; Anderson, J.A. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: A review. Plant Breed. 2009, 128, 1–26. [Google Scholar] [CrossRef]
- Bernardo, A.N.; Ma, H.X.; Zhang, D.D.; Bai, G.H. Single nucleotide polymorphism in wheat chromosome region harboring Fhb1 for Fusarium head blight resistance. Mol. Breed. 2012, 29, 477–488. [Google Scholar] [CrossRef]
- Chen, S.L.; Zhang, Z.L.; Sun, Y.Y.; Li, D.S.; Gao, D.R.; Zhan, K.H.; Cheng, S.H. Identification of quantitative trait loci for Fusarium head blight (FHB) resistance in the cross between wheat landrace N553 and elite cultivar Yangmai 13. Mol Breed. 2021, 41, 24. [Google Scholar] [CrossRef]
- Line, R.F.; Qayoum, A. Virulence, aggressiveness, evolution, and distribution of races of Puccinia striiformis (the cause of stripe rust of wheat) in North America. Tech. Bull.-United States Dep. Agric. 1992, 44, 1968–1987. [Google Scholar]
- Sheng, B.Q. Wheat powdery mildew was recorded using infection type in seedling stage. Plant Prot. 1984, 6, 49–53. [Google Scholar]
- Su, P.S.; Guo, X.X.; Fan, Y.H.; Wang, L.; Yu, G.H.; Ge, W.Y.; Zhao, L.F.; Ma, X.; Wu, J.J.; Li, A.F.; et al. Application of Brachypodium genotypes to the analysis of type II resistance to Fusarium head blight (FHB). Plant Sci. 2018, 272, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Bariana, H.S.; McIntosh, R.A. Cytogenetic studies in wheat. XV. Location of rust resistance genes in VPM1 and their genetic linkage with other disease resistance genes in chromosome 2A. Genome 1993, 36, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Saari, E.E.; Prescott, J.M. A scale for appraising the foliar intensity of wheat diseases. Plant Dis. Rep. 1975, 59, 377–380. [Google Scholar]
- Liu, L.Y.; Dong, Y.Y.; Huang, W.J.; Du, X.P.; Ren, B.Y.; Huang, L.S.; Zheng, Q.; Ma, H.Q. A Disease Index for Efficiently Detecting Wheat Fusarium Head Blight Using Sentinel-2 Multispectral Imagery. IEEE Access 2020, 8, 52181–52191. [Google Scholar] [CrossRef]
- Saghai-Maroof, M.A.; Soliman, K.M.; Jorgensen, R.A.; Allard, R.W. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. USA 1984, 81, 8014–8018. [Google Scholar] [CrossRef]
- Marchal, C.; Zhang, J.P.; Zhang, P.; Fenwick, P.; Steuernagel, B.; Adamski, N.M.; Boyd, L.; McIntosh, R.; Wulff, B.B.H.; Berry, S.; et al. BED-domain-containing immune receptors confer diverse resistance spectra to yellow rust. Nat. Plants 2018, 4, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, Z.J.; Li, G.R.; Zeng, Z.X.; Zhang, Y.; Zhou, J.P.; Liu, Z.H.; Ren, Z.L. Isolation of a new repetive DNA sequece from Secale africanum enables targeting of Secale chromatin in wheat background. Euphytica 2008, 159, 249–258. [Google Scholar] [CrossRef]
- Francis, H.A.; Leitch, A.R.; Koebner, R.M.D. Conversion of a Rapd-Generated Pcr Product, Containing a Novel Dispersed Repetitive Element, into a Fast and Robust Assay for the Presence of Rye Chromatin in Wheat. Theor. Appl. Genet. 1995, 90, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Datta, D.; Priyamvada, S.S.; Tiwari, R. A diagnostic PCR based assay for stripe rust resistance gene Yr10 in wheat. Acta Phytopathol. Entomol. Hung. 2009, 44, 11–18. [Google Scholar] [CrossRef]
- Klymiuk, V.; Yaniv, E.; Huang, L.; Raats, D.; Fatiukha, A.; Chen, S.S.; Feng, L.H.; Frenkel, Z.; Krugman, T.; Lidzbarsky, G.; et al. Cloning of the wheat Yr15 resistance gene sheds light on the plant tandem kinase-pseudokinase family. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Zou, J.W.; Jia, W.L.; Li, L.X.; Chen, X.; Jia, D.; Yan, C.S. Kasp marker assays for functional genes of important trait in 120 wheat cultivars (lines). Mol. Plant. Breed. 2019, 17, 3945–3959. [Google Scholar] [CrossRef]
