Comparative Analyses Reveal Peroxidases Play Important Roles in Soybean Tolerance to Aluminum Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions, Treatments, and Tissue Harvest
2.2. POD Activity Measurement
2.3. Determination of Al Concentration in Soybean Roots
2.4. Hematoxylin Staining of Soybean Roots
2.5. Statistical Analysis
2.6. Total RNA Isolation, Labeling, and Array Hybridization
2.7. Microarray Data Analysis
2.8. Quantitative Real-Time PCR (qRT-PCR)
3. Results
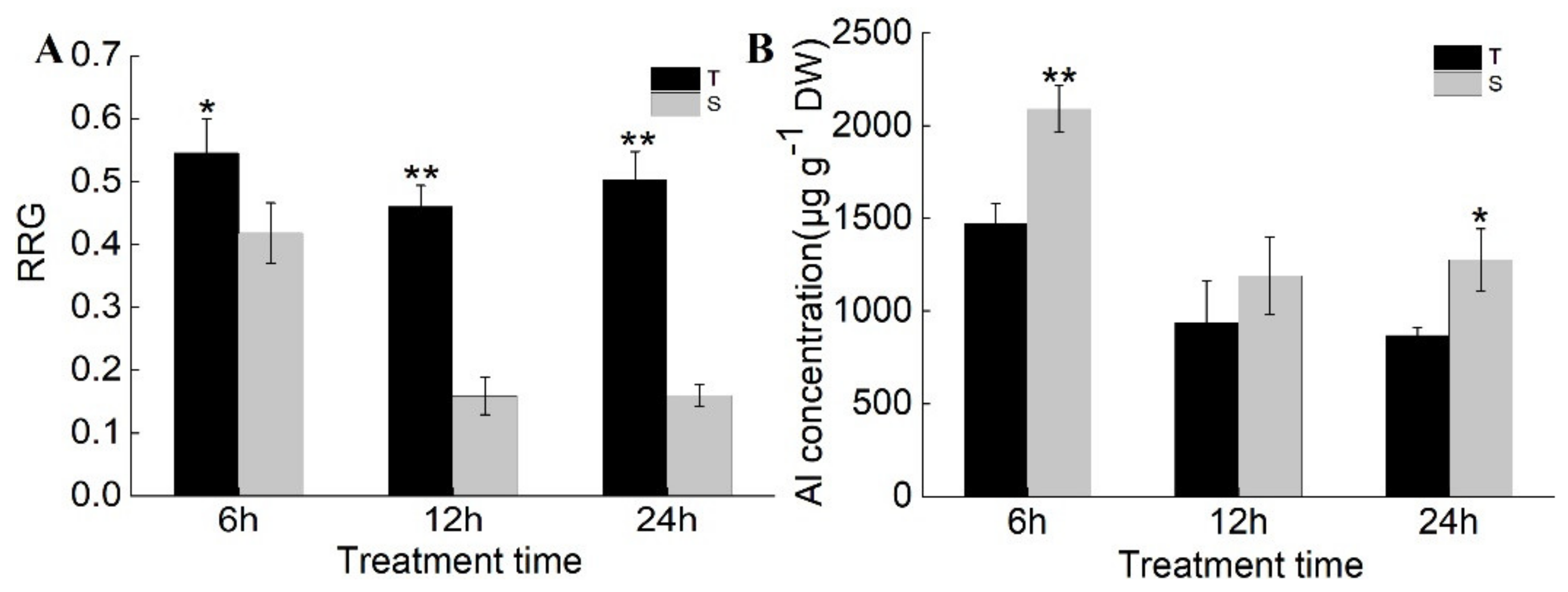
3.1. Phenotypic Difference in Al Tolerance Between Soybean Varieties of KF and GF
3.2. Genome-Wide Gene Expression Profiles in Soybean Roots in Response to Al Stress
3.3. Identification of Al-Responsive Genes in Soybean Roots
3.4. Identification of Candidate Genes for Soybean Tolerance to Al Stress
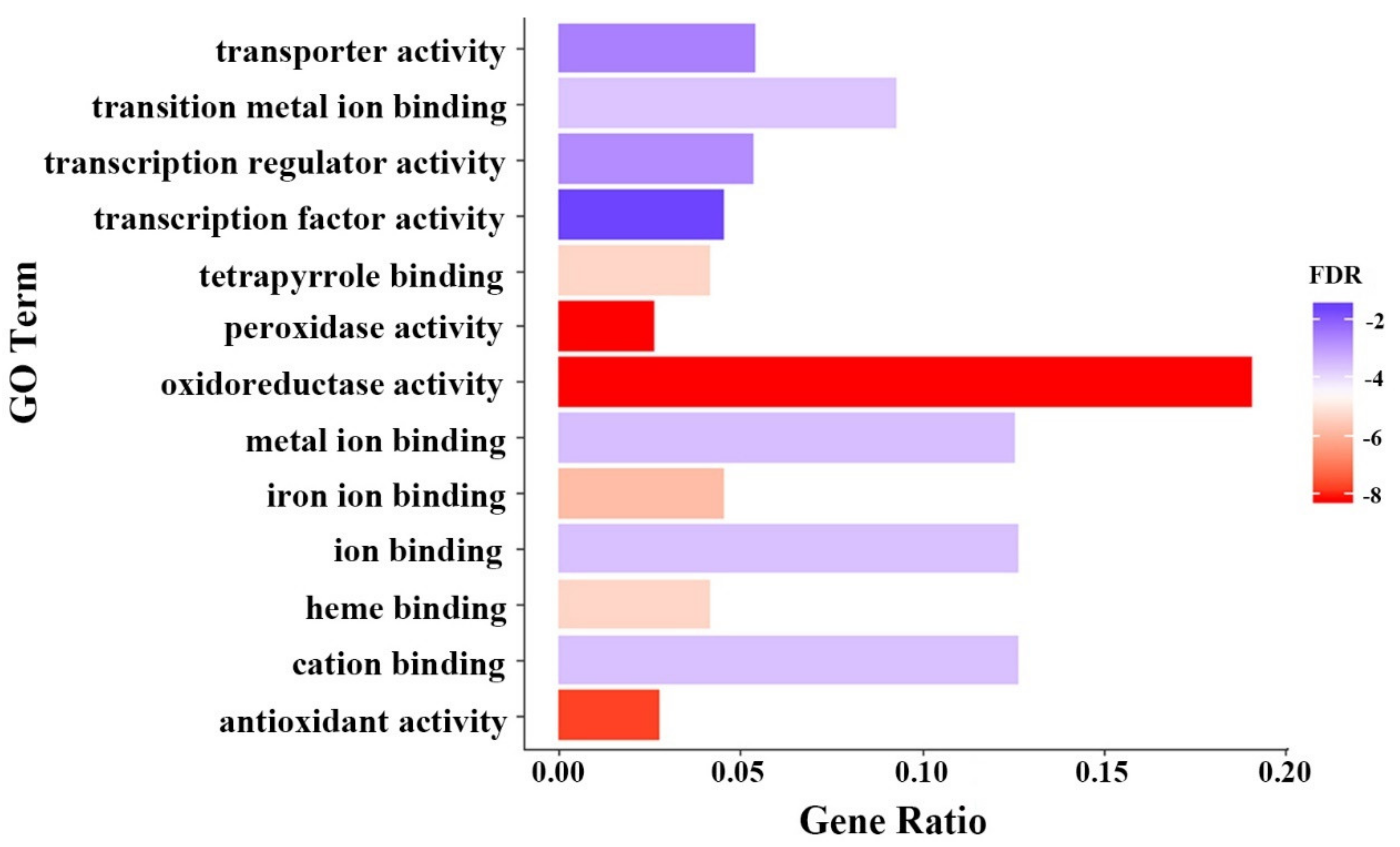
3.5. Gene Ontology (GO) Analysis of Al Tolerance Candidate Genes in Soybean
3.6. Validation of Microarray Data by Real-Time Quantitative PCR
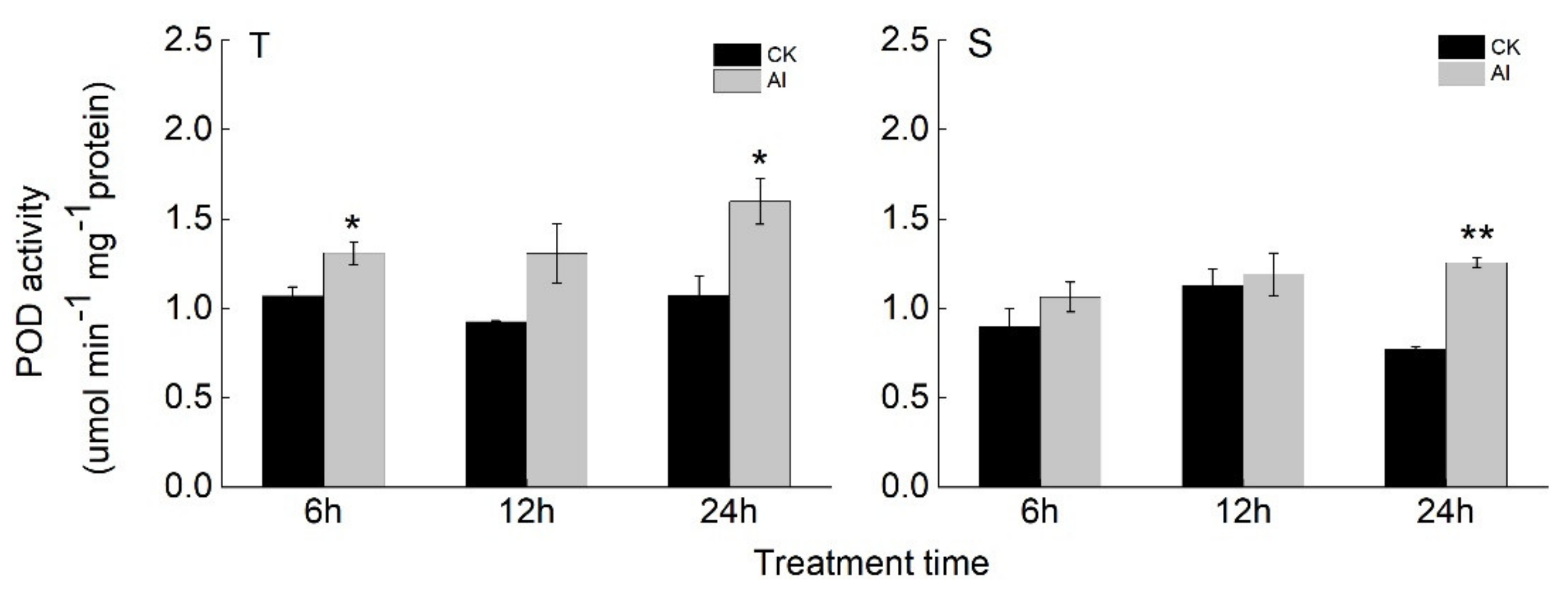
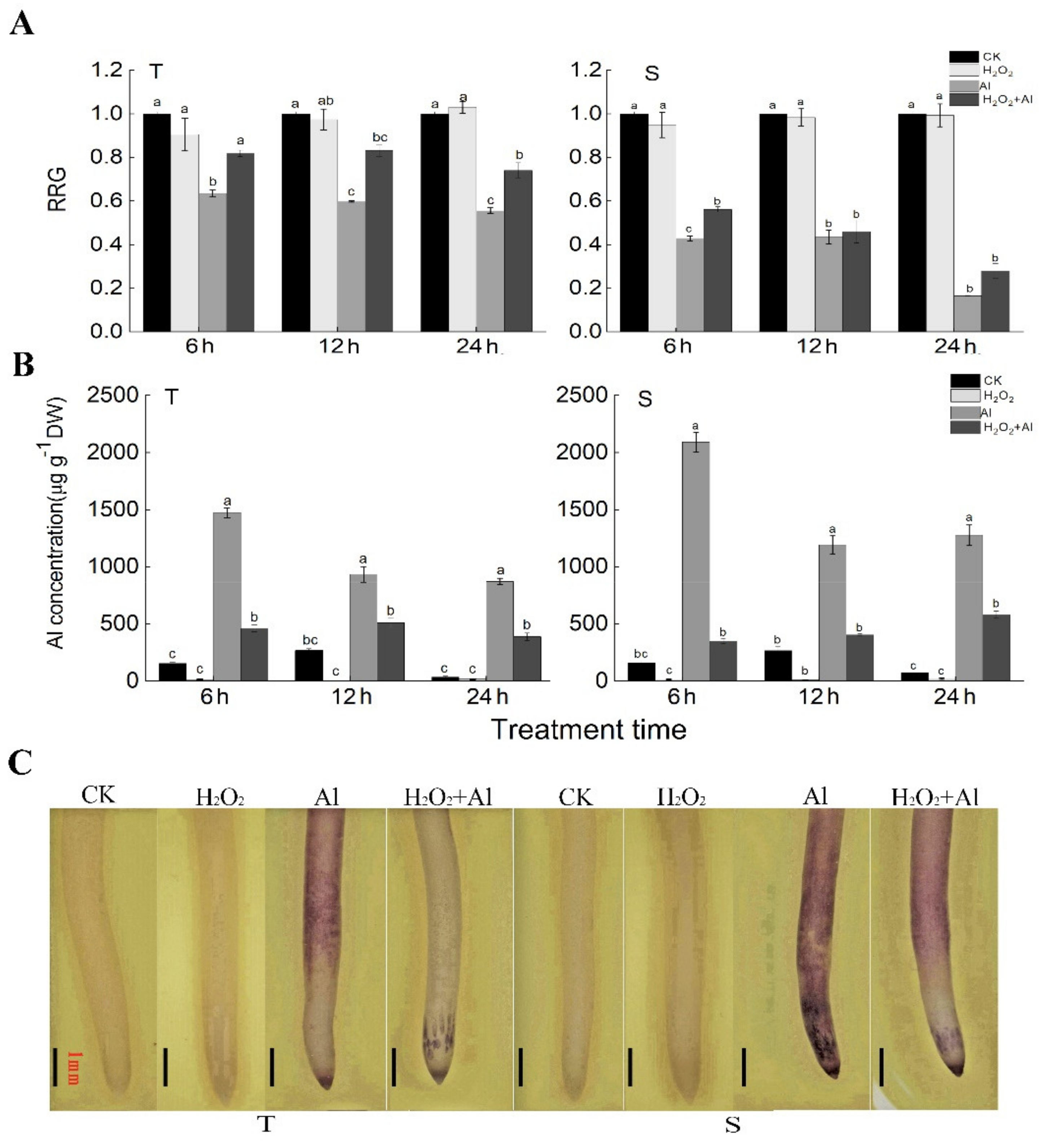
3.7. Role of Peroxidase in Soybean Tolerance to Al Stress
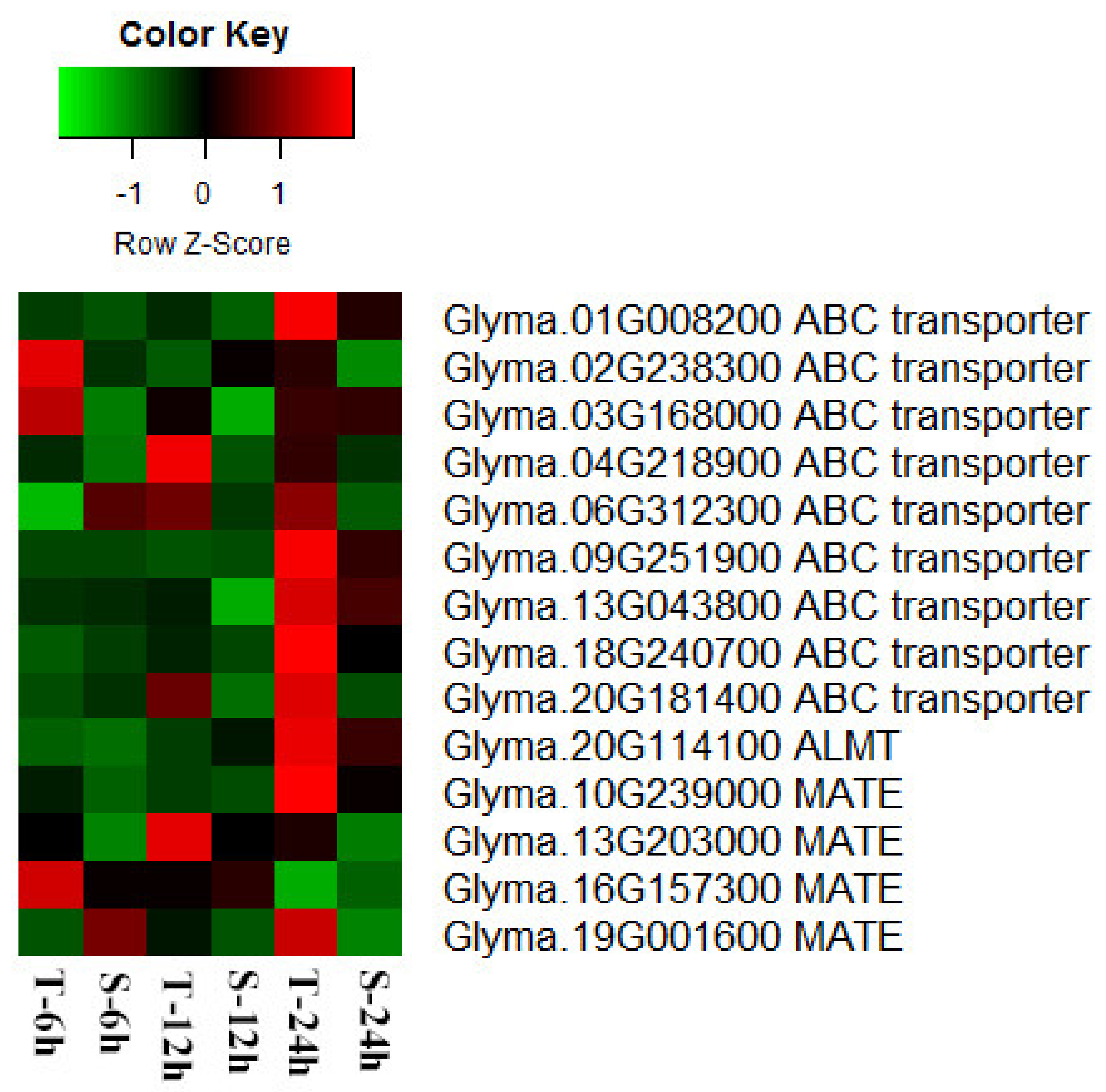
3.8. Al Tolerance Related Transporter Genes in Soybean Roots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | ATP-Binding Cassette |
| Al | Aluminum |
| ALMT | Aluminum-activated malate transporter |
| DEG | Differently expressed gene |
| DW | Dry weight |
| FDR | False discovery rate |
| FC | Fold change |
| Fructose-6-P | Fructose-6-phosphate |
| Fructose-1,6-bis-P1 | Fructose-1,6-bisphosphatase1 |
| GO | Gene Ontology |
| H2O2 | Hydrogen peroxide |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MATE | Multidrug and toxin extrusion |
| OAA | Oxaloacetate |
| POD | Peroxidase |
| PEP | Phosphoenolpyruvate |
| PEPC | Phosphoenolpyruvate Carboxylase |
| ROS | Reactive oxygen species |
| RG | Root growth |
| RRG | Relative root growth |
| RNA-Seq | RNA sequencing |
| S | Sensitive |
| SA | Salicylic acid |
| SEA | Singular enrichment analysis |
| TCA | Tricarboxylic acid cycle |
| TF | Transcription factor |
| T | Tolerant |
References
- Sinclair, J.B. Compendium of Soybean Diseases; American Phytopathological Society and University of Illinois: St. Paul, MN, USA, 1982. [Google Scholar]
- Wilson, R.F. Soybean: Market driven research needs. In Genetics and Genomics of Soybean; Springer: Berlin/Heidelberg, Germany, 2008; pp. 3–15. [Google Scholar]
- Kochian, L.V.; Hoekenga, O.A.; Pineros, M.A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef] [PubMed]
- Kochian, L.V.; Pineros, M.A.; Hoekenga, O.A. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 2005, 274, 175–195. [Google Scholar] [CrossRef]
- Ryan, P.R.; Kochian, L.V. Interaction between Aluminum Toxicity and Calcium Uptake at the Root Apex in Near-Isogenic Lines of Wheat (Triticum aestivum L.) Differing in Aluminum Tolerance. Plant Physiol. 1993, 102, 975. [Google Scholar] [CrossRef]
