Protein Hydrolysates and Mo-Biofortification Interactively Modulate Plant Performance and Quality of ‘Canasta’ Lettuce Grown in a Protected Environment
Abstract
:1. Introduction
2. Materials and Methods
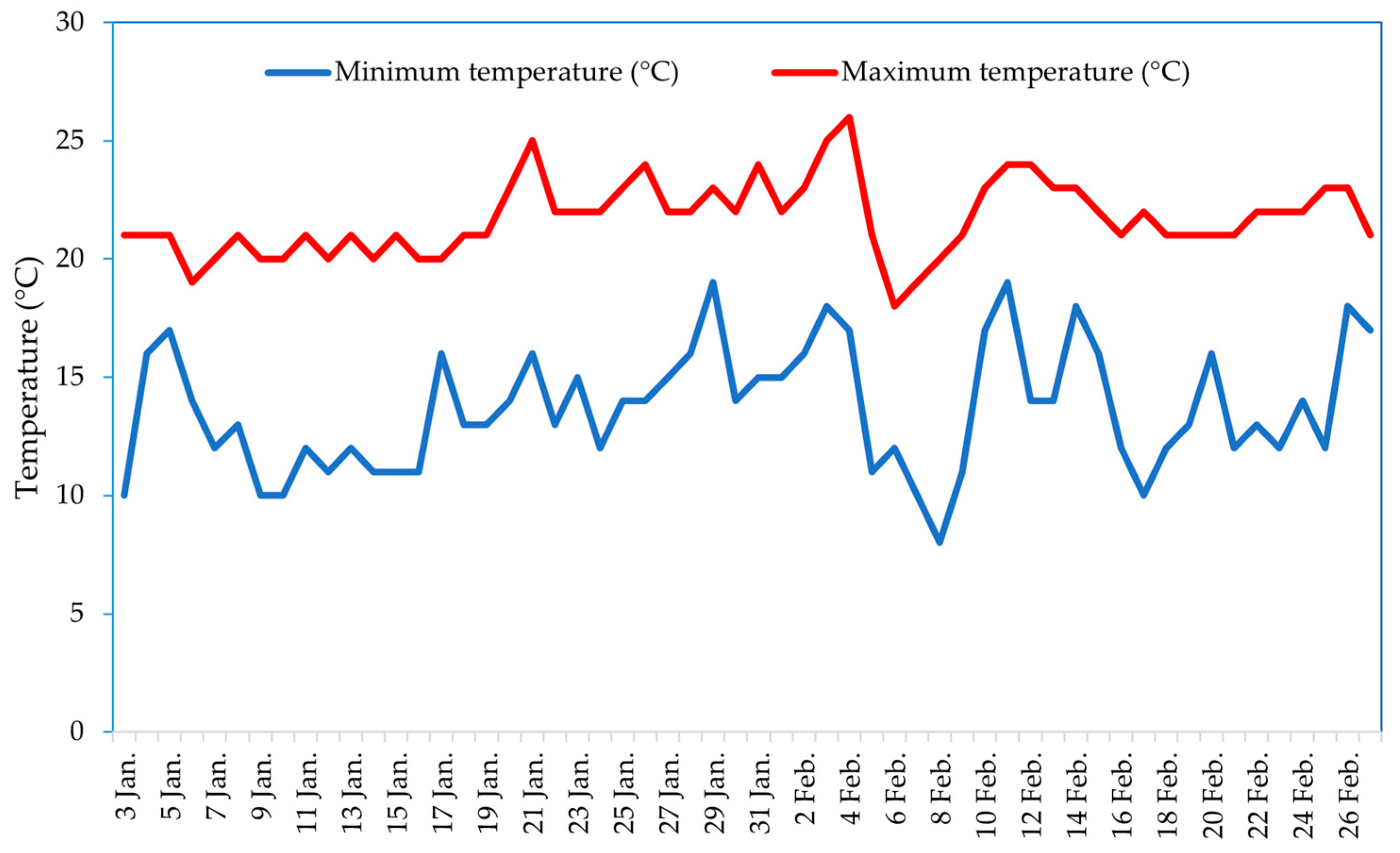
2.1. Plant Genetic Material and Experimental Site
2.2. Plant-Derived Protein Hydrolysates Application and Molybdenum Biofortification
2.3. Yield, Nutritional and Bioactive Attributes of Lettuce Plants
2.4. Plant Pigments and Mineral Profile
2.5. Nitrogen Indices
2.6. Statistics and Experimental Design
3. Results
3.1. Crop Yield and Biometric Features
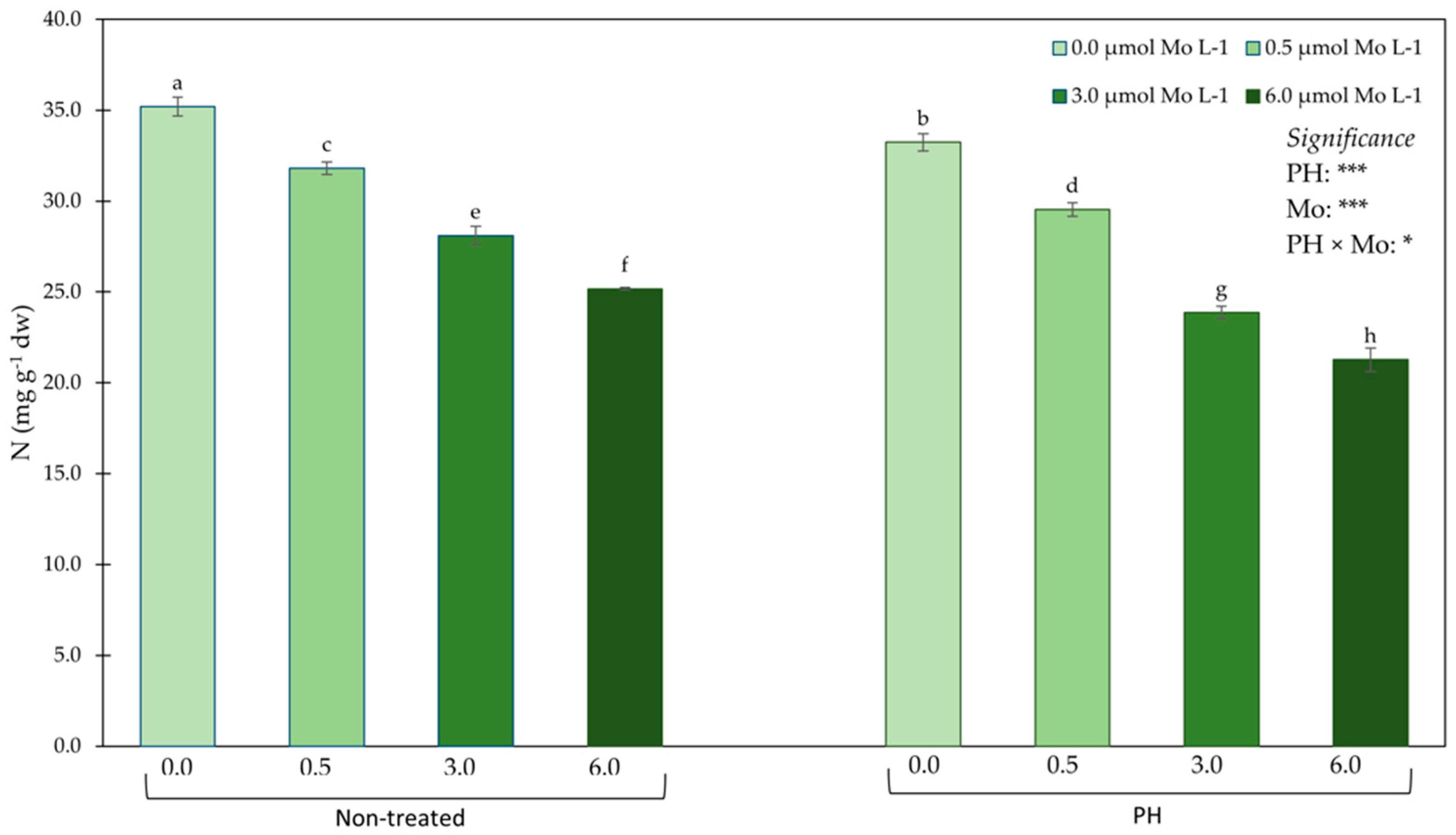
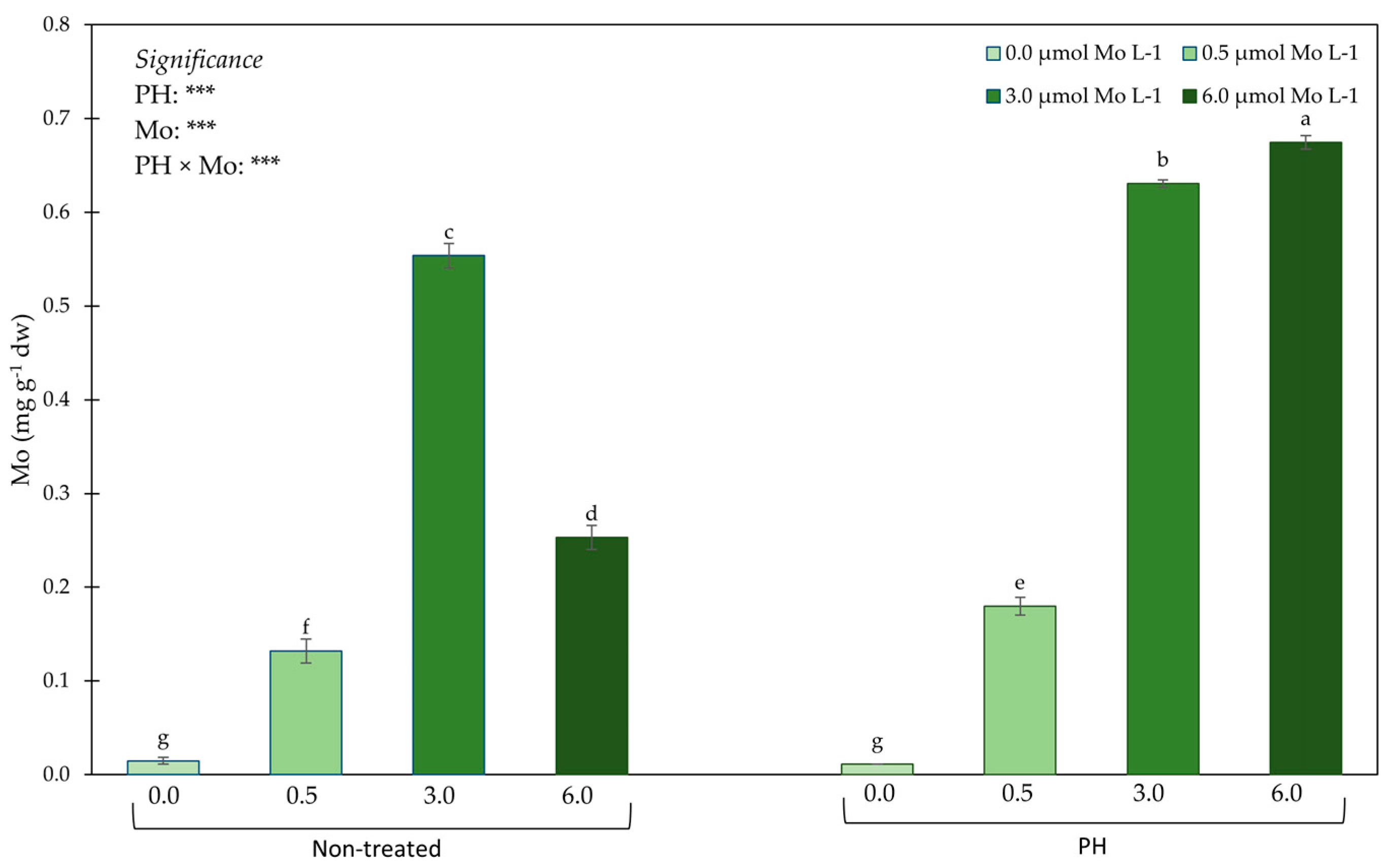
3.2. Mineral Profile in Leaf Tissues
3.3. Nutritional and Bioactive Compounds
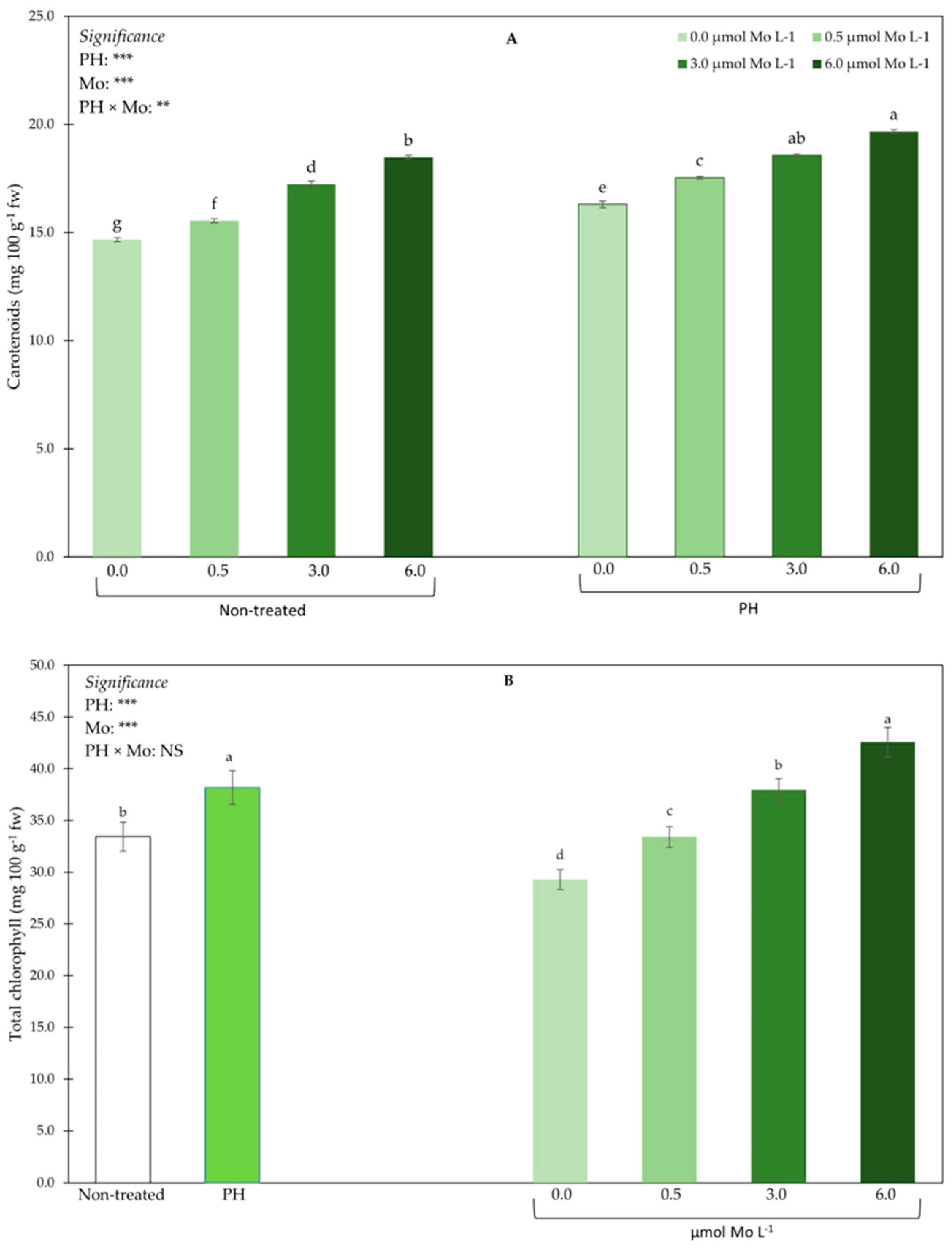
3.4. Pigments
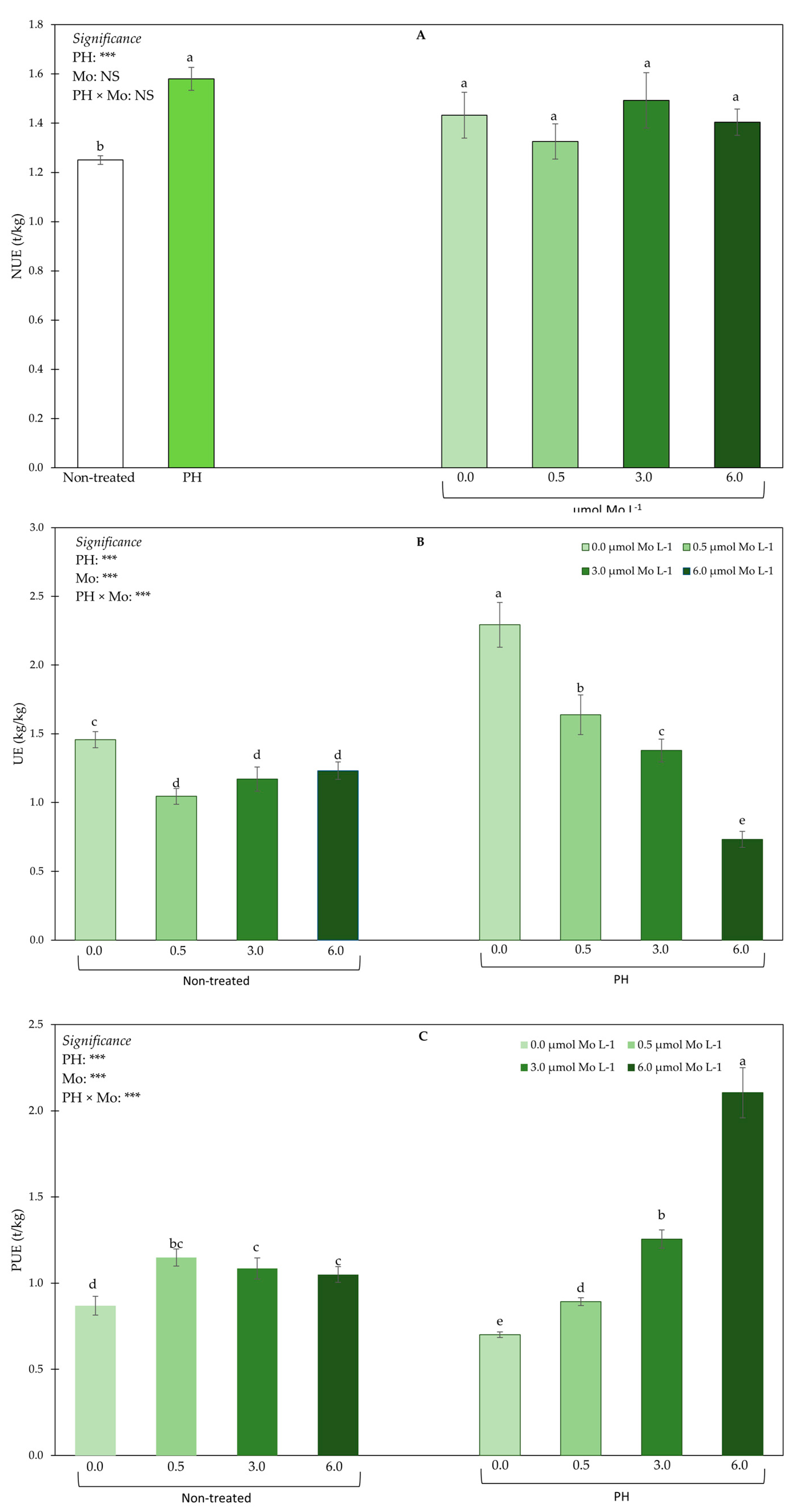
3.5. Nitrogen Indices
3.6. Heat Map Analysis of All Recorded Plant Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duhamel, M.; Vandenkoornhuyse, P. Sustainable agriculture: Possible trajectories from mutualistic symbiosis and plant neodomestication. Trends Plant Sci. 2013, 18, 597–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Jardin, P. Plant biostimulants: Definition; concept; main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Sabatino, L.; Fabio, D.; Torta, A.L.; Ferrara, G.; Iapichino, G. Effects of arbuscular mycorrhizal fungi on Gazania rigens pot plant cultivation in a mediterranean environment. Not. Bot. Horti Agrobot. Cluj Napoca 2019, 47, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Sabatino, L.; D’anna, F.; Torta, L.; Ferrara, G.; Iapichino, G. Arbuscular mycorrhizal inoculation and shading enhance crop performance and quality of greenhouse Begonia semperflorens. Acta Sci. Pol. Hortorum Cultus 2019, 18, 17–33. [Google Scholar] [CrossRef]
- Sabatino, L.; Iapichino, G.; Consentino, B.B.; D’Anna, F.; Rouphael, Y. Rootstock and Arbuscular Mycorrhiza Combinatorial Effects on Eggplant Crop Performance and Fruit Quality under Greenhouse Conditions. Agronomy 2020, 10, 693. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Colla, G.; Fiorentino, N.; Sabatino, L.; El-Nakhel, C.; Cozzolino, E. Appraisal of Combined Applications of Trichoderma virens and a Biopolymer-Based Biostimulant on Lettuce Agronomical, Physiological, and Qualitative Properties under Variable N Regimes. Agronomy 2020, 10, 196. [Google Scholar] [CrossRef] [Green Version]