- Helguera, M.; Khan, I.A.; Kolmer, J.; Lijavetzky, D.; Zhong, Q.L.; Dubcovsky, J. PCR assays for the Lr37-Yr17-Sr38 cluster of rust resistance genes and their use to develop isogenic hard red spring wheat lines. Crop. Sci. 2003, 43, 1839–1847. [Google Scholar] [CrossRef]
- Lagudah, E.S.; McFadden, H.; Singh, R.P.; Huerta-Espino, J.; Bariana, H.S.; Spielmeyer, W. Molecular genetic characterization of the Lr34/Yr18 slow rusting resistance gene region in wheat. Theor. Appl. Genet. 2006, 114, 21–30. [Google Scholar] [CrossRef]
- Lagudah, E.S.; Krattinger, S.G.; Herrera-Foessel, S.; Singh, R.P.; Huerta-Espino, J.; Spielmeyer, W.; Brown-Guedira, G.; Selter, L.L.; Keller, B. Gene-specific markers for the wheat gene Lr34/Yr18/Pm38 which confers resistance to multiple fungal pathogens. Theor. Appl. Genet. 2009, 119, 889–898. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.P.; Han, D.J.; Kang, Z.S.; Li, G.P.; Cao, A.Z.; Chen, P.D. SSR and STS markers for wheat stripe rust resistance gene Yr26. Euphytica 2008, 159, 359–366. [Google Scholar] [CrossRef]
- Wu, J.H.; Zeng, Q.D.; Wang, Q.L.; Liu, S.J.; Yu, S.Z.; Mu, J.M.; Huang, S.; Sela, H.A.; Distelfeld, A.; Huang, L.L.; et al. SNP-based pool genotyping and haplotype analysis accelerate fine-mapping of the wheat genomic region containing stripe rust resistance gene Yr26. Theor. Appl. Genet. 2018, 131, 1481–1496. [Google Scholar] [CrossRef]
- He, H.G.; Ji, Y.Y.; Zhu, S.Y.; Li, B.; Zhao, R.H.; Jiang, Z.N.; Bie, T.D. Genetic, Physical and Comparative Mapping of the Powdery Mildew Resistance Gene Pm21 Originating from Dasypyrum villosum. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, Q.L.; Wu, J.H.; Xue, W.B.; Zeng, Q.D.; Huang, L.L.; Kang, Z.S.; Han, D.J. Distribution of powdery mildew resistance gene Pm21 in Chinese winter wheat cultivars and breeding lines based on gene-specific marker. Sci. Agric. Sin. 2014, 47, 2078–2087. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Q.; Ni, Z.; Yang, T. Development of SCAR markers linked to the Pm21 gene conferring resistance to powdery mildew in common wheat. Plant Breed. 1999, 118, 215–219. [Google Scholar] [CrossRef]
- Brar, G.S.; Brule-Babel, A.L.; Ruan, Y.F.; Henriquez, M.A.; Pozniak, C.J.; Kutcher, H.R.; Hucl, P.J. Genetic factors affecting Fusarium head blight resistance improvement from introgression of exotic Sumai 3 alleles (including Fhb1, Fhb2, and Fhb5) in hard red spring wheat. BMC Plant Biol. 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Q.; Jin, S.J.; Zhang, D.D.; Bai, G.H. Development and validation of diagnostic markers for Fhb1 region, a major QTL for Fusarium head blight resistance in wheat. Theor. Appl. Genet. 2018, 131, 2371–2380. [Google Scholar] [CrossRef]
- Su, Z.Q.; Bernardo, A.; Tian, B.; Chen, H.; Wang, S.; Ma, H.X.; Cai, S.B.; Liu, D.T.; Zhang, D.D.; Li, T.; et al. A deletion mutation in TaHRC confers Fhb1 resistance to Fusarium head blight in wheat. Nat. Genet. 2019, 51, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.D.; Han, D.J.; Wang, Q.L.; Yuan, F.P.; Wu, J.H.; Zhang, L.; Wang, X.J.; Huang, L.L.; Chen, X.M.; Kang, Z.S. Stripe rust resistance and genes in Chinese wheat cultivars and breeding lines. Euphytica 2014, 196, 271–284. [Google Scholar] [CrossRef]
- Zhu, G.; Peng, M.; Liu, J.; Zou, X.; Wang, H.; Yang, L.; Gao, C.; Liu, Y. Evaluation on resistance of wheat varieties (lines) to Fusarium head blight, strip rust, powdery mildew and sharp eyespot in Hubei province. Plant Prot. 2020, 46, 204–209. [Google Scholar] [CrossRef]