- Ma, J.F. Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int. Rev. Cytol. 2007, 264, 225–252. [Google Scholar] [PubMed]
- Poschenrieder, C.; Gunsé, B.; Corrales, I.; Barceló, J. A glance into aluminum toxicity and resistance in plants. Sci. Total Environ. 2008, 400, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Rout, G.; Samantaray, S.; Das, P. Aluminium toxicity in plants: A review. Agronomie 2001, 21, 3–21. [Google Scholar] [CrossRef]
- Rengel, Z.; Zhang, W.H. Role of dynamics of intracellular calcium in aluminium-toxicity syndrome. New Phytol. 2003, 159, 295–314. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kobayashi, Y.; Devi, S.R.; Rikiishi, S.; Matsumoto, H. Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol. 2002, 128, 63–72. [Google Scholar] [CrossRef]
- Blancaflor, E.B.; Jones, D.L.; Gilroy, S. Alterations in the cytoskeleton accompany aluminum-induced growth inhibition and morphological changes in primary roots of maize. Plant Physiol. 1998, 118, 159–172. [Google Scholar] [CrossRef] [Green Version]
- Amenós, M.; Corrales, I.; Poschenrieder, C.; Illéš, P.; Baluška, F.; Barceló, J. Different effects of aluminum on the actin cytoskeleton and brefeldin A-sensitive vesicle recycling in root apex cells of two maize varieties differing in root elongation rate and aluminum tolerance. Plant Cell Physiol. 2009, 50, 528–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiner, K.A.; Hoddinott, J.; Taylor, G.J. Aluminum-induced deposition of (1,3)-β-glucans (callose) in Triticum aestivum L. Plant Soil 1994, 162, 273–280. [Google Scholar] [CrossRef]
- Ezaki, B.; TsugUa, S.; Matsumoto, H. Expression of a moderately anionic peroxidase is induced by aluminum treatment in tobacco cells: Possible involvement of peroxidase isozymes in aluminum ion stress. Physiol. Plant. 1996, 96, 21–28. [Google Scholar] [CrossRef]
- Richards, K.D.; Schott, E.J.; Sharma, Y.K.; Davis, K.R.; Gardner, R.C. Aluminum induces oxidative stress genes in Arabidopsis thaliana. Plant Physiol. 1998, 116, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Simonovicova, M.; Huttova, J.; Mistrik, I.; Siroka, B.; Tamas, L. Root growth inhibition by aluminum is probably caused by cell death due to peroxidase-mediated hydrogen peroxide production. Protoplasma 2004, 224, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, L.; Zeng, H.; Chen, L.Y.; Zheng, Y.; Li, C.X.; Yang, Z.P.; Wu, N.; Mu, X.; Dai, C.Y.; et al. iTRAQ-based proteomics screen for potential regulators of wheat (Triticum aestivum L.) root cell wall component response to Al stress. Gene 2018, 675, 301–311. [Google Scholar] [CrossRef]