- Consentino, B.B.; Virga, G.; La Placa, G.G.; Sabatino, L.; Rouphael, Y.; Ntatsi, G.; Iapichino, G.; La Bella, S.; Mauro, R.P.; D’Anna, F.; et al. Celery (Apium graveolens L.) Performances as Subjected to Different Sources of Protein Hydrolysates. Plants 2020, 9, 1633. [Google Scholar] [CrossRef]
- Di Mola, I.; Conti, S.; Cozzolino, E.; Melchionna, G.; Ottaiano, L.; Testa, A.; Sabatino, L.; Rouphael, Y.; Mori, M. Plant-Based Protein Hydrolysate Improves Salinity Tolerance in Hemp: Agronomical and Physiological Aspects. Agronomy 2021, 11, 342. [Google Scholar] [CrossRef]
- Schaafsma, G. Safety of protein hydrolysates, fractions thereof and bioactive peptides in human nutrition. Eur. J. Clin. Nutr. 2009, 63, 1161–1168. [Google Scholar] [CrossRef] [Green Version]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Fernández, V.; Eichert, T. Uptake of hydrophilic solutes through plant leaves: Current state of knowledge and perspectives of foliar fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef] [Green Version]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activities of two protein hydrolysates on the growth and nitrogen metabolism in maize seedlings. J. Plant. Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Matsumiya, Y.; Kubo, M. Soybean peptide: Novel plant growth promoting peptide from soybean. In Soybean and Nutrition; H El-Shemy, R., Ed.; Tech Europe Publisher: Giza, Egypt, 2011; pp. 215–230. [Google Scholar]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef] [Green Version]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 96, 28–38. [Google Scholar] [CrossRef]
- Bouis, H.E.; Welch, R.M. Biofortification-a sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci. 2010, 50, S-20–S-32. [Google Scholar] [CrossRef] [Green Version]
- D’Anna, F.; Sabatino, L. Morphological and agronomical characterization of eggplant genetic resources from the Sicily area. J. Food Agric. Environ. 2013, 11, 401–404. [Google Scholar]
- Azeez, M.A.; Adubi, A.O.; Durodola, F.A. Landraces and crop genetic improvement. In Rediscovery of Landraces as a Resource for the Future; InTechOpen Europe Publisher: Rijeka, Croatia, 2018. [Google Scholar]
- Fraga, C.G. Relevance, essentiality and toxicity of trace elements in human health. Mol. Aspects Med. 2005, 26, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Ierna, A.; Mauro, R.; Mauromicale, G. Improved yield and nutrient efficiency in two globe artichoke genotypes by balancing nitrogen and phosphorus supply. Agron. Sustain. Dev. 2012, 32, 773–780. [Google Scholar] [CrossRef]