- Wu, J.H.; Wang, Q.L.; Chen, X.M.; Wang, M.J.; Mu, J.M.; Lv, X.N.; Huang, L.L.; Han, D.J.; Kang, Z.S. Stripe rust resistance in wheat breeding lines developed for central Shaanxi, an overwintering region for Puccinia striiformis f. sp tritici in China. Can. J. Plant Pathol. 2016, 38, 317–324. [Google Scholar] [CrossRef]
- Li, Q.; Wang, B.T.; Chao, K.X.; Guo, J.; Song, J.R.; Yue, W.Y.; Li, Q. Molecular Detection of Stripe Rust Resistance Gene(s) in 115 Wheat Cultivars (lines) from the Yellow and Huai River Valley Wheat Region. J. Phytopathol. 2016, 164, 946–958. [Google Scholar] [CrossRef]
- Gebreslasie, Z.S.; Huang, S.; Zhan, G.M.; Badebo, A.; Zeng, Q.D.; Wu, J.H.; Wang, Q.L.; Liu, S.J.; Huang, L.L.; Wang, X.J.; et al. Stripe rust resistance genes in a set of Ethiopian bread wheat cultivars and breeding lines. Euphytica 2020, 216. [Google Scholar] [CrossRef]
- Yang, W.X.; Yang, F.P.; Dan, L.; He, Z.H.; Shang, X.W.; Xia, X.C. Molecular characterization of slow-rusting genes Lr34/Yr18 in Chinese wheat cultivars. Acta Agron. Sin. 2008, 34, 1109–1113. [Google Scholar] [CrossRef]
- Huang, L.; Xiao, X.Z.; Liu, B.; Gao, L.; Gong, G.S.; Chen, W.Q.; Zhang, M.; Liu, T.G. Identification of Stripe Rust Resistance Genes in Common Wheat Cultivars from the Huang-Huai-Hai Region of China. Plant Dis. 2020, 104, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.N.; Long, L.; Yao, F.J.; Wang, Y.Q.; Jiang, Q.T.; Kang, H.Y.; Chen, G.Y. Evaluation of Resistance to Stripe Rust and Molecular Detection of Important Known Yr Gene(s) of 152 Chinese Wheat Landraces from the Huang-huai-hai. Sci. Agric. Sin. 2020, 53, 3629–3637. [Google Scholar] [CrossRef]
- Singh, R.P.; Huerta-Espino, J.; William, H.M. Genetics and Breeding for Durable Resistance to Leaf and Stripe Rusts in Wheat. Turk. J. Agric. For. 2005, 29, 121–127. Available online: https://journals.tubitak.gov.tr/agriculture/issues/tar-05-29-2/tar-29-2-4-0402-2.pdf (accessed on 25 April 2021).
- Song, W.; Xie, C.J.; Du, J.K.; Xie, H.; Liu, Q.; Ni, Z.F.; Liu, Z.Y. A “one-marker-for-two-genes” approach for efficient molecular discrimination of Pm12 and Pm21 conferring resistance to powdery mildew in wheat. Mol. Breed. 2009, 23, 357–363. [Google Scholar] [CrossRef]
- Cheng, B.; Ding, Y.Q.; Gao, X.; Cao, N.; Xin, Z.H.; Zhang, L.Y. The diversity of powdery mildew resistance gene loci among wheat germplasm in Southwest China. Cereal Res. Commun. 2020, 48, 65–70. [Google Scholar] [CrossRef]
- Lu, N.; Lu, M.X.; Liu, P.; Xu, H.X.; Qiu, X.L.; Hu, S.S.; Wu, Y.A.; Bai, S.L.; Wu, J.Z.; Xue, S.L. Fine Mapping a Broad-Spectrum Powdery Mildew Resistance Gene in Chinese Landrace Datoumai, PmDTM, and Its Relationship with Pm24. Plant Dis. 2020, 104, 1709–1714. [Google Scholar] [CrossRef]
- Mergoum, M.; Frohberg, R.C.; Stack, R.W. Breeding hard red spring wheat for Fusarium head blight resistance. Wheat Prod. Stressed Environ. 2007, 12, 161–167. [Google Scholar] [CrossRef]
- Anderson, J.A.; Wiersma, J.J.; Linkert, G.L.; Kolmer, J.; Jin, Y.; Dill-Macky, R.; Wiersma, J.V.; Hareland, G.A. Registration of ‘Sabin’ wheat. J. Plant Regist. 2012, 6, 174–179. [Google Scholar] [CrossRef]
- Xu, F.; Wang, J.M.; Yang, G.Q.; Song, Y.L.; Liu, L.L.; Li, L.J.; Li, Y.H.; Han, Z.H.; Zhang, J.J. Evaluation of the comprehensive resistance to Fusarium head blight and detection of the resistance gene Fhb1 in main wheat cultivars in the Huanghuai winter wheat region. Plant Prot. 2020, 46, 84–92. [Google Scholar] [CrossRef]
- Zhu, Z.W.; Xu, D.A.; Cheng, S.H.; Gao, C.B.; Xia, X.C.; Hao, Y.F. Characterization of Fusarium Head Blight Resistance Gene Fhb1 and Its Putative Ancestor in Chinese Wheat Germplasm. Acta Agron. Sin. 2018, 4, 473–482. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).