- Ezaki, B.; Gardner, R.C.; Ezaki, Y.; Matsumoto, H. Expression of aluminum-induced genes in transgenic Arabidopsis plants can ameliorate aluminum stress and/or oxidative stress. Plant Physiol. 2000, 122, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Yang, Z.; How, J.; Xu, H.; Chen, L.; Li, K. Overexpression of a peroxidase gene (AtPrx64) of Arabidopsis thaliana in tobacco improves plant’s tolerance to aluminum stress. Plant Mol. Biol. 2017, 95, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.J.; Jin, C.W.; Liu, W.J.; Zhang, Y.S.; Lin, X.Y. Pretreatment with H2O2 Alleviates Aluminum-induced Oxidative Stress in Wheat Seedlings. J. Integr. Plant Biol. 2011, 53, 44–53. [Google Scholar] [CrossRef]
- Ryan, P.; Delhaize, E.; Jones, D. Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Biol. 2001, 52, 527–560. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef]
- Rengel, Z. Aluminium cycling in the soil-plant-animal-human continuum. Biometals 2004, 17, 669–689. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, M.D.; Poschenrieder, C.; Corrales, I.; Barceló, J. Change in apoplastic aluminum during the initial growth response to aluminum by roots of a tolerant maize variety. Plant Physiol. 1999, 119, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Delhaize, E.; Zhou, M.; Ryan, P.R. Biotechnological solutions for enhancing the aluminium resistance of crop plants. In Abiotic Stress in Plants—Mechanisms and Adaptations; InTech: Brisbane, Australia, 2011; pp. 119–142. [Google Scholar]
- Delhaize, E.; Ma, J.F.; Ryan, P.R. Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci. 2012, 17, 341–348. [Google Scholar] [CrossRef]
- Sasaki, T.; Yamamoto, Y.; Ezaki, B.; Katsuhara, M.; Ahn, S.J.; Ryan, P.R.; Delhaize, E.; Matsumoto, H. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004, 37, 645–653. [Google Scholar] [CrossRef]
- Pineros, M.A.; Cançado, G.M.; Kochian, L.V. Novel properties of the wheat aluminum tolerance organic acid transporter (TaALMT1) revealed by electrophysiological characterization in Xenopus oocytes: Functional and structural implications. Plant Physiol. 2008, 147, 2131–2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.-H.; Ryan, P.R.; Sasaki, T.; Yamamoto, Y.; Sullivan, W.; Tyerman, S.D. Characterization of the TaALMT1 protein as an Al3+-activated anion channel in transformed tobacco (Nicotiana tabacum L.) cells. Plant Cell Physiol. 2008, 49, 1316–1330. [Google Scholar] [CrossRef] [Green Version]
- Hoekenga, O.A.; Maron, L.G.; Pineros, M.A.; Cancado, G.M.; Shaff, J.; Kobayashi, Y.; Ryan, P.R.; Dong, B.; Delhaize, E.; Sasaki, T.; et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 9738–9743. [Google Scholar] [CrossRef] [Green Version]