- Tsongas, T.A.; Meglen, R.R.; Walravens, P.A.; Chappel, W.R. Molybdenum in the diet: An estimate of average daily intake in the United States. Am. J. Clin. Nutr. 1980, 33, 1103–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bender, D.; Kaczmarek, A.T.; Santamaria-Araujo, J.A.; Stueve, B.; Waltz, S.; Bartsch, D.; Kurian, L.; Cirak, S.; Schwarz, G. Impaired mitochondrial maturation of sulfite oxidase in a patient with severe sulfite oxidase deficiency. Hum. Mol. Genet. 2019, 28, 2885–2899. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.J.; Fridovich, I.; Rajagopalan, K.V. Hepatic sulfite oxidase. A functional role for molybdenum. J. Biol. Chem. 1971, 246, 374–382. [Google Scholar] [CrossRef]
- Johnson, J.L.; Rajagopalan, K.V.; Cohen, H.J. Molecular basis of the biological function of molybdenum. Effect of tungsten on xanthine oxidase and sulfite oxidase in the rat. J. Biol. Chem. 1974, 249, 859–866. [Google Scholar] [CrossRef]
- Gunnison, A.F. Sulphite toxicity: A critical review of in vitro and in vivo data. Food Cosmet. Toxicol. 1981, 19, 667–682. [Google Scholar] [CrossRef]
- Schwarz, G.; Belaidi, A.A. Interrelations between essential metal ions and human diseases. In Molybdenum in Human Health and Disease; Sigel, A., Sigel, H., Sigel, R., Eds.; Springer: Dordrecht, The Netherlands, 2013; Volume 13, pp. 415–450. [Google Scholar]
- Pennington, J.A.; Jones, J.W. Molybdenum, nickel, cobalt, vanadium, and strontium in total diets. J. Am. Diet. Assoc. 1987, 87, 1644–1650. [Google Scholar]
- Rose, M.; Baxter, M.; Brereton, N.; Baskaran, C. Dietary exposure to metals and other elements in the 2006 UK Total Diet Study and some trends over the last 30 years. Food Addit. Contam. 2010, 27, 1380–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luk, E.; Jensen, L.T.; Culotta, V.C. The many highways for intracellular trafficking of metals. J. Biol. Inorg. Chem. 2003, 8, 803–809. [Google Scholar] [CrossRef]
- Schwarz, G.; Mendel, R.R.; Ribbe, M.W. Molybdenum cofactors, enzymes and pathways. Nature 2009, 460, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Vatansever, R.; Ozyigit, I.I.; Filiz, E. Essential and beneficial trace elements in plants, and their transport in roots: A review. Biotechnol. Appl. Biochem. 2017, 181, 464–482. [Google Scholar] [CrossRef]
- Mendel, R.R.; Schwarz, G. Molybdoenzymes and molybdenum cofactor in plants. Crit. Rev. Plant. Sci. 1999, 18, 33–69. [Google Scholar] [CrossRef]