- Ligaba, A.; Katsuhara, M.; Ryan, P.R.; Shibasaka, M.; Matsumoto, H. The BnALMT1 and BnALMT2 genes from rape encode aluminum-activated malate transporters that enhance the aluminum resistance of plant cells. Plant Physiol. 2006, 142, 1294–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontecha, G.; Silva-Navas, J.; Benito, C.; Mestres, M.; Espino, F.; Hernandez-Riquer, M.; Gallego, F. Candidate gene identification of an aluminum-activated organic acid transporter gene at the Alt4 locus for aluminum tolerance in rye (Secale cereale L.). Theor. Appl. Genet. 2007, 114, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.C.; Shirley, N.J.; Saeed, M.; Pallotta, M.; Gustafson, J.P. An ALMT1 Gene Cluster Controlling Aluminum Tolerance at the Alt4 Locus of Rye (Secale cereale L.). Genetics 2008, 179, 669–682. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Pineros, M.A.; Tian, J.; Yao, Z.; Sun, L.; Liu, J.; Shaff, J.; Coluccio, A.; Kochian, L.V.; Liao, H. Low pH, aluminum, and phosphorus coordinately regulate malate exudation through GmALMT1 to improve soybean adaptation to acid soils. Plant Physiol. 2013, 161, 1347–1361. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, J.; Yamaji, N.; Wang, H.; Mitani, N.; Murata, Y.; Sato, K.; Katsuhara, M.; Takeda, K.; Ma, J.F. An aluminum-activated citrate transporter in barley. Plant Cell Physiol. 2007, 48, 1081–1091. [Google Scholar] [CrossRef] [Green Version]
- Magalhaes, J.V.; Liu, J.; Guimaraes, C.T.; Lana, U.G.; Alves, V.M.; Wang, Y.H.; Schaffert, R.E.; Hoekenga, O.A.; Pineros, M.A.; Shaff, J.E.; et al. A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat. Genet. 2007, 39, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Magalhaes, J.V.; Shaff, J.; Kochian, L.V. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009, 57, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maron, L.G.; Piñeros, M.A.; Guimarães, C.T.; Magalhaes, J.V.; Pleiman, J.K.; Mao, C.; Shaff, J.; Belicuas, S.N.; Kochian, L.V. Two functionally distinct members of the MATE (multi-drug and toxic compound extrusion) family of transporters potentially underlie two major aluminum tolerance QTLs in maize. Plant J. 2010, 61, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Yokosho, K.; Yamaji, N.; Ma, J.F. An Al-inducible MATE gene is involved in external detoxification of Al in rice. Plant J. 2011, 68, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Yang, J.L.; Zhou, Y.; Pineros, M.A.; Kochian, L.V.; Li, G.X.; Zheng, S.J. A de novo synthesis citrate transporter, Vigna umbellata multidrug and toxic compound extrusion, implicates in Al-activated citrate efflux in rice bean (Vigna umbellata) root apex. Plant Cell Environ. 2011, 34, 2138–2148. [Google Scholar] [CrossRef]
- Sawaki, Y.; Kihara-Doi, T.; Kobayashi, Y.; Nishikubo, N.; Kawazu, T.; Kobayashi, Y.; Koyama, H.; Sato, S. Characterization of Al-responsive citrate excretion and citrate-transporting MATEs in Eucalyptus camaldulensis. Planta 2013, 237, 979–989. [Google Scholar] [CrossRef]