- Bittner, F. Molybdenum metabolism in plants and crosstalk to iron. Front. Plant Sci. 2014, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Moncada, A.; Miceli, A.; Sabatino, L.; Iapichino, G.; D’Anna, F.; Vetrano, F. Effect of molybdenum enrichment on yield and quality of lettuce, escarole and curly endive grown in floating system. Agronomy 2018, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Sabatino, L.; D’Anna, F.; Iapichino, G.; Moncada, A.; D’Anna, E.; De Pasquale, C. Interactive effects of genotype and molybdenum supply on yield and overall fruit quality of tomato. Front. Plant Sci. 2019, 9, 1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longbottom, M.L.; Dry, P.R.; Sedgley, M. Effects of sodiummolybdate foliar sprays on molybdenum concentration in the vegetative and reproductive structures and on yield components of Vitis vinifera cv. Merlot. J. Grape Wine Res. 2010, 16, 477–490. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, X.; Sandhu, A.K.; Gu, L. Effects of exogenous abscisic acid on yield, antioxidant capacities, and phytochemical contents of greenhouse grown lettuces. J. Agricult. Food Chem. 2010, 58, 6503–6509. [Google Scholar] [CrossRef] [PubMed]
- Luz, G.L.; Medeiros, S.L.P.; Manfron, P.A.; Amaral, A.D.; Muller, L.; Torrez, M.G.; Mentges, L.A. The nitrate issue in hydroponic lettuce and the human health. Ciência Rural. 2008, 38, 2388–2394. [Google Scholar] [CrossRef] [Green Version]
- Romani, A.; Pinelli, P.; Galardi, C.; Sani, G.; Cimato, A.; Heimler, D. Polyphenols in greenhouse and open-air-grown lettuce. Food Chem. 2002, 79, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Prohens-Tomás, J.; Nuez, F. Lettuce. In Vegetables I: Asteraceae, Brassicaceae, Chenopodicaceae, and Cucurbitaceae; International Publishing: Cham, Switzerland, 2008; pp. 75–116. [Google Scholar]
- Tesi, R. Orticoltura Mediterranea Sostenibile; Pàtron Editore: Bologna, Italy, 2010. [Google Scholar]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Serna, M.; Hernández, F.; Coll, F.; Coll, Y.; Amorós, A. Effects of brassinosteroid analogues on total phenols, antioxidant activity, sugars, organic acids and yield of field grown endive (Cichorium endivia L.). J. Sci. Food Agric. 2013, 93, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Costache, M.A.; Campeanu, G.; Neata, G. Studies concerning the extraction of chlorophyll and total carotenoids from vegetables. Rom. Biotechnol. Lett. 2012, 17, 7702–7708. [Google Scholar]
- Morand, P.; Gullo, J.L. Mineralisation des tissus vegetaux en vue du dosage de P, Ca, Mg, Na, K. Ann. Agron. 1970, 21, 229–236. [Google Scholar]