- Tovkach, A.; Ryan, P.R.; Richardson, A.E.; Lewis, D.C.; Rathjen, T.M.; Ramesh, S.; Tyerman, S.D.; Delhaize, E. Transposon-mediated alteration of TaMATE1B expression in wheat confers constitutive citrate efflux from root apices. Plant Physiol. 2013, 161, 880–892. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Oliveira, A.L.; Martins-Lopes, P.; Tolra, R.; Poschenrieder, C.; Tarquis, M.; Guedes-Pinto, H.; Benito, C. Molecular characterization of the citrate transporter gene TaMATE1 and expression analysis of upstream genes involved in organic acid transport under Al stress in bread wheat (Triticum aestivum). Physiol. Plant. 2014, 152, 441–452. [Google Scholar] [CrossRef]
- Wu, X.; Li, R.; Shi, J.; Wang, J.; Sun, Q.; Zhang, H.; Xing, Y.; Qi, Y.; Zhang, N.; Guo, Y.-D. Brassica oleracea MATE Encodes a Citrate Transporter and Enhances Aluminum Tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 1426–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Yi, R.; Li, L.; Liang, Z.; Zeng, T.; Zhang, Y.; Huang, H.; Zhang, X.; Yin, X.; Cai, Z.; et al. GsMATE encoding a multidrug and toxic compound extrusion transporter enhances aluminum tolerance in Arabidopsis thaliana. BMC Plant Biol. 2018, 18, 212. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Nagao, S.; Sato, K.; Ito, H.; Furukawa, J.; Takeda, K. Molecular mapping of a gene responsible for Al-activated secretion of citrate in barley. J. Exp. Bot. 2004, 55, 1335–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, B.; Korir, P.; Zhao, T.; Yu, D.; Chen, S.; Gai, J. Mapping quantitative trait loci associated with aluminum toxin tolerance in NJRIKY recombinant inbred line population of soybean (Glycine max). J. Integr. Plant Biol. 2008, 50, 1089–1095. [Google Scholar] [CrossRef]
- Sharma, A.D.; Sharma, H.; Lightfoot, D.A. The genetic control of tolerance to aluminum toxicity in the ‘Essex’ by ‘Forrest’ recombinant inbred line population. Theor. Appl. Genet. 2011, 122, 687–694. [Google Scholar] [CrossRef]
- Famoso, A.N.; Zhao, K.; Clark, R.T.; Tung, C.-W.; Wright, M.H.; Bustamante, C.; Kochian, L.V.; McCouch, S.R. Genetic architecture of aluminum tolerance in rice (Oryza sativa) determined through genome-wide association analysis and QTL mapping. PLoS Genet. 2011, 7, e1002221. [Google Scholar] [CrossRef] [Green Version]
- Korir, P.C.; Zhang, J.; Wu, K.; Zhao, T.; Gai, J. Association mapping combined with linkage analysis for aluminum tolerance among soybean cultivars released in Yellow and Changjiang River Valleys in China. Theor. Appl. Genet. 2013, 126, 1659–1675. [Google Scholar] [CrossRef]
- Daspute, A.A.; Sadhukhan, A.; Tokizawa, M.; Kobayashi, Y.; Panda, S.K.; Koyama, H. Transcriptional Regulation of Aluminum-Tolerance Genes in Higher Plants: Clarifying the Underlying Molecular Mechanisms. Front. Plant Sci. 2017, 8, 1358. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, N.; Chen, P.; Gao, M.; Liu, J.; Wang, Y.; Zhao, T.; Li, Y.; Gai, J. Overexpression of a Soybean Ariadne-Like Ubiquitin Ligase Gene GmARI1 Enhances Aluminum Tolerance in Arabidopsis. PLoS ONE 2014, 9, e111120. [Google Scholar] [CrossRef] [Green Version]