- Fogg, D.N.; Wilkinson, A.N. The colorimetric determination of phosphorus. Analyst 1958, 83, 406–414. [Google Scholar] [CrossRef]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Giordano, M.; El-Nakhel, C.; Sacco, A.; Rouphael, Y.; Colla, G.; Mori, M. Plant-based biostimulants influence the agronomical, physiological, and qualitative responses of baby rocket leaves under diverse nitrogen conditions. Plants 2019, 8, 522. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Biacs, P.A.; Daood, H.G.; Kadar, I. Effect of Mo, Se, Zn, and Cr treatments on the yield, element concentration, and carotenoid content of carrot. J. Agric. Food Chem. 1995, 43, 589–591. [Google Scholar] [CrossRef]
- Vieira, R.F.; Salgado, L.T.; Ferreira, A.C.B. Performance of common bean using seeds harvested from plants fertilized with high rates of molybdenum. J. Plant Nutr. 2005, 28, 363–377. [Google Scholar] [CrossRef]
- Liu, X.Q.; Lee, K.S. Effect of mixed amino acids on crop growth. In Agricultural Science; Aflakpui, G., Ed.; InTechOpen Europe Publisher: Rijeka, Croatia, 2012; pp. 119–158. [Google Scholar]
- Tsouvaltzis, P.; Koukounaras, A.; Siomos, S.A. Application of amino acids improves lettuce crop uniformity and inhibits nitrate accumulation induced by the supplemental inorganic nitrogen fertilization. Int. J. Agric. Biol. 2014, 16, 951–955. [Google Scholar]
- Zhen, Y.; Liu, S.; Liu, S. Effect of appling molybdenum fertilizer on nitrate content in lettuce and you-mai vegetable. Soil Fertil. Sci. China 2007, 1, 51–53. [Google Scholar]
- Cantliffe, D.J.; MacDonald, G.E.; Peck, N.H. Reduction of nitrate accumulation by molybdenum in spinach grown at low pH. Commun. Soil Sci. Plant Anal. 1974, 5, 273–282. [Google Scholar] [CrossRef]
- Cox, D.A. Poinsettia cultivars differ in their response to molybdenum deficiency. HortScience 1992, 27, 892–893. [Google Scholar] [CrossRef] [Green Version]
- Cox, D.A. Foliar-applied molybdenum for preventing or correcting molybdenum deficiency of poinsettia. Hortscience 1992, 8, 894–895. [Google Scholar] [CrossRef]
- Schwarz, G. Molybdenum cofactor and human disease. Curr. Opin. Chem. Biol. 2016, 179, 87. [Google Scholar] [CrossRef] [PubMed]
- Valenciano, J.B.; Boto, J.A.; Marcelo, V. Chickpea (Cicer arietinum L.) response to zinc, boron and molybdenum application under field conditions. N.Z.J. Crop Hortic. Sci. 2011, 39, 217–229. [Google Scholar] [CrossRef]