- Duressa, D.; Soliman, K.; Taylor, R.; Senwo, Z. Proteomic Analysis of Soybean Roots under Aluminum Stress. Int. J. Plant Genom. 2011, 2011, 282531. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Zhang, H.; Liu, N.; Gao, L.; Kong, L.; Yang, Z. Transcriptomic responses to aluminum stress in soybean roots. Genome 2011, 54, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Y.; Wang, W.; Gai, J.; Li, Y. Genome-wide analysis of MATE transporters and expression patterns of a subgroup of MATE genes in response to aluminum toxicity in soybean. BMC Genom. 2016, 17, 223. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Jiang, L.; Su, N.; Wang, J.K.; Deng, P.; Ma, J.F.; Zhai, H.Q.; Wan, J.M. The genetic basic and fine-mapping of a stable quantitative-trait loci for aluminium tolerance in rice. Planta 2007, 227, 255–262. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Delhaize, E.; Ryan, P.R.; Hebb, D.M.; Yamamoto, Y.; Sasaki, T.; Matsumoto, H. Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proc. Natl. Acad. Sci. USA 2004, 101, 15249–15254. [Google Scholar] [CrossRef] [Green Version]
- Irizarry, R.A.; Hobbs, B.; Collin, F.; Beazer-Barclay, Y.D.; Antonellis, K.J.; Scherf, U.; Speed, T.P. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003, 4, 249–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usadel, B.; Nagel, A.; Thimm, O.; Redestig, H.; Blaesing, O.E.; Palacios-Rojas, N.; Selbig, J.; Hannemann, J.; Piques, M.C.; Steinhauser, D.; et al. Extension of the visualization tool MapMan to allow statistical analysis of arrays, display of corresponding genes, and comparison with known responses. Plant Physiol. 2005, 138, 1195–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, A.I.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A free, open-source system for microarray data management and analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Z.; Zhou, X.; Ling, Y.; Zhang, Z.; Su, Z. agriGO: A GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010, 38, W64–W70. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2016, 45, D1040–D1045. [Google Scholar] [CrossRef] [Green Version]
- Maron, L.G.; Kirst, M.; Mao, C.; Milner, M.J.; Menossi, M.; Kochian, L.V. Transcriptional profiling of aluminum toxicity and tolerance responses in maize roots. New Phytol. 2008, 179, 116–128. [Google Scholar] [CrossRef] [Green Version]
- Suo, Y.; Gao, S.; Baranzoni, G.M.; Xie, Y.; Liu, Y. Comparative transcriptome RNA-Seq analysis of Listeria monocytogenes with sodium lactate adaptation. Food Control 2018, 91, 193–201. [Google Scholar] [CrossRef]