- Br Brodrick, S.J.; Giller, K.E. Genotypic difference in molybdenum accumulation affects N2-fixation in tropical Phaseolus vulgaris. J. Exp. Bot. 1991, 42, 1339–1343. [Google Scholar] [CrossRef]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Giordano, M.; El-Nakhel, C.; Cuciniello, A.; Cenvinzo, V.; Colla, G.; Rouphael, Y. Protein Hydrolysate or Plant Extract-based Biostimulants Enhanced Yield and Quality Performances of Greenhouse Perennial Wall Rocket Grown in Different Seasons. Plants 2019, 8, 208. [Google Scholar] [CrossRef] [Green Version]
- Giordano, M.; El-Nakhel, C.; Caruso, G.; Cozzolino, E.; De Pascale, S.; Kyriacou, M.C.; Rouphael, Y. Stand-Alone and Combinatorial Effects of Plant-based Biostimulants on the Production and Leaf Quality of Perennial Wall Rocket. Plants 2020, 9, 922. [Google Scholar] [CrossRef] [PubMed]
- Boertje, G.A. Molybdenum and lettuce. In Annual Report—Glasshouse Crops Research and Experiment Station; Glasshouse Crops Research and Experiment Station: Naaldwijk, The Netherlands, 1969. [Google Scholar]
- Randall, P.J. Changes in nitrate and nitrate reductase levels on restoration of molybdenum to molybdenum-deficient plants. Aust. J. Agric. Res. 1969, 20, 635–642. [Google Scholar] [CrossRef]
- Carillo, P.; Colla, G.; Fusco, G.M.; Dell’Aversana, E.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cozzolino, E.; Mori, M.; Reynaud, H.; et al. Morphological and Physiological Responses Induced by Protein Hydrolysate-Based Biostimulant and Nitrogen Rates in Greenhouse Spinach. Agronomy 2019, 9, 450. [Google Scholar] [CrossRef] [Green Version]
- Li, L.I.U.; Wei, X.I.A.O.; JI, M.L.; Chao, Y.A.N.G.; Ling, L.I.; GAO, D.S.; FU, X.L. Effects of molybdenum on nutrition, quality, and flavour compounds of strawberry (Fragaria× ananassa Duch. cv. Akihime) fruit. J. Integr. Agric. 2017, 16, 1502–1512. [Google Scholar] [CrossRef]
- Xi, W.; Zhang, Q.; Lu, X.; Wei, C.; Yu, S.; Zhou, Z. Improvement of flavour quality and consumer acceptance during postharvest ripening in greenhouse peaches by carbon dioxide enrichment. Food Chem. 2014, 164, 219–227. [Google Scholar] [CrossRef]
- Bergmann, W. Nutritional disorders of plants. In Visual and Analytical Diagnosis; Gustav Fischer Verlag: Jena, Germany, 1992. [Google Scholar]
- Gupta, U.C. Symptoms of molybdenum deficiency and toxicity in crops. In Molybdenum in Agriculture; U.C. Gupta Cambridge, Cambridge University Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; Colla, G.; Mori, M. Effect of Vegetal- and Seaweed Extract-Based Biostimulants on Agronomical and Leaf Quality Traits of Plastic Tunnel-Grown Baby Lettuce under Four Regimes of Nitrogen Fertilization. Agronomy 2019, 9, 571. [Google Scholar] [CrossRef] [Green Version]