- Kumari, M.; Taylor, G.J.; Deyholos, M.K. Transcriptomic responses to aluminum stress in roots of Arabidopsis thaliana. Mol. Genet. Genom. 2008, 279, 339–357. [Google Scholar] [CrossRef]
- Eticha, D.; Zahn, M.; Bremer, M.; Yang, Z.; Rangel, A.F.; Rao, I.M.; Horst, W.J. Transcriptomic analysis reveals differential gene expression in response to aluminium in common bean (Phaseolus vulgaris) genotypes. Ann. Bot. 2010, 105, 1119–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsutsui, T.; Yamaji, N.; Huang, C.F.; Motoyama, R.; Nagamura, Y.; Ma, J.F. Comparative genome-wide transcriptional analysis of Al-responsive genes reveals novel Al tolerance mechanisms in rice. PLoS ONE 2012, 7, e48197. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.; Zhu, Y.; Zou, J.; Zhao, F.-J.; Huang, C.-F. Genome-wide transcriptomic and phylogenetic analyses reveal distinct aluminum-tolerance mechanisms in the aluminum-accumulating species buckwheat (Fagopyrum tataricum). BMC Plant Biol. 2015, 15, 1. [Google Scholar] [CrossRef] [Green Version]
- Nunesnesi, A.; Santos, B.D.; Inostrozablancheteau, C.; Fernie, A.R.; Araújo, W.L. The complex role of mitochondrial metabolism in plant aluminum resistance. Trends Plant Sci. 2014, 19, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Begum, H.H.; Osaki, M.; Shinano, T.; Miyatake, H.; Wasaki, J.; Yamamura, T.; Watanabe, T. The Function of a Maize-Derived Phosphoenol pyruvate Carboxylase (PEPC) in Phosphorus-Deficient Transgenic Rice. Soil Sci. Plant Nutr. 2005, 51, 497–506. [Google Scholar] [CrossRef]
- Ma, B.; Gao, L.; Zhang, H.; Cui, J.; Shen, Z. Aluminum-induced oxidative stress and changes in antioxidant defenses in the roots of rice varieties differing in Al tolerance. Plant Cell Rep. 2012, 31, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.R.; Yamamoto, Y.; Matsumoto, H. An intracellular mechanism of aluminum tolerance associated with high antioxidant status in cultured tobacco cells. J. Inorg. Biochem. 2003, 97, 59–68. [Google Scholar] [CrossRef]
- Tamás, L.; Budíková, S.; Huttová, J.; Mistrík, I.; Šimonovičová, M.; Široká, B. Aluminum-induced cell death of barley-root border cells is correlated with peroxidase- and oxalate oxidase-mediated hydrogen peroxide production. Plant Cell Rep. 2005, 24, 189–194. [Google Scholar] [CrossRef]
- Gruber, V.; Blanchet, S.; Diet, A.; Zahaf, O.; Boualem, A.; Kakar, K.; Alunni, B.; Udvardi, M.; Frugier, F.; Crespi, M. Identification of transcription factors involved in root apex responses to salt stress in Medicago truncatula. Mol. Genet. Genom. 2009, 281, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-Y.; Hwang, E.Y.; Seok, H.-Y.; Tarte, V.N.; Jeong, M.S.; Jang, S.B.; Moon, Y.-H. Arabidopsis AtERF71/HRE2 functions as transcriptional activator via cis-acting GCC box or DRE/CRT element and is involved in root development through regulation of root cell expansion. Plant Cell Rep. 2015, 34, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.