- Luziatelli, F.; Ficca, A.G.; Colla, G.; Svecova, E.; Ruzzi, M. Effects of a protein hydrolysate-based biostimulant and two micronutrient based fertilizers on plant growth and epiphytic bacterial populations of lettuce. Acta Hortic. 2016, 1148, 43–48. [Google Scholar] [CrossRef]
- Yu, M.; Hu, X.C.; Wang, Y.H. Effect of molybdenum on the precursors of chlorophyll biosynthesis in winter wheat cultivars under low temperature. Sci. Agric. Sin. 2006, 39, 702–708. [Google Scholar]
- Zhang, M.; Hu, C.; Sun, X.; Zhao, X.; Tan, Q.; Li, Y.Z.N. Molybdenum affects photosynthesis and ionic homeostasis of Chinese cabbage under salinity stress. Commun. Soil Sci. Plant Anal. 2014, 45, 2660–2672. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Nocerino, S.; Rouphael, Y.; Colla, G.; El-Nakhel, C.; Mori, M. Nitrogen Use and Uptake Efficiency and Crop Performance of Baby Spinach (Spinacia oleracea L.) and Lamb’s Lettuce (Valerianella locusta L.) Grown under Variable Sub-Optimal N Regimes Combined with Plant-Based Biostimulant Application. Agronomy 2020, 10, 278. [Google Scholar] [CrossRef] [Green Version]

| Treatments | HFW (g) | HH (cm) | SD (mm) | NL (no.) | ||||
|---|---|---|---|---|---|---|---|---|
| Biostimulant | ||||||||
| Non-treated | 500.1 | b | 23.2 | b | 27.1 | a | 26.4 | a |
| PHs | 630.7 | a | 26.0 | a | 26.8 | a | 26.5 | a |
| Biofortification (µmol Mo L−1) | ||||||||
| 0 | 572.9 | a | 24.4 | a | 29.8 | a | 25.9 | a |
| 0.5 | 530.1 | a | 24.8 | a | 27.8 | b | 26.7 | a |
| 3 | 597.0 | a | 24.4 | a | 26.5 | c | 27.8 | a |
| 6 | 561.6 | a | 24.7 | a | 23.5 | d | 25.5 | a |
| Significance | ||||||||
| PHs | *** | *** | NS | NS | ||||
| Mo | NS | NS | *** | NS | ||||
| PHs × Mo | NS | NS | NS | NS | ||||
| Treatments | P (mg g−1 dw) | K (mg g−1 dw) | Ca (mg g−1 dw) | Mg (mg g−1 dw) | ||||
|---|---|---|---|---|---|---|---|---|
| Biostimulant | ||||||||
| Non-treated | 4.20 | a | 32.48 | b | 1.77 | a | 10.32 | b |
| PHs | 4.24 | a | 40.13 | a | 1.75 | a | 11.50 | a |
| Biofortification (µmol Mo L−1) | ||||||||
| 0.0 | 4.23 | a | 36.09 | a | 1.72 | a | 11.12 | a |
| 0.5 | 4.25 | a | 36.10 | a | 1.78 | a | 10.75 | a |
| 3.0 | 4.20 | a | 36.87 | a | 1.76 | a | 11.26 | a |
| 6.0 | 4.20 | a | 36.14 | a | 1.78 | a | 10.49 | a |
| Significance | ||||||||
| PH | NS | *** | NS | *** | ||||
| Mo | NS | NS | NS | NS | ||||
| PHs × Mo | NS | NS | NS | NS | ||||
| Treatments | Ascorbic Acid (mg g−1 fw) | a* | b* | L* | ||||
|---|---|---|---|---|---|---|---|---|
| Biostimulant | ||||||||
| Non-treated | 32.80 | b | −15.67 | a | 29.80 | a | 59.76 | a |
| PHs | 34.97 | a | −18.19 | b | 28.86 | a | 57.16 | a |
| Biofortification (µmol Mo L−1) | ||||||||
| 0 | 27.62 | d | −17.18 | a | 31.18 | a | 59.31 | a |
| 0.5 | 32.03 | c | −16.84 | a | 29.79 | a | 57.83 | a |
| 3 | 36.75 | b | −16.46 | a | 29.08 | a | 58.47 | a |
| 6 | 39.13 | a | −17.29 | a | 27.34 | a | 58.22 | a |
| Significance | ||||||||
| PHs | *** | *** | NS | NS | ||||
| Mo | *** | NS | NS | NS | ||||
| PHs × Mo | NS | NS | NS | NS | ||||
| Treatments | HDM (%) | SSC (Brix°) | Total Sugars (% fw) | Total Phenolics (µg g−1 fw) | ||||
|---|---|---|---|---|---|---|---|---|
| Biostimulant × Biofortification (µmol Mo L−1) | ||||||||
| Non-treated × 0.0 | 14.53 | c | 2.13 | d | 1.45 | f | 37.03 | g |
| Non-treated × 0.5 | 13.87 | d | 2.20 | d | 1.62 | e | 44.13 | f |
| Non-treated × 3.0 | 14.53 | c | 2.30 | c | 1.92 | d | 55.07 | d |
| Non-treated × 6.0 | 15.11 | b | 2.33 | c | 2.17 | c | 75.23 | b |
| PHs × 0.0 | 15.68 | a | 2.15 | d | 1.48 | f | 36.57 | g |
| PHs × 0.5 | 15.11 | b | 2.40 | b | 1.92 | d | 47.07 | e |
| PHs × 3.0 | 14.59 | c | 2.42 | b | 2.24 | b | 59.53 | c |
| PHs × 6.0 | 13.24 | e | 2.53 | a | 2.54 | a | 80.60 | a |
| Significance | ||||||||
| PHs | NS | *** | *** | *** | ||||
| Mo | *** | *** | *** | *** | ||||
| PHs × Mo | *** | * | *** | * | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabatino, L.; Consentino, B.B.; Rouphael, Y.; De Pasquale, C.; Iapichino, G.; D’Anna, F.; La Bella, S. Protein Hydrolysates and Mo-Biofortification Interactively Modulate Plant Performance and Quality of ‘Canasta’ Lettuce Grown in a Protected Environment. Agronomy 2021, 11, 1023. https://doi.org/10.3390/agronomy11061023
Sabatino L, Consentino BB, Rouphael Y, De Pasquale C, Iapichino G, D’Anna F, La Bella S. Protein Hydrolysates and Mo-Biofortification Interactively Modulate Plant Performance and Quality of ‘Canasta’ Lettuce Grown in a Protected Environment. Agronomy. 2021; 11(6):1023. https://doi.org/10.3390/agronomy11061023
Chicago/Turabian StyleSabatino, Leo, Beppe Benedetto Consentino, Youssef Rouphael, Claudio De Pasquale, Giovanni Iapichino, Fabio D’Anna, and Salvatore La Bella. 2021. "Protein Hydrolysates and Mo-Biofortification Interactively Modulate Plant Performance and Quality of ‘Canasta’ Lettuce Grown in a Protected Environment" Agronomy 11, no. 6: 1023. https://doi.org/10.3390/agronomy11061023