-Y.; Yang, C.-Y.; Ma, Q.-B.; Li, X.-P.; Dong, W.-W.; Nian, H. Identification of wild soybean miRNAs and their target genes responsive to aluminum stress. BMC Plant Biol. 2012, 12, 1. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Brader, G.; Palva, E.T. The WRKY70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eulgem, T.; Somssich, I.E. Networks of WRKY transcription factors in defense signaling. Curr. Opin. Plant Biol. 2007, 10, 366–371. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.J.; Yan, J.Y.; Xu, X.Y.; Li, G.X.; Zheng, S.J. WRKY46 functions as a transcriptional repressor of ALMT1, regulating aluminum-induced malate secretion in Arabidopsis. Plant J. 2013, 76, 825–835. [Google Scholar] [CrossRef]
- Kochian, L.V.; Pineros, M.A.; Liu, J.; Magalhaes, J.V. Plant adaptation to Acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Larsen, P.B.; Geisler, M.J.; Jones, C.A.; Williams, K.M.; Cancel, J.D. ALS3 encodes a phloem-localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis. Plant J. 2005, 41, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Yamaji, N.; Mitani, N.; Yano, M.; Nagamura, Y.; Ma, J.F. A bacterial-type ABC transporter is involved in aluminum tolerance in rice. Plant Cell 2009, 21, 655–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber, B.D.; Ryan, P.R.; Richardson, A.E.; Tyerman, S.D.; Ramesh, S.; Hebb, D.M.; Howitt, S.M.; Delhaize, E. HvALMT1 from barley is involved in the transport of organic anions. J. Exp. Bot. 2010, 61, 1455–1467. [Google Scholar] [CrossRef]
- Ryan, P.R.; Raman, H.; Gupta, S.; Sasaki, T.; Yamamoto, Y.; Delhaize, E. The multiple origins of aluminium resistance in hexaploid wheat include Aegilops tauschii and more recent cis mutations to TaALMT1. Plant J. 2010, 64, 446–455. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.B.; Gong, L.; Chen, A.L.; Liu, N.; Li, S.; Sun, H.R.; Yang, Z.M.; You, J.F. Functional characterization of three MATE genes in relation to aluminum-induced citrate efflux from soybean root. Plant Soil 2019, 443, 121–138. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Wang, X.; Wang, N.; Li, Y.; Jin, T.; Gai, J.; Li, Y. Comparative Analyses Reveal Peroxidases Play Important Roles in Soybean Tolerance to Aluminum Toxicity. Agronomy 2021, 11, 670. https://doi.org/10.3390/agronomy11040670
Liu J, Wang X, Wang N, Li Y, Jin T, Gai J, Li Y. Comparative Analyses Reveal Peroxidases Play Important Roles in Soybean Tolerance to Aluminum Toxicity. Agronomy. 2021; 11(4):670. https://doi.org/10.3390/agronomy11040670
Chicago/Turabian StyleLiu, Juge, Xiangting Wang, Ning Wang, Yang Li, Ting Jin, Junyi Gai, and Yan Li. 2021. "Comparative Analyses Reveal Peroxidases Play Important Roles in Soybean Tolerance to Aluminum Toxicity" Agronomy 11, no. 4: 670. https://doi.org/10.3390/agronomy11040670





